 Found 914 hits with Last Name = 'noble' and Initial = 'sa'
Found 914 hits with Last Name = 'noble' and Initial = 'sa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50026917
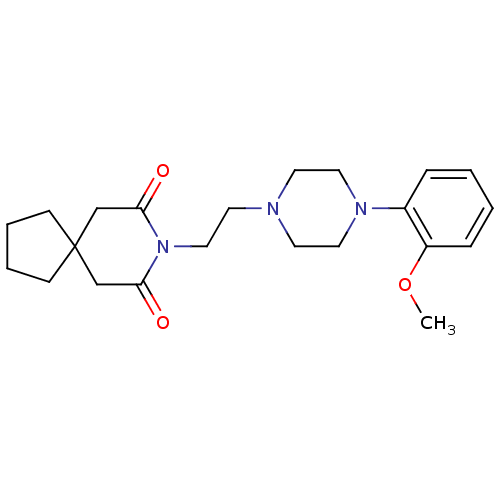
(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354066

(CHEMBL1836324)Show InChI InChI=1S/C18H25N3O2S3/c1-2-26(22,23)20-12-7-3-6-11-19-18-21-17-14-8-4-5-9-15(14)24-13-10-16(17)25-18/h4-5,8-9,20H,2-3,6-7,10-13H2,1H3,(H,19,21) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354068
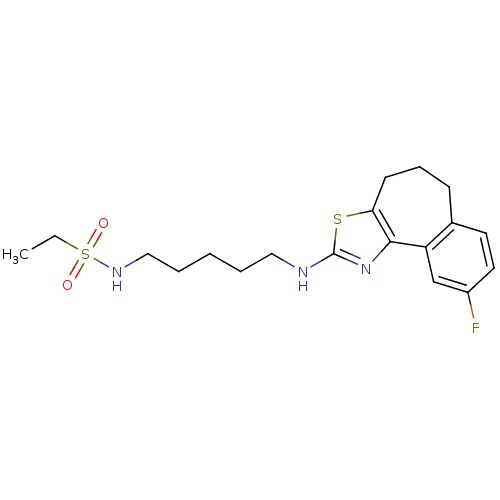
(CHEMBL1836322)Show SMILES CCS(=O)(=O)NCCCCCNc1nc-2c(CCCc3ccc(F)cc-23)s1 Show InChI InChI=1S/C19H26FN3O2S2/c1-2-27(24,25)22-12-5-3-4-11-21-19-23-18-16-13-15(20)10-9-14(16)7-6-8-17(18)26-19/h9-10,13,22H,2-8,11-12H2,1H3,(H,21,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143664
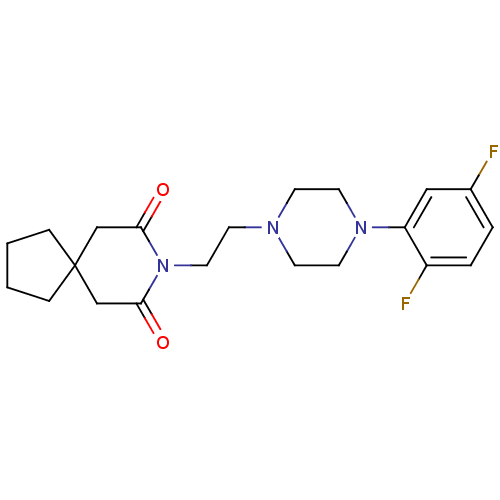
(8-{2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(F)c(c1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-17(23)18(13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354077
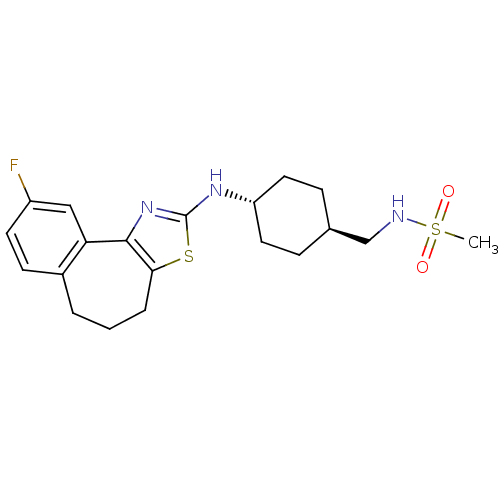
(CHEMBL1836331)Show SMILES CS(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCCc3ccc(F)cc-23)s1 |r,wU:6.5,wD:9.12,(32.91,-10.2,;31.56,-10.97,;30.79,-12.31,;32.33,-12.32,;30.22,-10.2,;28.88,-10.97,;27.54,-10.19,;26.21,-10.96,;24.87,-10.19,;24.87,-8.64,;26.21,-7.88,;27.55,-8.65,;23.53,-7.86,;22.18,-8.61,;20.82,-7.98,;19.81,-9.09,;20.55,-10.39,;19.93,-11.88,;18.42,-12.26,;17.2,-11.32,;17.17,-9.72,;15.78,-9.21,;15.53,-7.74,;16.68,-6.78,;16.42,-5.26,;18.08,-7.31,;18.32,-8.78,;22.02,-10.09,)| Show InChI InChI=1S/C20H26FN3O2S2/c1-28(25,26)22-12-13-5-9-16(10-6-13)23-20-24-19-17-11-15(21)8-7-14(17)3-2-4-18(19)27-20/h7-8,11,13,16,22H,2-6,9-10,12H2,1H3,(H,23,24)/t13-,16- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354079

(CHEMBL1836329)Show SMILES COc1cccc2-c3nc(N[C@H]4CC[C@H](CNS(C)(=O)=O)CC4)sc3CCOc12 |r,wU:14.14,wD:11.10,(19.34,4.17,;20.79,3.64,;21.98,4.62,;21.73,6.09,;22.88,7.04,;24.28,6.52,;24.53,5.05,;26.01,4.74,;27.03,5.85,;28.38,5.22,;29.74,5.97,;31.07,5.19,;31.08,3.64,;32.42,2.86,;33.75,3.64,;35.09,2.86,;36.43,3.63,;37.77,2.85,;39.11,3.63,;36.99,1.52,;38.54,1.51,;33.76,5.18,;32.42,5.95,;28.22,3.73,;26.75,3.44,;26.13,1.95,;24.63,1.57,;23.41,2.5,;23.37,4.11,)| Show InChI InChI=1S/C20H27N3O4S2/c1-26-16-5-3-4-15-18-17(10-11-27-19(15)16)28-20(23-18)22-14-8-6-13(7-9-14)12-21-29(2,24)25/h3-5,13-14,21H,6-12H2,1-2H3,(H,22,23)/t13-,14- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50122803

