 Found 129 hits with Last Name = 'naoe' and Initial = 'y'
Found 129 hits with Last Name = 'naoe' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093793
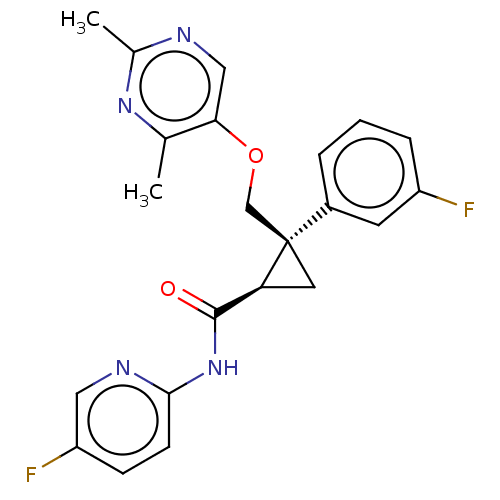
(E-2006 | Lemborexant)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R expressed in HEK-293 cells assessed as inhibition of orexin A-induced calcium accumulation by FLIPR assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093788
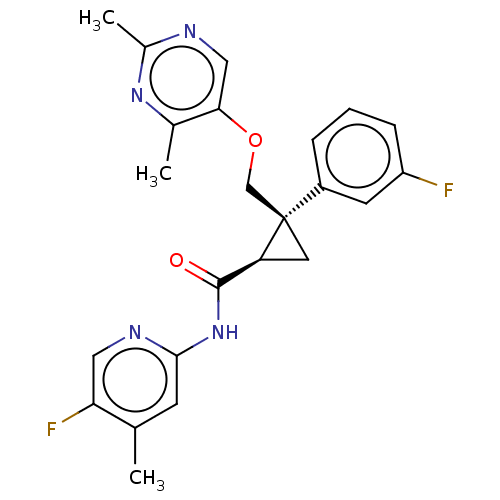
(CHEMBL3585957)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cc(C)c(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C19H28N2O/c22-19(17-9-5-2-6-10-17)20-18-12-14-21(15-18)13-11-16-7-3-1-4-8-16/h1,3-4,7-8,17-18H,2,5-6,9-15H2,(H,20,22)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093815

(CHEMBL3585948)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(Cl)cn2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C57H82Cl2N2O8/c1-33(2)14-12-15-34(3)43-20-21-44-40-19-18-38-26-35(22-24-56(38,10)45(40)23-25-57(43,44)11)16-13-17-39(36-27-41(50(64)46(58)29-36)52(66)60-31-48(62)68-54(4,5)6)37-28-42(51(65)47(59)30-37)53(67)61-32-49(63)69-55(7,8)9/h17,27-30,33-35,38,40,43-45,64-65H,12-16,18-26,31-32H2,1-11H3,(H,60,66)(H,61,67)/t34?,35-,38?,40?,43+,44?,45?,56-,57+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093791
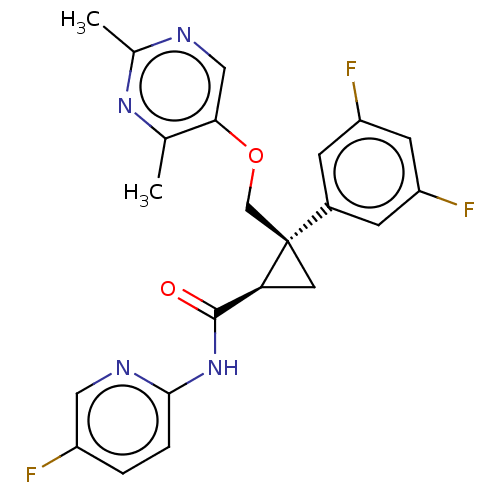
(CHEMBL3585955)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cc(F)cc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H19F3N4O2/c1-12-19(10-26-13(2)28-12)31-11-22(14-5-16(24)7-17(25)6-14)8-18(22)21(30)29-20-4-3-15(23)9-27-20/h3-7,9-10,18H,8,11H2,1-2H3,(H,27,29,30)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093795

(CHEMBL3585952)Show SMILES COc1cc(NC(=O)[C@@H]2C[C@@]2(COc2cnc(C)nc2C)c2ccccc2)ncc1F |r| Show InChI InChI=1S/C23H23FN4O3/c1-14-20(12-25-15(2)27-14)31-13-23(16-7-5-4-6-8-16)10-17(23)22(29)28-21-9-19(30-3)18(24)11-26-21/h4-9,11-12,17H,10,13H2,1-3H3,(H,26,28,29)/t17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093793

(E-2006 | Lemborexant)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093788

