

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179201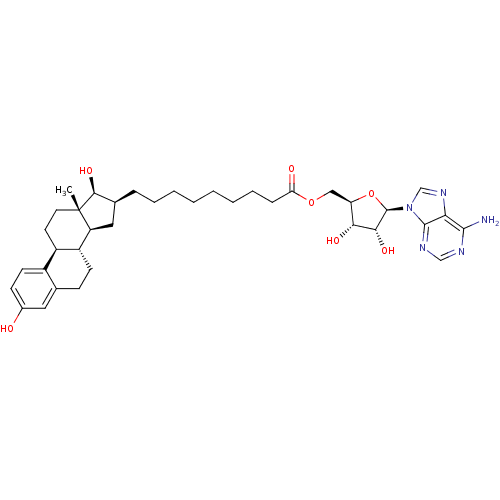 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50464579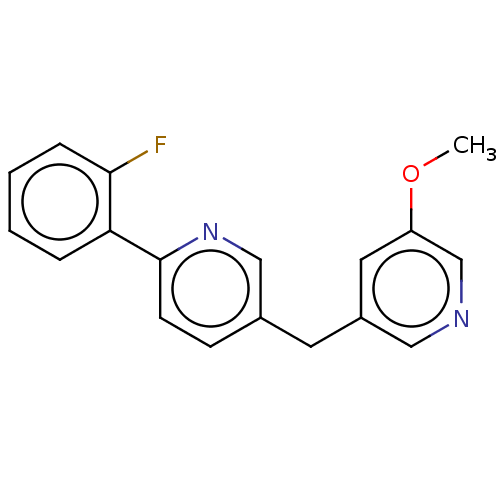 (CHEMBL4281975) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329192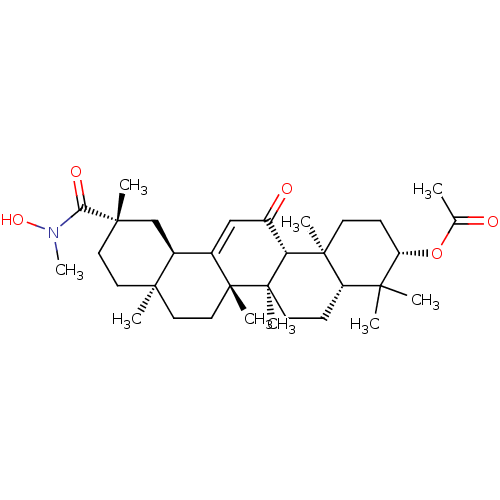 ((3beta,18beta,20beta)-3-Acetoxy-N-methyl-N-hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329192 ((3beta,18beta,20beta)-3-Acetoxy-N-methyl-N-hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329187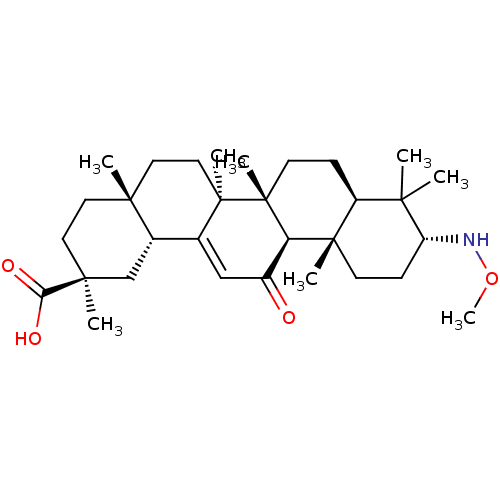 ((3alpha,18beta,20beta)-3-Methoxyamino-11-oxo-olean...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339809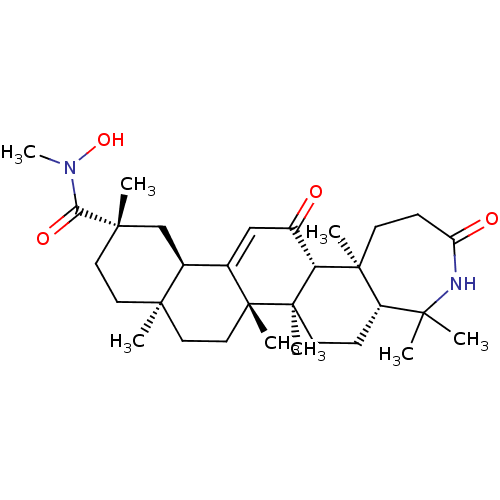 ((5aR,7aR,7bS,9aS,12S,13aR,15aR,15bS)-2,3,4,5,5a,6,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339805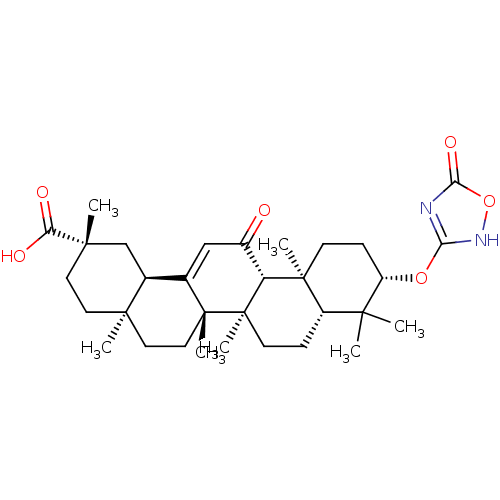 ((3beta,18beta,20beta)-[3-(1,2,4-Oxadiazole-5(2H)-o...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329188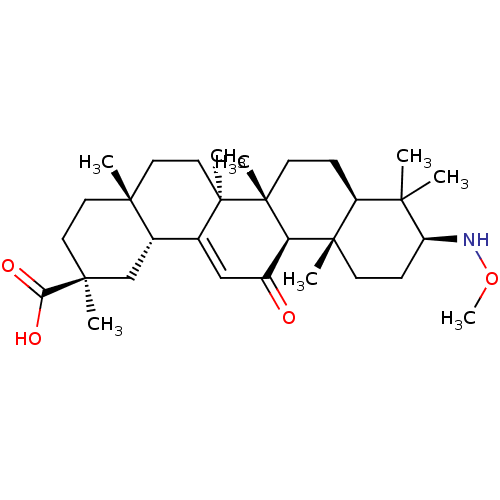 ((3beta,18beta,20beta)-3-Methoxyamino-11-oxo-olean-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50028166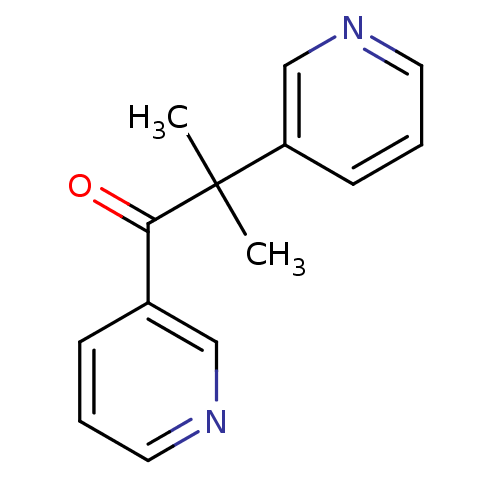 (CHEMBL934 | METYRAPONE | US9138393, Metyrapone | U...