((SNAP-8719)[4-(4-Amino-6,7-dimethoxy-quinazolin-2-...)Show SMILES C[C@H](CN1CCN(CC1)c1cc(F)c(F)cc1F)N1C(=O)CC2(CCCC2)CC1=O Show InChI InChI=1S/C22H28F3N3O2/c1-15(28-20(29)12-22(13-21(28)30)4-2-3-5-22)14-26-6-8-27(9-7-26)19-11-17(24)16(23)10-18(19)25/h10-11,15H,2-9,12-14H2,1H3/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354070
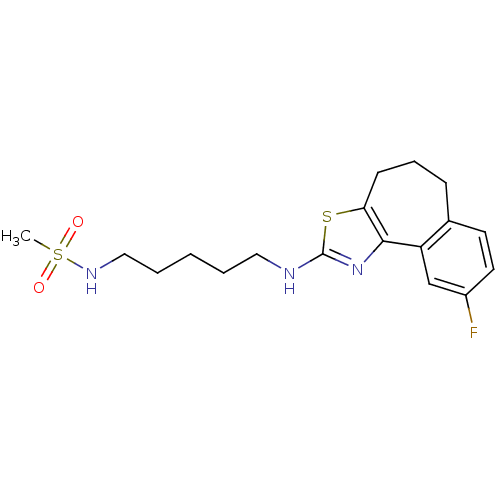
(CHEMBL1836319)Show SMILES CS(=O)(=O)NCCCCCNc1nc-2c(CCCc3ccc(F)cc-23)s1 Show InChI InChI=1S/C18H24FN3O2S2/c1-26(23,24)21-11-4-2-3-10-20-18-22-17-15-12-14(19)9-8-13(15)6-5-7-16(17)25-18/h8-9,12,21H,2-7,10-11H2,1H3,(H,20,22) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354083

(CHEMBL1836102 | N-{[(1r,4r)-4-{[(4-aminoquinazolin...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3ccc4ccccc4c3)CC2)nc2ccccc12 |r,wU:9.9,wD:6.5,(22.1,-1.37,;22.11,-2.91,;23.44,-3.67,;23.45,-5.22,;24.79,-5.98,;26.12,-5.21,;27.46,-5.97,;27.46,-7.51,;28.79,-8.28,;30.12,-7.51,;31.45,-8.29,;32.79,-7.52,;34.12,-8.29,;33.35,-9.62,;34.89,-9.63,;35.45,-7.52,;35.45,-5.99,;36.78,-5.22,;38.12,-5.99,;39.45,-5.22,;40.78,-5.99,;40.78,-7.53,;39.45,-8.3,;38.12,-7.53,;36.79,-8.3,;30.12,-5.97,;28.79,-5.2,;22.12,-5.99,;20.78,-5.22,;19.45,-6,;18.11,-5.23,;18.11,-3.69,;19.44,-2.92,;20.78,-3.68,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-23-7-3-4-8-24(23)30-26(31-25)28-16-18-9-11-19(12-10-18)17-29-34(32,33)22-14-13-20-5-1-2-6-21(20)15-22/h1-8,13-15,18-19,29H,9-12,16-17H2,(H3,27,28,30,31)/t18-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to NPY5 receptor |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50354072

(CHEMBL1836317)Show SMILES CS(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1 |r,wU:6.5,wD:9.12,(30.35,-14.16,;30.37,-12.61,;31.92,-12.63,;31.16,-11.28,;29.04,-11.82,;29.06,-10.28,;27.73,-9.49,;26.38,-10.24,;25.06,-9.46,;25.08,-7.91,;26.42,-7.14,;27.75,-7.94,;23.75,-7.13,;22.4,-7.89,;21.04,-7.26,;20.02,-8.37,;20.76,-9.68,;20.14,-11.16,;18.63,-11.54,;17.42,-10.61,;17.38,-9,;15.99,-8.49,;15.74,-7.02,;16.89,-6.07,;18.29,-6.59,;18.53,-8.06,;22.23,-9.38,)| Show InChI InChI=1S/C19H25N3O2S3/c1-27(23,24)20-12-13-6-8-14(9-7-13)21-19-22-18-15-4-2-3-5-16(15)25-11-10-17(18)26-19/h2-5,13-14,20H,6-12H2,1H3,(H,21,22)/t13-,14- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at rat NPY5 receptor |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354072