(CHEMBL3585957)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cc(C)c(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C19H28N2O/c22-19(17-9-5-2-6-10-17)20-18-12-14-21(15-18)13-11-16-7-3-1-4-8-16/h1,3-4,7-8,17-18H,2,5-6,9-15H2,(H,20,22)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093790

(CHEMBL3585956)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccc(F)c(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H19F3N4O2/c1-12-19(10-26-13(2)28-12)31-11-22(14-3-5-17(24)18(25)7-14)8-16(22)21(30)29-20-6-4-15(23)9-27-20/h3-7,9-10,16H,8,11H2,1-2H3,(H,27,29,30)/t16-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093798
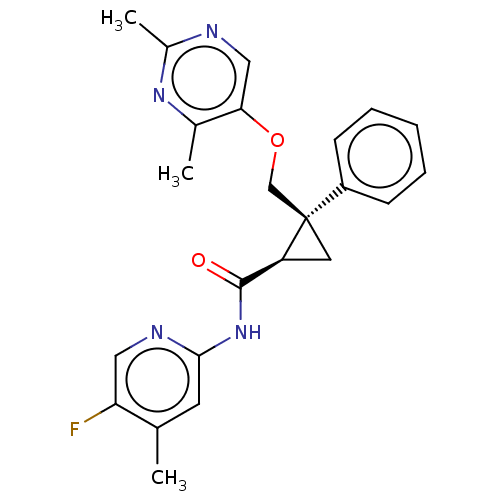
(CHEMBL3585951)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cc(C)c(F)cn2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C19H22N2O/c22-19(17-9-5-2-6-10-17)20-18-12-14-21(15-18)13-11-16-7-3-1-4-8-16/h1-10,18H,11-15H2,(H,20,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50292929
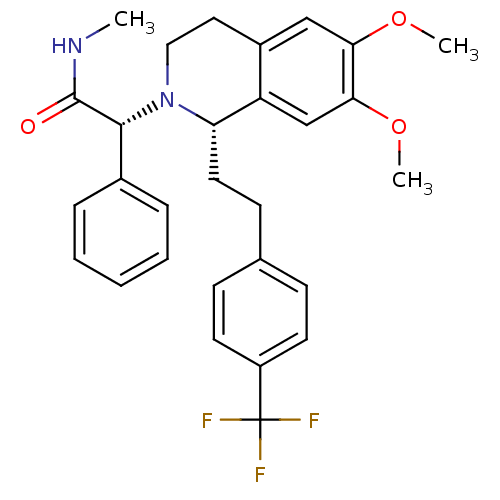
(CHEMBL455136 | almorexant)Show SMILES CNC(=O)[C@H](N1CCc2cc(OC)c(OC)cc2[C@@H]1CCc1ccc(cc1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H31F3N2O3/c1-33-28(35)27(20-7-5-4-6-8-20)34-16-15-21-17-25(36-2)26(37-3)18-23(21)24(34)14-11-19-9-12-22(13-10-19)29(30,31)32/h4-10,12-13,17-18,24,27H,11,14-16H2,1-3H3,(H,33,35)/t24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029064
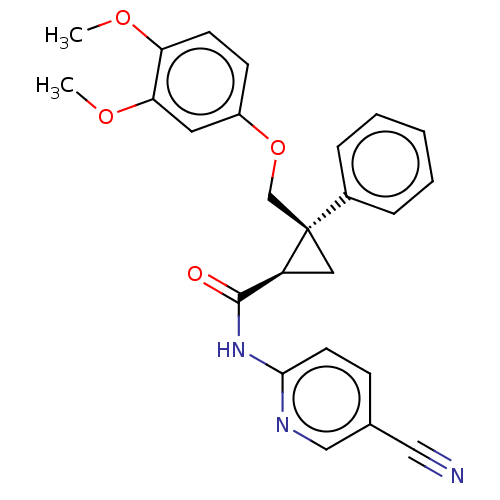
(CHEMBL3343259)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C25H23N3O4/c1-30-21-10-9-19(12-22(21)31-2)32-16-25(18-6-4-3-5-7-18)13-20(25)24(29)28-23-11-8-17(14-26)15-27-23/h3-12,15,20H,13,16H2,1-2H3,(H,27,28,29)/t20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029065
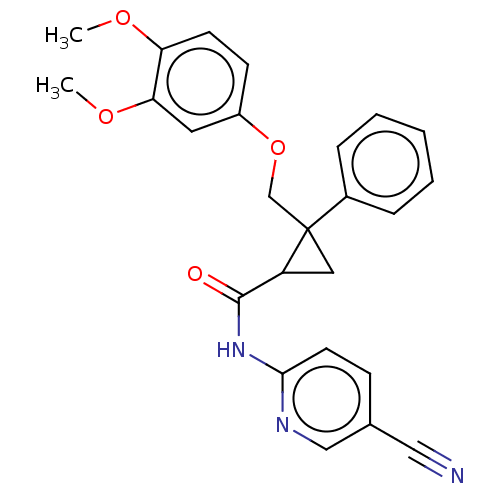
(CHEMBL3343260)Show SMILES COc1ccc(OCC2(CC2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)cc1OC Show InChI InChI=1S/C25H23N3O4/c1-30-21-10-9-19(12-22(21)31-2)32-16-25(18-6-4-3-5-7-18)13-20(25)24(29)28-23-11-8-17(14-26)15-27-23/h3-12,15,20H,13,16H2,1-2H3,(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093793