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339811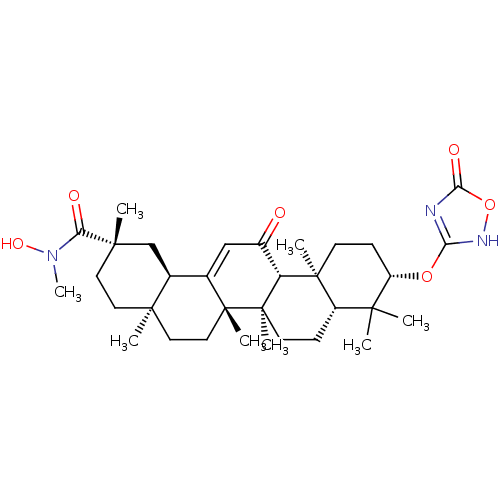 ((3beta,18beta,20beta)-N-Hydroxy-N-methyl-[3-(1,2,4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50464575 (CHEMBL4282375) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339812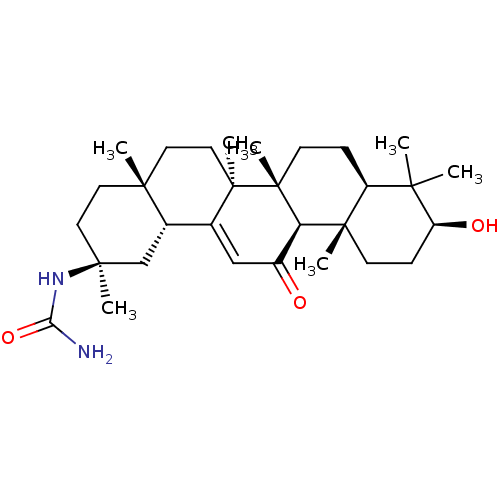 (CHEMBL1689283 | N-[(3beta,18beta,20beta)-3-Hydroxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50237104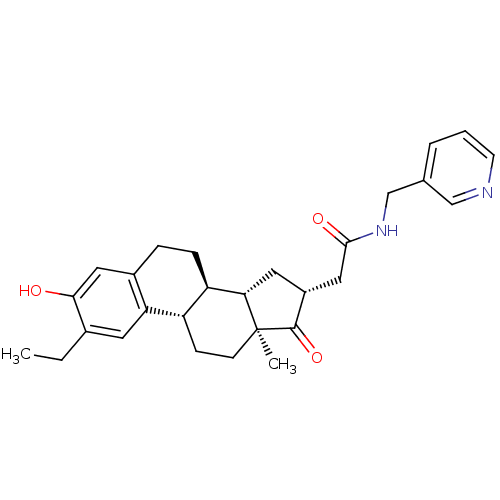 (2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50464576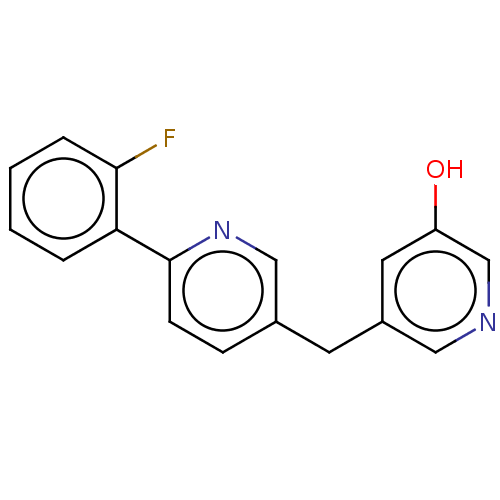 (CHEMBL4290660) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339808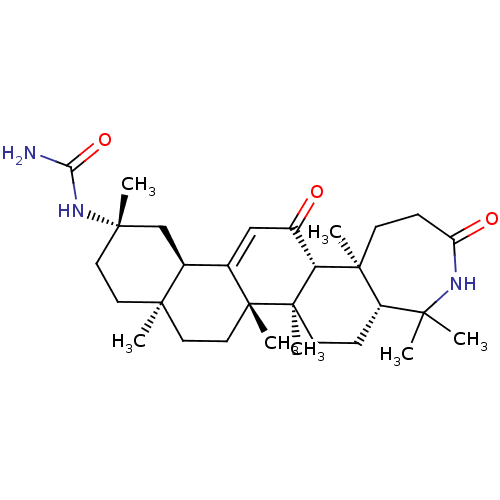 (CHEMBL1689279 | N-[(5aR,7aR,7bS,9aS,12S,13aR,15aR,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50267362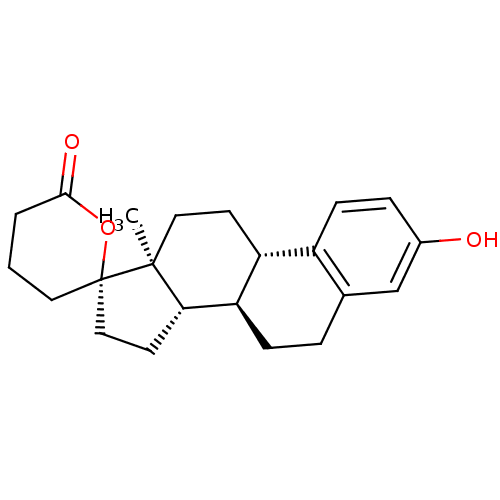 ((2'S,8R,9S,13S,14S)-3-hydroxy-13-methyl-4',5',6,7,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 (unknown origin) | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329188 ((3beta,18beta,20beta)-3-Methoxyamino-11-oxo-olean-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [1,2-3H]-cortisone to cortisol by scintillation count... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339813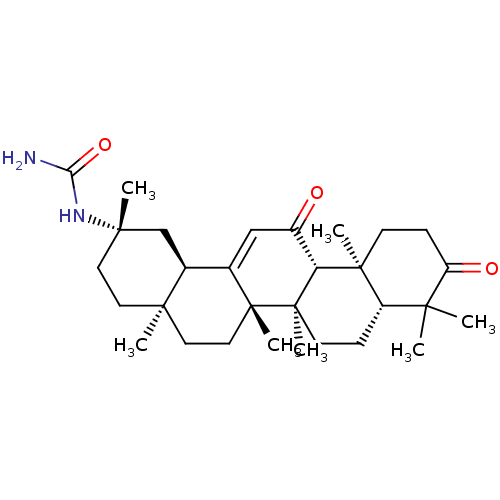 (CHEMBL1689284 | N-[(3beta,18beta,20beta)-3,11-Diox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50130177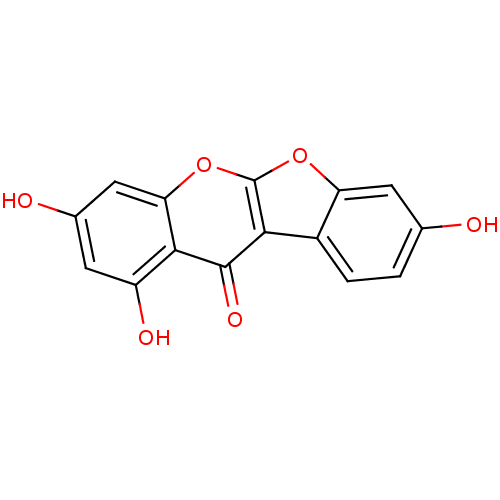 (2,6,8-Trihydroxy-10,11-dioxa-benzo[b]fluoren-5-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human 17beta-HSD1 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estrone and NADPH by scintillation counting... | J Nat Prod 80: 965-974 (2017) Article DOI: 10.1021/acs.jnatprod.6b00950 BindingDB Entry DOI: 10.7270/Q2XD148R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358116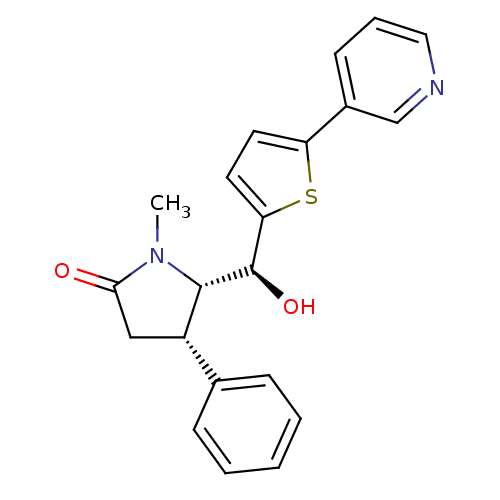 (CHEMBL1915968) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 (unknown origin) | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179201 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426580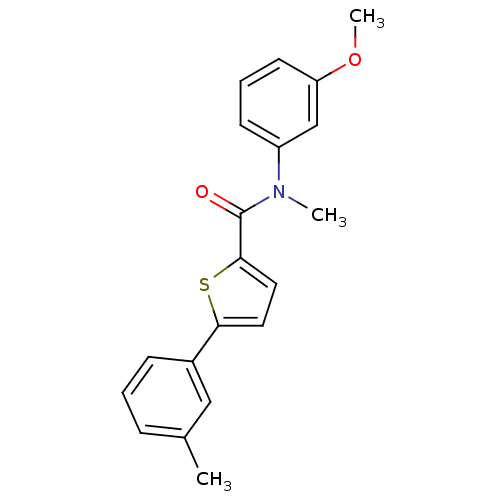 (CHEMBL2324690) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188387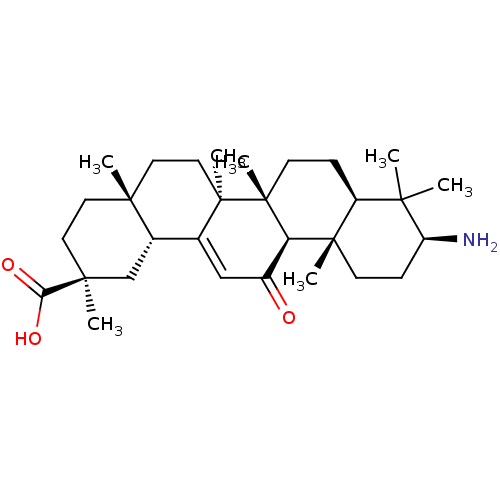 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329193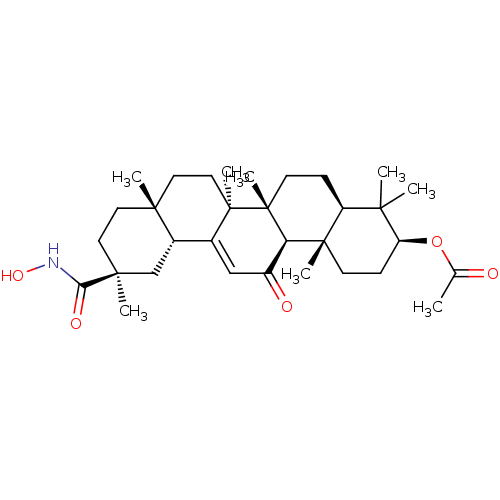 ((3beta,18beta,20beta)-3-Acetoxy-N-hydroxy-11-oxo-o...