(CHEMBL1836317)Show SMILES CS(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1 |r,wU:6.5,wD:9.12,(30.35,-14.16,;30.37,-12.61,;31.92,-12.63,;31.16,-11.28,;29.04,-11.82,;29.06,-10.28,;27.73,-9.49,;26.38,-10.24,;25.06,-9.46,;25.08,-7.91,;26.42,-7.14,;27.75,-7.94,;23.75,-7.13,;22.4,-7.89,;21.04,-7.26,;20.02,-8.37,;20.76,-9.68,;20.14,-11.16,;18.63,-11.54,;17.42,-10.61,;17.38,-9,;15.99,-8.49,;15.74,-7.02,;16.89,-6.07,;18.29,-6.59,;18.53,-8.06,;22.23,-9.38,)| Show InChI InChI=1S/C19H25N3O2S3/c1-27(23,24)20-12-13-6-8-14(9-7-13)21-19-22-18-15-4-2-3-5-16(15)25-11-10-17(18)26-19/h2-5,13-14,20H,6-12H2,1H3,(H,21,22)/t13-,14- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418564
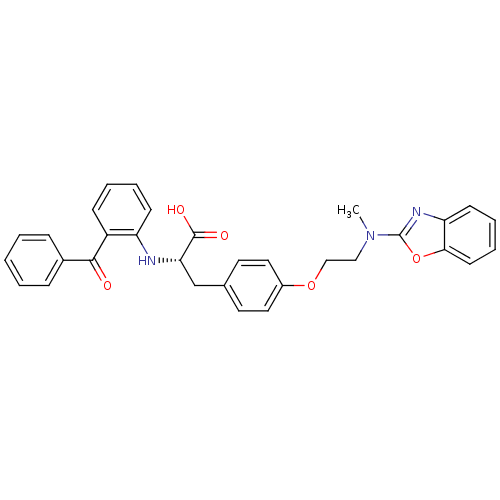
(CHEMBL423026)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354078
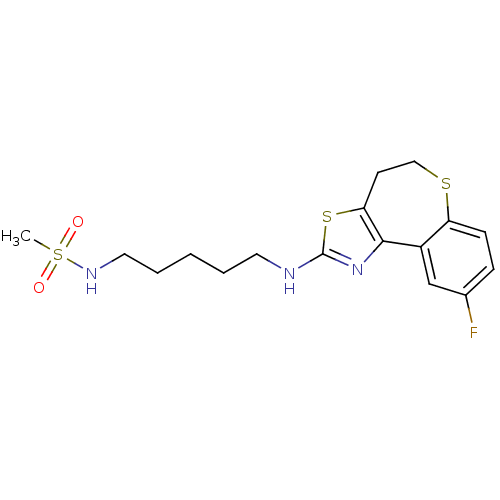
(CHEMBL1836330)Show SMILES CS(=O)(=O)NCCCCCNc1nc-2c(CCSc3ccc(F)cc-23)s1 Show InChI InChI=1S/C17H22FN3O2S3/c1-26(22,23)20-9-4-2-3-8-19-17-21-16-13-11-12(18)5-6-14(13)24-10-7-15(16)25-17/h5-6,11,20H,2-4,7-10H2,1H3,(H,19,21) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354073
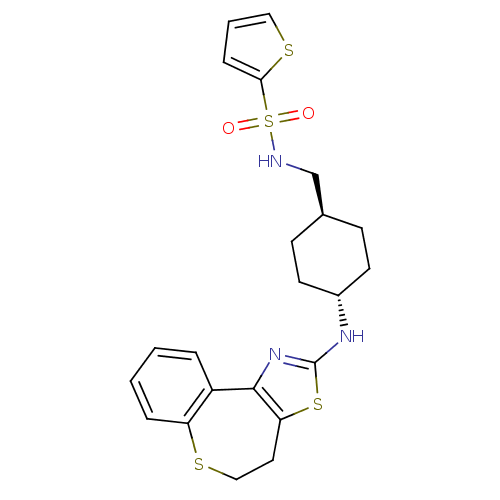
(CHEMBL1836316)Show SMILES O=S(=O)(NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1)c1cccs1 |r,wU:5.4,wD:8.11,(42.05,-18.71,;40.51,-18.69,;41.3,-17.37,;39.18,-17.9,;39.2,-16.36,;37.88,-15.57,;36.53,-16.33,;35.21,-15.54,;35.23,-14,;36.57,-13.24,;37.9,-14.03,;33.9,-13.22,;32.56,-13.98,;31.2,-13.36,;30.19,-14.46,;30.93,-15.76,;30.31,-17.24,;28.81,-17.62,;27.6,-16.69,;27.56,-15.09,;26.18,-14.58,;25.93,-13.12,;27.07,-12.17,;28.47,-12.69,;28.71,-14.15,;32.39,-15.46,;40.49,-20.23,;41.73,-21.16,;41.24,-22.62,;39.69,-22.6,;39.24,-21.12,)| Show InChI InChI=1S/C22H25N3O2S4/c26-31(27,20-6-3-12-29-20)23-14-15-7-9-16(10-8-15)24-22-25-21-17-4-1-2-5-18(17)28-13-11-19(21)30-22/h1-6,12,15-16,23H,7-11,13-14H2,(H,24,25)/t15-,16- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165417
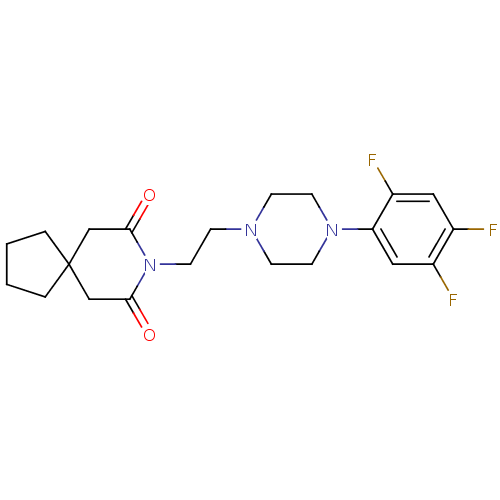
(8-{2-[4-(2,4,5-Trifluoro-phenyl)-piperazin-1-yl]-e...)Show SMILES Fc1cc(F)c(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H26F3N3O2/c22-15-11-17(24)18(12-16(15)23)26-8-5-25(6-9-26)7-10-27-19(28)13-21(14-20(27)29)3-1-2-4-21/h11-12H,1-10,13-14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354081