(E-2006 | Lemborexant)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R expressed in HEK-293 cells assessed as inhibition of orexin A-induced calcium accumulation by FLIPR assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093795

(CHEMBL3585952)Show SMILES COc1cc(NC(=O)[C@@H]2C[C@@]2(COc2cnc(C)nc2C)c2ccccc2)ncc1F |r| Show InChI InChI=1S/C23H23FN4O3/c1-14-20(12-25-15(2)27-14)31-13-23(16-7-5-4-6-8-16)10-17(23)22(29)28-21-9-19(30-3)18(24)11-26-21/h4-9,11-12,17H,10,13H2,1-3H3,(H,26,28,29)/t17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093798

(CHEMBL3585951)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cc(C)c(F)cn2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C19H22N2O/c22-19(17-9-5-2-6-10-17)20-18-12-14-21(15-18)13-11-16-7-3-1-4-8-16/h1-10,18H,11-15H2,(H,20,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093793

(E-2006 | Lemborexant)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cccc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093791

(CHEMBL3585955)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2cc(F)cc(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H19F3N4O2/c1-12-19(10-26-13(2)28-12)31-11-22(14-5-16(24)7-17(25)6-14)8-18(22)21(30)29-20-4-3-15(23)9-27-20/h3-7,9-10,18H,8,11H2,1-2H3,(H,27,29,30)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093900

(CHEMBL3585946)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12?,13-,14?,15?,16?,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50094022
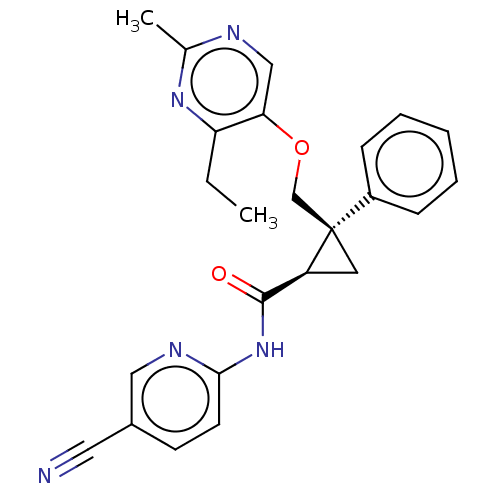
(CHEMBL3585940)Show SMILES CCc1nc(C)ncc1OC[C@]1(C[C@H]1C(=O)Nc1ccc(cn1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-3-20-21(14-26-16(2)28-20)31-15-24(18-7-5-4-6-8-18)11-19(24)23(30)29-22-10-9-17(12-25)13-27-22/h4-10,13-14,19H,3,11,15H2,1-2H3,(H,27,29,30)/t19-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50029044
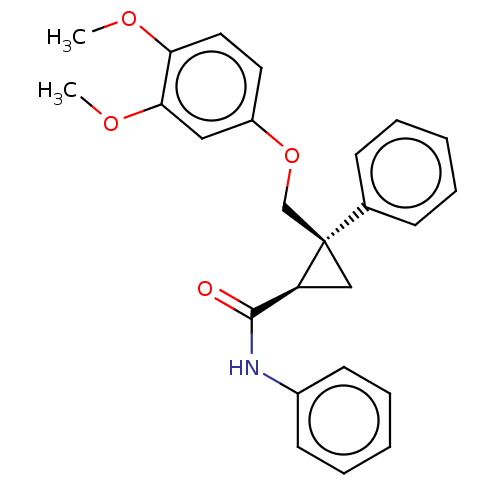
(CHEMBL3343242)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ccccc2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C25H25NO4/c1-28-22-14-13-20(15-23(22)29-2)30-17-25(18-9-5-3-6-10-18)16-21(25)24(27)26-19-11-7-4-8-12-19/h3-15,21H,16-17H2,1-2H3,(H,26,27)/t21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX1R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029044