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50329187 ((3alpha,18beta,20beta)-3-Methoxyamino-11-oxo-olean...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [1,2-3H]-cortisone to cortisol by scintillation count... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426587 (CHEMBL2324361) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426579 (CHEMBL2324360) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426581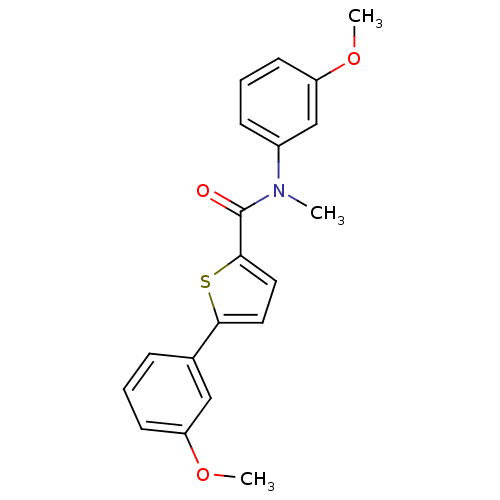 (CHEMBL2324679) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426580 (CHEMBL2324690) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50464578 (CHEMBL4280014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of rat hepatic 11beta-HSD1 | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vienna University of Technology Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in rat liver assessed as cortisone level | Bioorg Med Chem 18: 433-54 (2010) Article DOI: 10.1016/j.bmc.2009.10.036 BindingDB Entry DOI: 10.7270/Q2BP02W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339806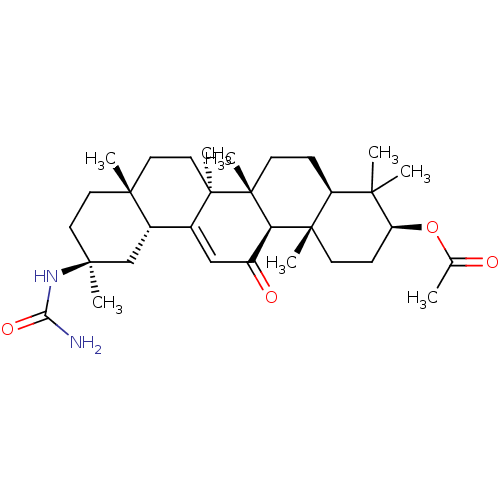 ((3beta,18beta,20beta)-N-[-3-(Acetoxy)-11-oxo-30-no...