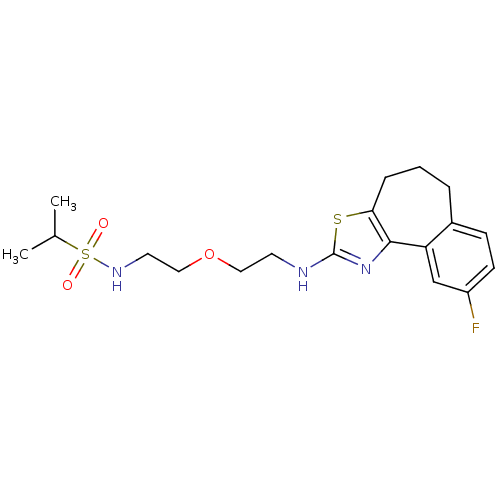
(CHEMBL1836327)Show SMILES CC(C)S(=O)(=O)NCCOCCNc1nc-2c(CCCc3ccc(F)cc-23)s1 Show InChI InChI=1S/C19H26FN3O3S2/c1-13(2)28(24,25)22-9-11-26-10-8-21-19-23-18-16-12-15(20)7-6-14(16)4-3-5-17(18)27-19/h6-7,12-13,22H,3-5,8-11H2,1-2H3,(H,21,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354071
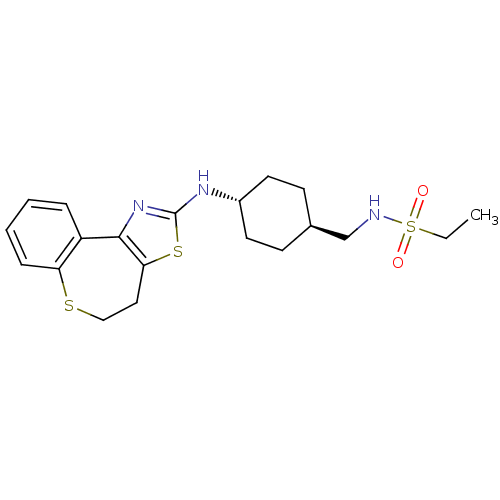
(CHEMBL1836318)Show SMILES CCS(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1 |r,wU:7.6,wD:10.13,(6.6,-30,;5.27,-29.21,;5.29,-27.67,;6.84,-27.69,;6.08,-26.34,;3.97,-26.88,;3.99,-25.34,;2.66,-24.55,;1.31,-25.3,;-.01,-24.52,;.01,-22.97,;1.35,-22.21,;2.68,-23,;-1.32,-22.19,;-2.66,-22.95,;-4.03,-22.33,;-5.04,-23.43,;-4.3,-24.74,;-4.92,-26.21,;-6.42,-26.6,;-7.64,-25.66,;-7.67,-24.07,;-9.06,-23.55,;-9.31,-22.09,;-8.16,-21.14,;-6.77,-21.66,;-6.52,-23.12,;-2.83,-24.44,)| Show InChI InChI=1S/C20H27N3O2S3/c1-2-28(24,25)21-13-14-7-9-15(10-8-14)22-20-23-19-16-5-3-4-6-17(16)26-12-11-18(19)27-20/h3-6,14-15,21H,2,7-13H2,1H3,(H,22,23)/t14-,15- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418568
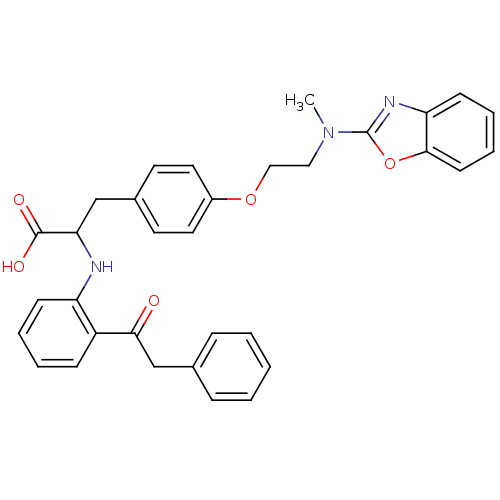
(CHEMBL146822)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2C(=O)Cc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H31N3O5/c1-36(33-35-28-13-7-8-14-31(28)41-33)19-20-40-25-17-15-24(16-18-25)21-29(32(38)39)34-27-12-6-5-11-26(27)30(37)22-23-9-3-2-4-10-23/h2-18,29,34H,19-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354064
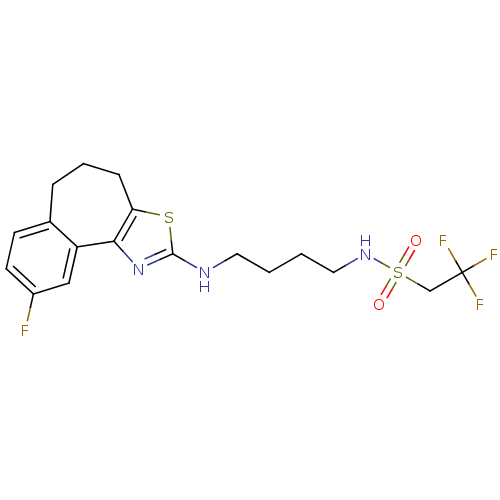
(CHEMBL1836326)Show SMILES Fc1ccc2CCCc3sc(NCCCCNS(=O)(=O)CC(F)(F)F)nc3-c2c1 Show InChI InChI=1S/C18H21F4N3O2S2/c19-13-7-6-12-4-3-5-15-16(14(12)10-13)25-17(28-15)23-8-1-2-9-24-29(26,27)11-18(20,21)22/h6-7,10,24H,1-5,8-9,11H2,(H,23,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50000012
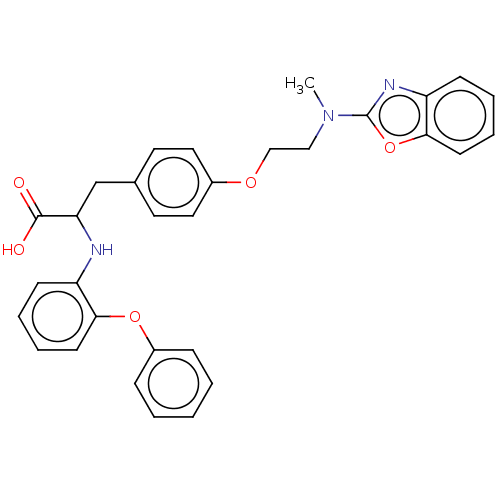
(CHEMBL147826)Show SMILES CN(CCOc1ccc(CC(Nc2ccccc2Oc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C31H29N3O5/c1-34(31-33-26-12-6-8-14-29(26)39-31)19-20-37-23-17-15-22(16-18-23)21-27(30(35)36)32-25-11-5-7-13-28(25)38-24-9-3-2-4-10-24/h2-18,27,32H,19-21H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50064451
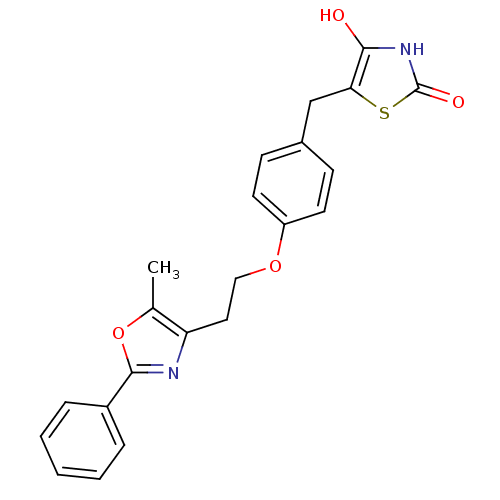