(CHEMBL3343242)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ccccc2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C25H25NO4/c1-28-22-14-13-20(15-23(22)29-2)30-17-25(18-9-5-3-6-10-18)16-21(25)24(27)26-19-11-7-4-8-12-19/h3-15,21H,16-17H2,1-2H3,(H,26,27)/t21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50094039

(CHEMBL3585938)Show SMILES CCn1cc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C149H249N47O42/c1-9-10-11-12-16-45-118(209)238-78-106(191-132(222)100(70-81(4)5)186-133(223)101(71-84-32-14-13-15-33-84)187-137(227)105(77-199)190-135(225)103(75-197)171-115(204)73-154)144(234)194-66-29-42-108(194)139(229)180-95(51-57-117(207)208)128(218)188-102(72-85-74-165-79-169-85)134(224)179-92(47-53-112(156)201)126(216)175-90(39-26-63-167-148(161)162)130(220)192-119(82(6)7)141(231)181-93(48-54-113(157)202)127(217)177-91(46-52-111(155)200)125(215)174-89(38-25-62-166-147(159)160)122(212)173-87(35-18-22-59-151)121(211)178-94(50-56-116(205)206)129(219)189-104(76-198)136(226)176-88(36-19-23-60-152)123(213)182-96(37-20-24-61-153)142(232)196-68-31-44-110(196)145(235)195-67-30-41-107(195)138(228)170-83(8)120(210)172-86(34-17-21-58-150)124(214)185-99(69-80(2)3)131(221)183-97(49-55-114(158)203)143(233)193-65-28-43-109(193)140(230)184-98(146(236)237)40-27-64-168-149(163)164/h13-15,32-33,74,79-83,86-110,119,197-199H,9-12,16-31,34-73,75-78,150-154H2,1-8H3,(H2,155,200)(H2,156,201)(H2,157,202)(H2,158,203)(H,165,169)(H,170,228)(H,171,204)(H,172,210)(H,173,212)(H,174,215)(H,175,216)(H,176,226)(H,177,217)(H,178,211)(H,179,224)(H,180,229)(H,181,231)(H,182,213)(H,183,221)(H,184,230)(H,185,214)(H,186,223)(H,187,227)(H,188,218)(H,189,219)(H,190,225)(H,191,222)(H,192,220)(H,205,206)(H,207,208)(H,236,237)(H4,159,160,166)(H4,161,162,167)(H4,163,164,168)/t83-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107?,108+,109?,110?,119-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093792
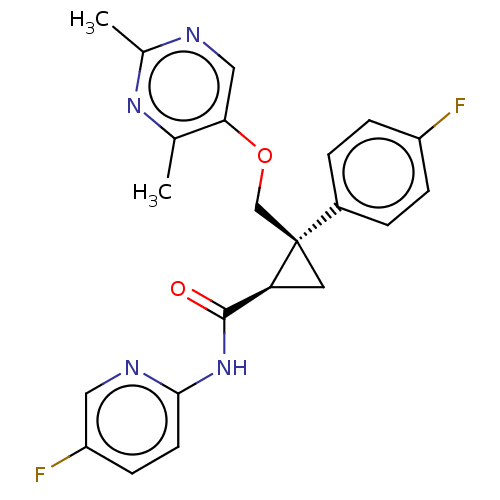
(CHEMBL3585954)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccc(F)cc2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-3-5-16(23)6-4-15)9-18(22)21(29)28-20-8-7-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50094006
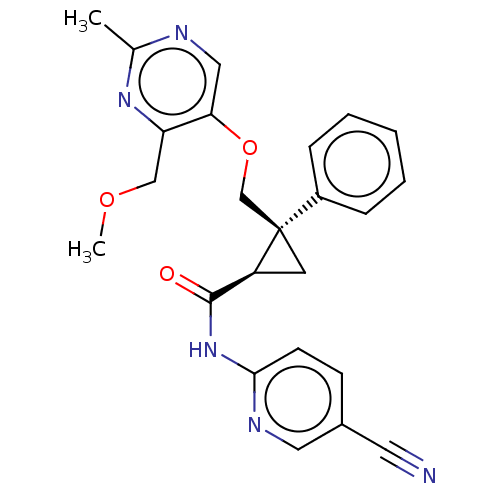
(CHEMBL3585942)Show SMILES COCc1nc(C)ncc1OC[C@]1(C[C@H]1C(=O)Nc1ccc(cn1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C21H21NO4/c23-19-11-10-18(26-19)20-17-9-5-4-8-16(17)12-13-22(20)21(24)25-14-15-6-2-1-3-7-15/h1-9,18,20H,10-14H2/t18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22950