(5-{4-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]-be...)Show SMILES Cc1oc(nc1CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O4S/c1-14-18(23-21(28-14)16-5-3-2-4-6-16)11-12-27-17-9-7-15(8-10-17)13-19-20(25)24-22(26)29-19/h2-10,25H,11-13H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354076
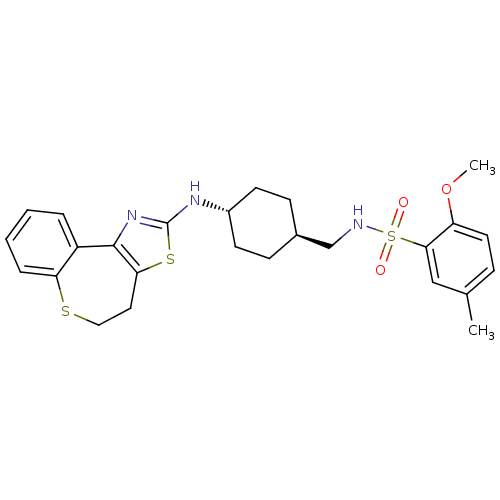
(CHEMBL1836103)Show SMILES COc1ccc(C)cc1S(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1 |r,wU:14.14,wD:17.21,(38.95,-3.6,;37.61,-2.84,;36.28,-3.62,;36.3,-5.16,;34.96,-5.95,;33.62,-5.18,;32.29,-5.96,;33.61,-3.64,;34.95,-2.87,;34.94,-1.33,;36.48,-1.32,;35.7,.01,;33.6,-.57,;33.59,.98,;32.25,1.74,;30.92,.96,;29.58,1.72,;29.58,3.26,;30.9,4.05,;32.24,3.28,;28.23,4.02,;26.91,3.24,;25.54,3.83,;24.55,2.71,;25.3,1.43,;24.71,-.06,;23.22,-.47,;21.99,.44,;21.93,2.03,;20.53,2.52,;20.26,3.98,;21.39,4.95,;22.79,4.45,;23.06,3,;26.76,1.75,)| Show InChI InChI=1S/C26H31N3O3S3/c1-17-7-12-21(32-2)24(15-17)35(30,31)27-16-18-8-10-19(11-9-18)28-26-29-25-20-5-3-4-6-22(20)33-14-13-23(25)34-26/h3-7,12,15,18-19,27H,8-11,13-14,16H2,1-2H3,(H,28,29)/t18-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418575
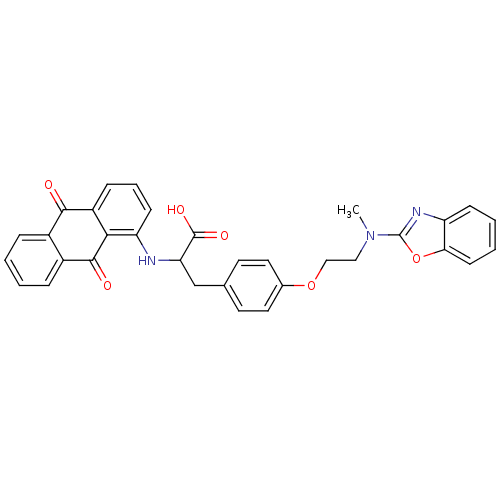
(CHEMBL358137)Show SMILES CN(CCOc1ccc(CC(Nc2cccc3C(=O)c4ccccc4C(=O)c23)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C33H27N3O6/c1-36(33-35-25-10-4-5-12-28(25)42-33)17-18-41-21-15-13-20(14-16-21)19-27(32(39)40)34-26-11-6-9-24-29(26)31(38)23-8-3-2-7-22(23)30(24)37/h2-16,27,34H,17-19H2,1H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418563
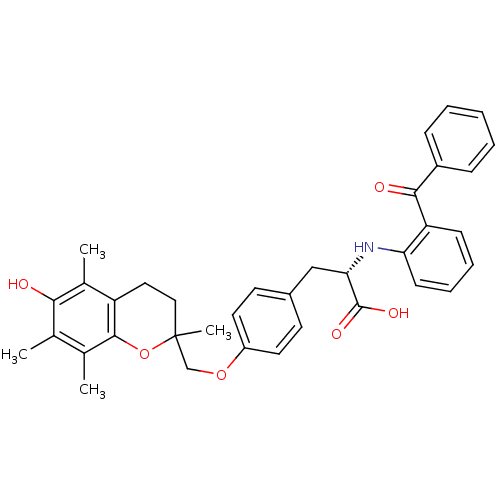
(CHEMBL148301)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(C[C@H](Nc4ccccc4C(=O)c4ccccc4)C(O)=O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C36H37NO6/c1-22-23(2)34-28(24(3)32(22)38)18-19-36(4,43-34)21-42-27-16-14-25(15-17-27)20-31(35(40)41)37-30-13-9-8-12-29(30)33(39)26-10-6-5-7-11-26/h5-17,31,37-38H,18-21H2,1-4H3,(H,40,41)/t31-,36?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165418
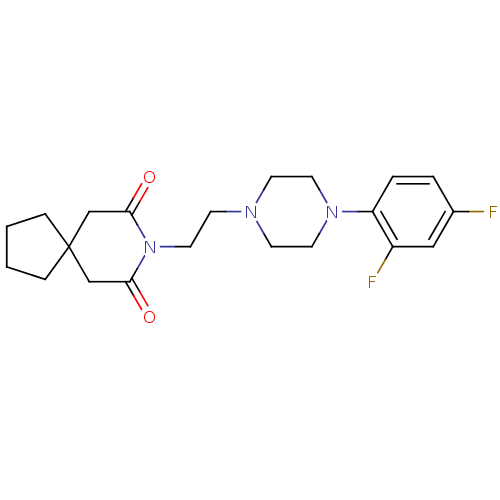
(8-{2-[4-(2,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(N2CCN(CCN3C(=O)CC4(CCCC4)CC3=O)CC2)c(F)c1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-18(17(23)13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50354083