(1-[(3R,4S)-4-hydroxy-1-(naphthalen-2-yloxy)pentan-...)Show SMILES C[C@H](O)[C@@H](CCOc1ccc2ccccc2c1)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O3/c1-13(23)18(22-11-17(19(20)24)21-12-22)8-9-25-16-7-6-14-4-2-3-5-15(14)10-16/h2-7,10-13,18,23H,8-9H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 9.80 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093780
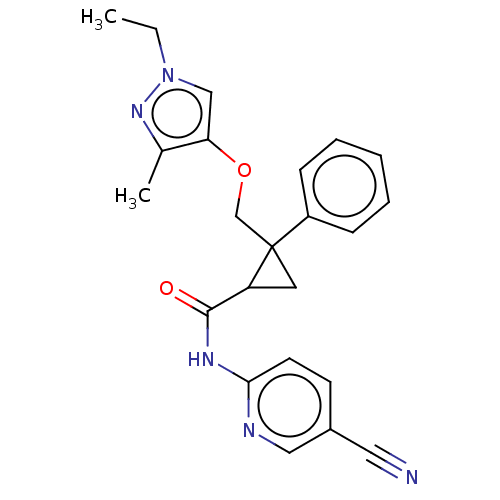
(CHEMBL3585936)Show SMILES CCn1cc(OCC2(CC2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)c(C)n1 Show InChI InChI=1S/C23H23N5O2/c1-3-28-14-20(16(2)27-28)30-15-23(18-7-5-4-6-8-18)11-19(23)22(29)26-21-10-9-17(12-24)13-25-21/h4-10,13-14,19H,3,11,15H2,1-2H3,(H,25,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22948
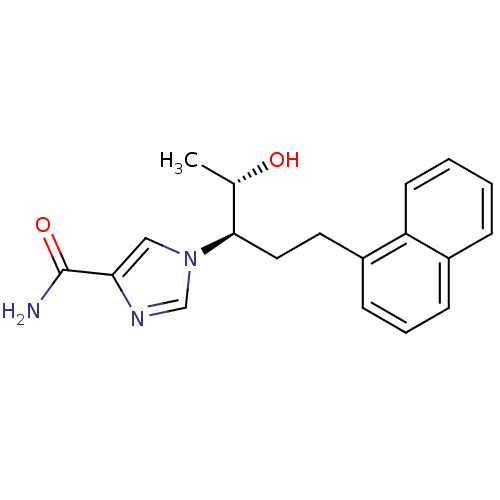
(1-[(1R,2S)-2-hydroxy-1-(2-naphthalen-1-ylethyl)pro...)Show SMILES C[C@H](O)[C@@H](CCc1cccc2ccccc12)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C19H21N3O2/c1-13(23)18(22-11-17(19(20)24)21-12-22)10-9-15-7-4-6-14-5-2-3-8-16(14)15/h2-8,11-13,18,23H,9-10H2,1H3,(H2,20,24)/t13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22949
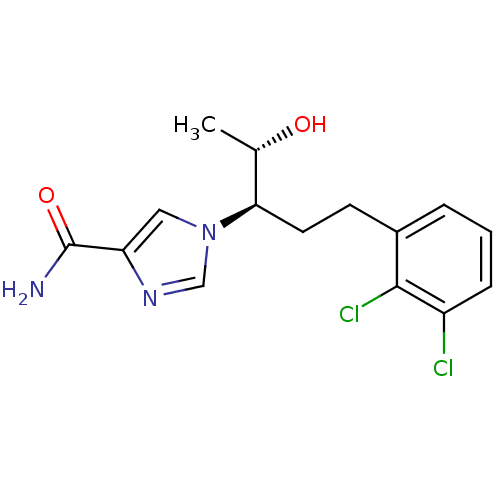
(1-[(3R,4S)-1-(2,3-dichlorophenyl)-4-hydroxypentan-...)Show SMILES C[C@H](O)[C@@H](CCc1cccc(Cl)c1Cl)n1cnc(c1)C(N)=O |r| Show InChI InChI=1S/C15H17Cl2N3O2/c1-9(21)13(20-7-12(15(18)22)19-8-20)6-5-10-3-2-4-11(16)14(10)17/h2-4,7-9,13,21H,5-6H2,1H3,(H2,18,22)/t9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
J Med Chem 47: 2728-31 (2004)
Article DOI: 10.1021/jm0499559
BindingDB Entry DOI: 10.7270/Q22F7KQT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093790

(CHEMBL3585956)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccc(F)c(F)c2)c(C)n1 |r| Show InChI InChI=1S/C22H19F3N4O2/c1-12-19(10-26-13(2)28-12)31-11-22(14-3-5-17(24)18(25)7-14)8-16(22)21(30)29-20-6-4-15(23)9-27-20/h3-7,9-10,16H,8,11H2,1-2H3,(H,27,29,30)/t16-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029064

(CHEMBL3343259)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C25H23N3O4/c1-30-21-10-9-19(12-22(21)31-2)32-16-25(18-6-4-3-5-7-18)13-20(25)24(29)28-23-11-8-17(14-26)15-27-23/h3-12,15,20H,13,16H2,1-2H3,(H,27,28,29)/t20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029060

(CHEMBL3343255)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2nc(C)cs2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C23H24N2O4S/c1-15-13-30-22(24-15)25-21(26)18-12-23(18,16-7-5-4-6-8-16)14-29-17-9-10-19(27-2)20(11-17)28-3/h4-11,13,18H,12,14H2,1-3H3,(H,24,25,26)/t18-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093794
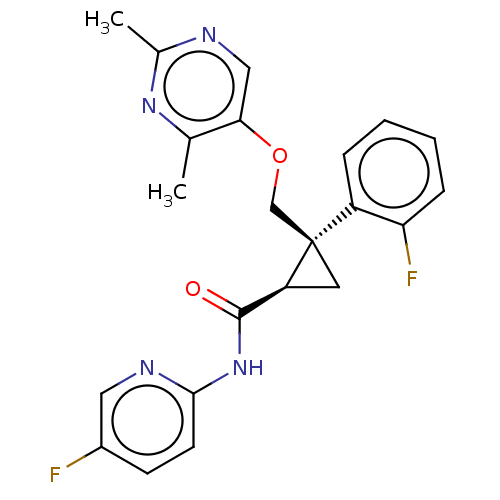
(CHEMBL3585953)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccccc2F)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(16-5-3-4-6-18(16)24)9-17(22)21(29)28-20-8-7-15(23)10-26-20/h3-8,10-11,17H,9,12H2,1-2H3,(H,26,28,29)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50094007
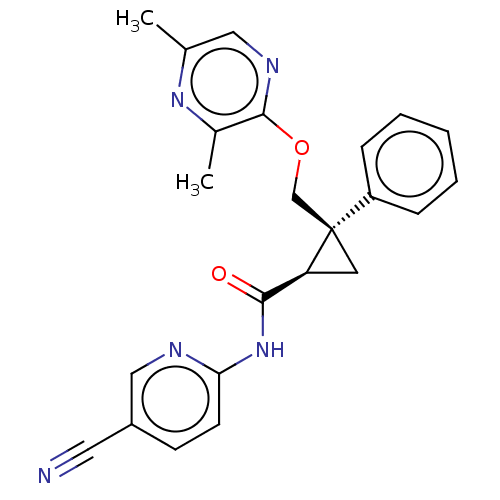
(CHEMBL3585941)Show SMILES Cc1cnc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C22H21NO4/c1-15-13-19(27-21(15)24)20-18-10-6-5-9-17(18)11-12-23(20)22(25)26-14-16-7-3-2-4-8-16/h2-10,13,19-20H,11-12,14H2,1H3/t19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029062
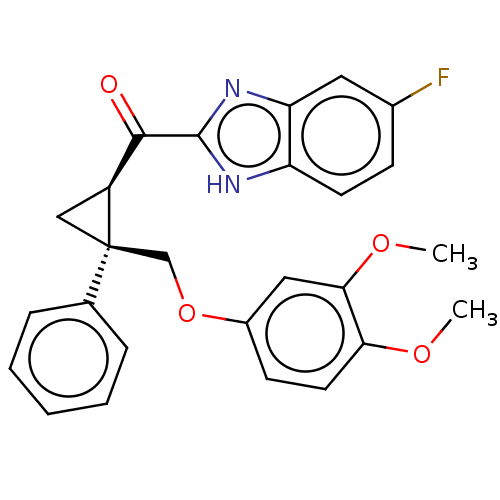
(CHEMBL3343257)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)c2nc3cc(F)ccc3[nH]2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C26H23FN2O4/c1-31-22-11-9-18(13-23(22)32-2)33-15-26(16-6-4-3-5-7-16)14-19(26)24(30)25-28-20-10-8-17(27)12-21(20)29-25/h3-13,19H,14-15H2,1-2H3,(H,28,29)/t19-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029061
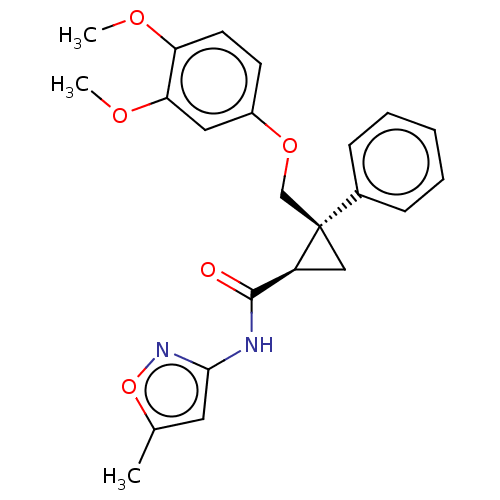
(CHEMBL3343256)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2cc(C)on2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C23H24N2O5/c1-15-11-21(25-30-15)24-22(26)18-13-23(18,16-7-5-4-6-8-16)14-29-17-9-10-19(27-2)20(12-17)28-3/h4-12,18H,13-14H2,1-3H3,(H,24,25,26)/t18-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093800
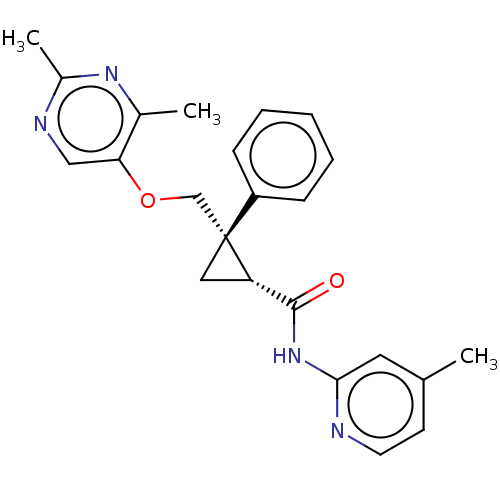
(CHEMBL3585950)Show SMILES Cc1ccnc(NC(=O)[C@@H]2C[C@@]2(COc2cnc(C)nc2C)c2ccccc2)c1 |r| Show InChI InChI=1S/C23H24N4O2/c1-15-9-10-24-21(11-15)27-22(28)19-12-23(19,18-7-5-4-6-8-18)14-29-20-13-25-17(3)26-16(20)2/h4-11,13,19H,12,14H2,1-3H3,(H,24,27,28)/t19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029063