(CHEMBL1836102 | N-{[(1r,4r)-4-{[(4-aminoquinazolin...)Show SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3ccc4ccccc4c3)CC2)nc2ccccc12 |r,wU:9.9,wD:6.5,(22.1,-1.37,;22.11,-2.91,;23.44,-3.67,;23.45,-5.22,;24.79,-5.98,;26.12,-5.21,;27.46,-5.97,;27.46,-7.51,;28.79,-8.28,;30.12,-7.51,;31.45,-8.29,;32.79,-7.52,;34.12,-8.29,;33.35,-9.62,;34.89,-9.63,;35.45,-7.52,;35.45,-5.99,;36.78,-5.22,;38.12,-5.99,;39.45,-5.22,;40.78,-5.99,;40.78,-7.53,;39.45,-8.3,;38.12,-7.53,;36.79,-8.3,;30.12,-5.97,;28.79,-5.2,;22.12,-5.99,;20.78,-5.22,;19.45,-6,;18.11,-5.23,;18.11,-3.69,;19.44,-2.92,;20.78,-3.68,)| Show InChI InChI=1S/C26H29N5O2S/c27-25-23-7-3-4-8-24(23)30-26(31-25)28-16-18-9-11-19(12-10-18)17-29-34(32,33)22-14-13-20-5-1-2-6-21(20)15-22/h1-8,13-15,18-19,29H,9-12,16-17H2,(H3,27,28,30,31)/t18-,19- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of serotonin transporter |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418571
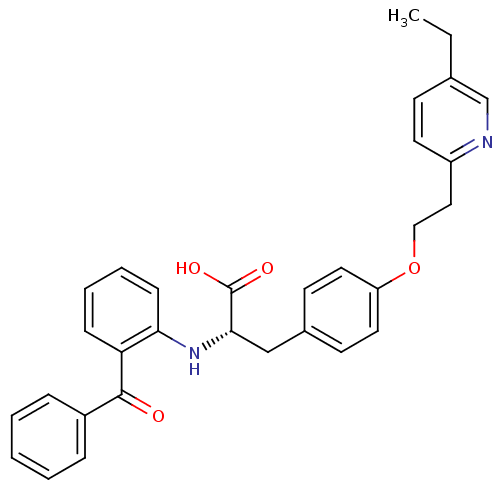
(CHEMBL346219)Show SMILES CCc1ccc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)nc1 Show InChI InChI=1S/C31H30N2O4/c1-2-22-12-15-25(32-21-22)18-19-37-26-16-13-23(14-17-26)20-29(31(35)36)33-28-11-7-6-10-27(28)30(34)24-8-4-3-5-9-24/h3-17,21,29,33H,2,18-20H2,1H3,(H,35,36)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354080
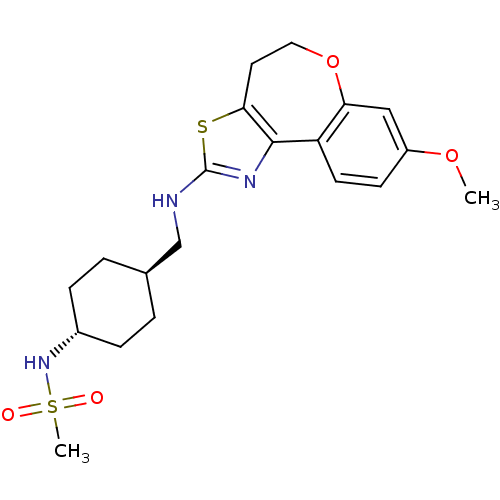
(CHEMBL1836328)Show SMILES COc1ccc2-c3nc(NC[C@H]4CC[C@@H](CC4)NS(C)(=O)=O)sc3CCOc2c1 |r,wU:11.10,wD:14.17,(14,4.11,;14.26,2.58,;15.71,2.05,;16.87,3,;18.26,2.48,;18.51,1.01,;20,.7,;21.01,1.81,;22.36,1.18,;23.72,1.93,;23.74,3.48,;25.1,4.23,;25.13,5.78,;26.49,6.53,;27.8,5.73,;27.78,4.19,;26.43,3.44,;29.16,6.48,;30.48,5.69,;31.84,6.44,;29.69,4.36,;31.24,4.33,;22.21,-.31,;20.74,-.61,;20.11,-2.09,;18.61,-2.48,;17.39,-1.54,;17.36,.06,;15.96,.58,)| Show InChI InChI=1S/C20H27N3O4S2/c1-26-15-7-8-16-17(11-15)27-10-9-18-19(16)22-20(28-18)21-12-13-3-5-14(6-4-13)23-29(2,24)25/h7-8,11,13-14,23H,3-6,9-10,12H2,1-2H3,(H,21,22)/t13-,14- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354082
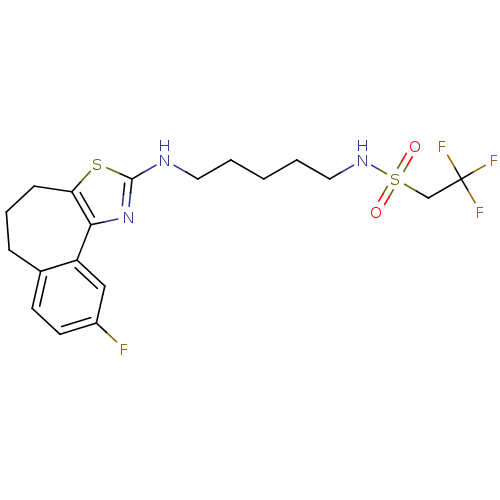
(CHEMBL1836321)Show SMILES Fc1ccc2CCCc3sc(NCCCCCNS(=O)(=O)CC(F)(F)F)nc3-c2c1 Show InChI InChI=1S/C19H23F4N3O2S2/c20-14-8-7-13-5-4-6-16-17(15(13)11-14)26-18(29-16)24-9-2-1-3-10-25-30(27,28)12-19(21,22)23/h7-8,11,25H,1-6,9-10,12H2,(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50143674