(CHEMBL3343258)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2cc(ccn2)C#N)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C25H23N3O4/c1-30-21-9-8-19(13-22(21)31-2)32-16-25(18-6-4-3-5-7-18)14-20(25)24(29)28-23-12-17(15-26)10-11-27-23/h3-13,20H,14,16H2,1-2H3,(H,27,28,29)/t20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093801

(CHEMBL3585949)Show SMILES Cc1ccc(NC(=O)[C@@H]2C[C@@]2(COc2cnc(C)nc2C)c2ccccc2)nc1 |r| Show InChI InChI=1S/C23H24N4O2/c1-15-9-10-21(25-12-15)27-22(28)19-11-23(19,18-7-5-4-6-8-18)14-29-20-13-24-17(3)26-16(20)2/h4-10,12-13,19H,11,14H2,1-3H3,(H,25,27,28)/t19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029059
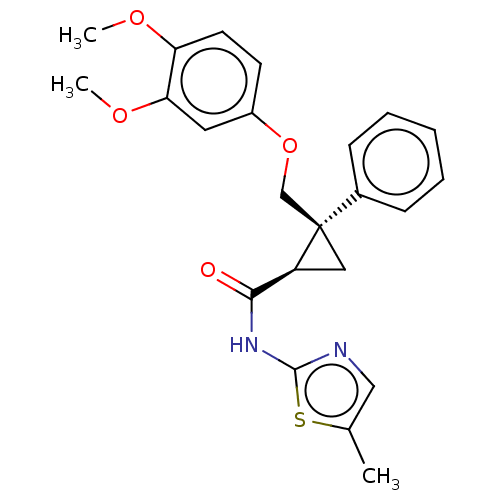
(CHEMBL3343254)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ncc(C)s2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C23H24N2O4S/c1-15-13-24-22(30-15)25-21(26)18-12-23(18,16-7-5-4-6-8-16)14-29-17-9-10-19(27-2)20(11-17)28-3/h4-11,13,18H,12,14H2,1-3H3,(H,24,25,26)/t18-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029048

(CHEMBL3343246)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2ccccn2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C24H24N2O4/c1-28-20-12-11-18(14-21(20)29-2)30-16-24(17-8-4-3-5-9-17)15-19(24)23(27)26-22-10-6-7-13-25-22/h3-14,19H,15-16H2,1-2H3,(H,25,26,27)/t19-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093792

(CHEMBL3585954)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(F)cn2)c2ccc(F)cc2)c(C)n1 |r| Show InChI InChI=1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-3-5-16(23)6-4-15)9-18(22)21(29)28-20-8-7-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50093815