(8-{2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-5-1-2-6-18(17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-3-4-8-21/h1-2,5-6H,3-4,7-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50165419
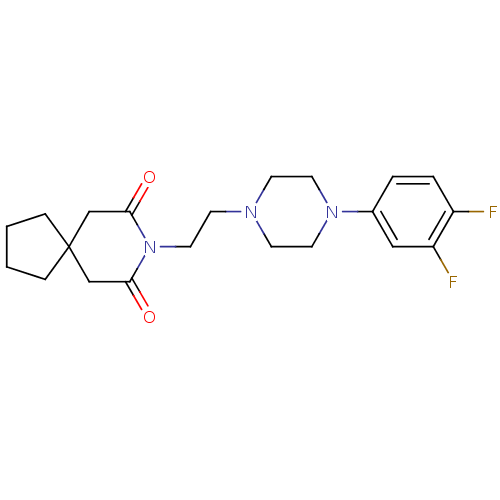
(8-{2-[4-(3,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-17-4-3-16(13-18(17)23)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50143664

(8-{2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(F)c(c1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-17(23)18(13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354067

(CHEMBL1836323)Show InChI InChI=1S/C17H23N3O2S3/c1-25(21,22)19-11-6-2-5-10-18-17-20-16-13-7-3-4-8-14(13)23-12-9-15(16)24-17/h3-4,7-8,19H,2,5-6,9-12H2,1H3,(H,18,20) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418557
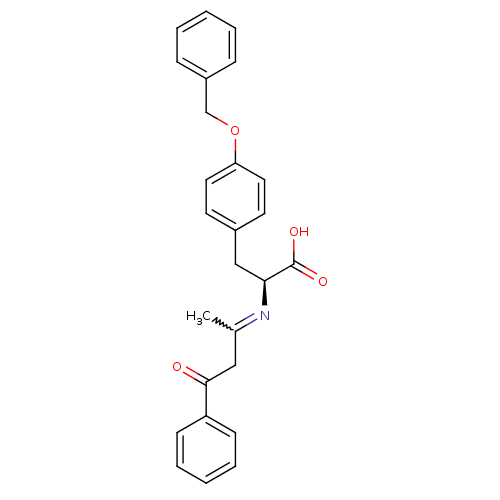
(CHEMBL1785028)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:1.0| Show InChI InChI=1S/C26H25NO4/c1-19(16-25(28)22-10-6-3-7-11-22)27-24(26(29)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418557

(CHEMBL1785028)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(O)=O |r,w:1.0| Show InChI InChI=1S/C26H25NO4/c1-19(16-25(28)22-10-6-3-7-11-22)27-24(26(29)30)17-20-12-14-23(15-13-20)31-18-21-8-4-2-5-9-21/h2-15,24H,16-18H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354075

(CHEMBL1836314)Show SMILES O=S(=O)(NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1)c1cccc2ccccc12 |r,wU:5.4,wD:8.11,(6.06,-16.55,;4.52,-16.53,;5.31,-15.2,;3.19,-15.74,;3.21,-14.19,;1.88,-13.4,;.53,-14.15,;-.79,-13.37,;-.77,-11.83,;.57,-11.06,;1.9,-11.85,;-2.1,-11.04,;-3.45,-11.8,;-4.81,-11.18,;-5.82,-12.29,;-5.08,-13.59,;-5.7,-15.07,;-7.21,-15.46,;-8.42,-14.52,;-8.46,-12.92,;-9.85,-12.4,;-10.1,-10.94,;-8.95,-9.99,;-7.55,-10.51,;-7.31,-11.97,;-3.62,-13.29,;4.5,-18.07,;5.82,-18.85,;5.81,-20.39,;4.46,-21.16,;3.13,-20.36,;1.79,-21.11,;.47,-20.34,;.49,-18.79,;1.83,-18.04,;3.15,-18.82,)| Show InChI InChI=1S/C28H29N3O2S3/c32-36(33,26-11-5-7-20-6-1-2-8-22(20)26)29-18-19-12-14-21(15-13-19)30-28-31-27-23-9-3-4-10-24(23)34-17-16-25(27)35-28/h1-11,19,21,29H,12-18H2,(H,30,31)/t19-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50143661

(8-{2-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-ethyl}-8...)Show SMILES Fc1ccc(cc1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H28FN3O2/c22-17-3-5-18(6-4-17)24-12-9-23(10-13-24)11-14-25-19(26)15-21(16-20(25)27)7-1-2-8-21/h3-6H,1-2,7-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Alpha-1D adrenergic receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50026917

(8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...)Show SMILES COc1ccccc1N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C22H31N3O3/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22/h2-3,6-7H,4-5,8-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Dopamine receptor D2 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354063

(CHEMBL1836332)Show SMILES CS(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCOc3ccccc-23)s1 |r,wU:6.5,wD:9.12,(55.11,-10.15,;53.77,-10.92,;53,-12.26,;54.54,-12.26,;52.43,-10.14,;51.09,-10.91,;49.75,-10.14,;48.42,-10.91,;47.08,-10.13,;47.07,-8.59,;48.42,-7.82,;49.76,-8.6,;45.74,-7.8,;44.39,-8.55,;43.03,-7.93,;42.02,-9.03,;42.76,-10.34,;42.14,-11.82,;40.63,-12.21,;39.41,-11.27,;39.38,-9.67,;37.99,-9.15,;37.74,-7.69,;38.89,-6.73,;40.29,-7.25,;40.53,-8.72,;44.23,-10.04,)| Show InChI InChI=1S/C19H25N3O3S2/c1-27(23,24)20-12-13-6-8-14(9-7-13)21-19-22-18-15-4-2-3-5-16(15)25-11-10-17(18)26-19/h2-5,13-14,20H,6-12H2,1H3,(H,21,22)/t13-,14- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354074

(CHEMBL1836315)Show SMILES Cn1cnc(c1)S(=O)(=O)NC[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCSc3ccccc-23)s1 |r,wU:11.11,wD:14.18,(18.91,-20.53,;19.83,-19.3,;21.38,-19.32,;21.87,-17.85,;20.63,-16.93,;19.38,-17.82,;20.65,-15.38,;22.19,-15.4,;21.44,-14.06,;19.32,-14.59,;19.34,-13.05,;18.02,-12.26,;16.67,-13.02,;15.34,-12.23,;15.37,-10.69,;16.7,-9.92,;18.03,-10.72,;14.04,-9.9,;12.69,-10.66,;11.33,-10.04,;10.32,-11.15,;11.05,-12.45,;10.43,-13.93,;8.93,-14.32,;7.72,-13.38,;7.68,-11.78,;6.29,-11.26,;6.04,-9.8,;7.19,-8.85,;8.59,-9.37,;8.83,-10.84,;12.52,-12.15,)| Show InChI InChI=1S/C22H27N5O2S3/c1-27-13-20(23-14-27)32(28,29)24-12-15-6-8-16(9-7-15)25-22-26-21-17-4-2-3-5-18(17)30-11-10-19(21)31-22/h2-5,13-16,24H,6-12H2,1H3,(H,25,26)/t15-,16- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50165417

(8-{2-[4-(2,4,5-Trifluoro-phenyl)-piperazin-1-yl]-e...)Show SMILES Fc1cc(F)c(cc1F)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H26F3N3O2/c22-15-11-17(24)18(12-16(15)23)26-8-5-25(6-9-26)7-10-27-19(28)13-21(14-20(27)29)3-1-2-4-21/h11-12H,1-10,13-14H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for rat Dopamine receptor D3 |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50165418

(8-{2-[4-(2,4-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(N2CCN(CCN3C(=O)CC4(CCCC4)CC3=O)CC2)c(F)c1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-18(17(23)13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50143664

(8-{2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-ethy...)Show SMILES Fc1ccc(F)c(c1)N1CCN(CCN2C(=O)CC3(CCCC3)CC2=O)CC1 Show InChI InChI=1S/C21H27F2N3O2/c22-16-3-4-17(23)18(13-16)25-10-7-24(8-11-25)9-12-26-19(27)14-21(15-20(26)28)5-1-2-6-21/h3-4,13H,1-2,5-12,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 3076-9 (2005)
Article DOI: 10.1021/jm0491391
BindingDB Entry DOI: 10.7270/Q2445M0K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50354065
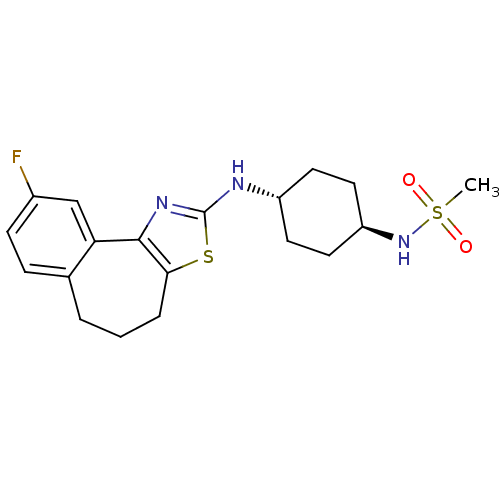
(CHEMBL1836325)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nc-2c(CCCc3ccc(F)cc-23)s1 |r,wU:5.4,wD:8.11,(26.46,-46.27,;25.36,-45.19,;26.16,-43.86,;24.61,-43.83,;24.14,-46.14,;22.8,-45.35,;21.45,-46.11,;20.12,-45.32,;20.14,-43.78,;21.48,-43.01,;22.81,-43.8,;18.81,-42.99,;17.47,-43.75,;16.1,-43.13,;15.08,-44.24,;15.82,-45.55,;15.2,-47.03,;13.69,-47.42,;12.48,-46.48,;12.44,-44.87,;11.05,-44.36,;10.8,-42.89,;11.95,-41.93,;11.69,-40.41,;13.35,-42.46,;13.59,-43.93,;17.3,-45.25,)| Show InChI InChI=1S/C19H24FN3O2S2/c1-27(24,25)23-15-9-7-14(8-10-15)21-19-22-18-16-11-13(20)6-5-12(16)3-2-4-17(18)26-19/h5-6,11,14-15,23H,2-4,7-10H2,1H3,(H,21,22)/t14-,15- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY5 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 21: 5436-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.124
BindingDB Entry DOI: 10.7270/Q2RN387K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049244
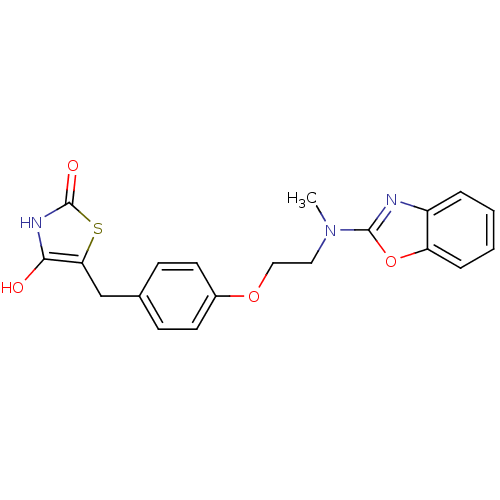
(5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C20H19N3O4S/c1-23(19-21-15-4-2-3-5-16(15)27-19)10-11-26-14-8-6-13(7-9-14)12-17-18(24)22-20(25)28-17/h2-9,24H,10-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data