(CHEMBL3585948)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(Cl)cn2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C57H82Cl2N2O8/c1-33(2)14-12-15-34(3)43-20-21-44-40-19-18-38-26-35(22-24-56(38,10)45(40)23-25-57(43,44)11)16-13-17-39(36-27-41(50(64)46(58)29-36)52(66)60-31-48(62)68-54(4,5)6)37-28-42(51(65)47(59)30-37)53(67)61-32-49(63)69-55(7,8)9/h17,27-30,33-35,38,40,43-45,64-65H,12-16,18-26,31-32H2,1-11H3,(H,60,66)(H,61,67)/t34?,35-,38?,40?,43+,44?,45?,56-,57+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50094006

(CHEMBL3585942)Show SMILES COCc1nc(C)ncc1OC[C@]1(C[C@H]1C(=O)Nc1ccc(cn1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C21H21NO4/c23-19-11-10-18(26-19)20-17-9-5-4-8-16(17)12-13-22(20)21(24)25-14-15-6-2-1-3-7-15/h1-9,18,20H,10-14H2/t18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50094038
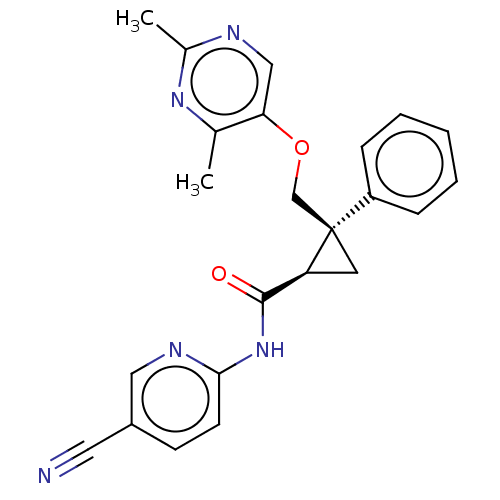
(CHEMBL3585939)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2ccc(cn2)C#N)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C21H21NO4/c23-19-11-10-18(26-19)20-17-9-5-4-8-16(17)12-13-22(20)21(24)25-14-15-6-2-1-3-7-15/h1-9,18,20H,10-14H2/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093976

(CHEMBL3585944)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cccnc2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C25H38O4/c1-16(2)8-6-12-24(4)13-7-9-20-19(24)11-10-17(3)25(20,5)15-21(26)18-14-22(27)29-23(18)28/h11,14,17,20-21,23,26,28H,1,6-10,12-13,15H2,2-5H3/t17-,20-,21-,23?,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50029057

(CHEMBL3343252)Show SMILES COc1ccc(OC[C@]2(C[C@H]2C(=O)Nc2cccnc2)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C24H24N2O4/c1-28-21-11-10-19(13-22(21)29-2)30-16-24(17-7-4-3-5-8-17)14-20(24)23(27)26-18-9-6-12-25-15-18/h3-13,15,20H,14,16H2,1-2H3,(H,26,27)/t20-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis |
Bioorg Med Chem 22: 6071-88 (2014)
Article DOI: 10.1016/j.bmc.2014.08.034
BindingDB Entry DOI: 10.7270/Q20R9R0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50093897
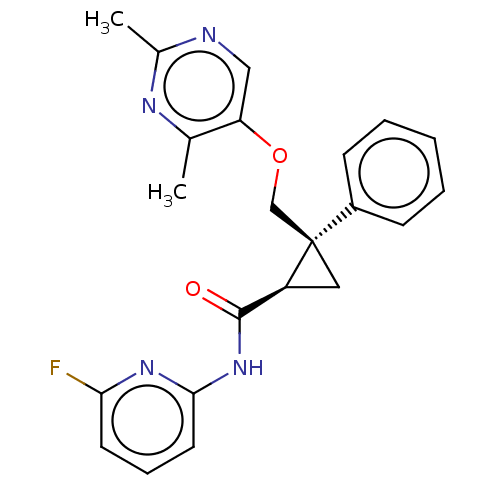
(CHEMBL3585947)Show SMILES Cc1ncc(OC[C@]2(C[C@H]2C(=O)Nc2cccc(F)n2)c2ccccc2)c(C)n1 |r| Show InChI InChI=1S/C52H54N4O2.2BrH/c1-3-11-33(12-4-1)29-55-23-21-51-39-15-7-9-17-41(39)53-47(51)45-37(27-43(51)55)35(31-55)19-25-57-49(45)54-42-18-10-8-16-40(42)52-22-24-56(30-34-13-5-2-6-14-34)32-36-20-26-58-50(53)46(48(52)54)38(36)28-44(52)56;;/h1-20,37-38,43-50H,21-32H2;2*1H/q+2;;/p-2/t37-,38-,43-,44-,45+,46+,47-,48-,49+,50+,51?,52?,55?,56?;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2R by radioligand displacement binding assay |
J Med Chem 58: 4648-64 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00217
BindingDB Entry DOI: 10.7270/Q2125VD7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data