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426579 (CHEMBL2324360) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426581 (CHEMBL2324679) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50464577 (CHEMBL4278914) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using [1,2-3H]-11-deoxycorticosterone as substrate after 6 hrs by HPTLC analysis | Eur J Med Chem 143: 591-597 (2018) Article DOI: 10.1016/j.ejmech.2017.11.018 BindingDB Entry DOI: 10.7270/Q2SF2ZTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50329195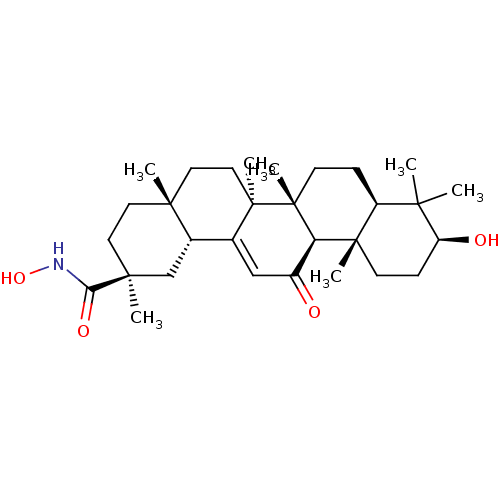 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-N,10-dihyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Natural Resources and Applied Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-3H]-cortisol to cortisone by scintillation c... | Bioorg Med Chem 18: 7522-41 (2010) Article DOI: 10.1016/j.bmc.2010.08.046 BindingDB Entry DOI: 10.7270/Q2PR7W7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426586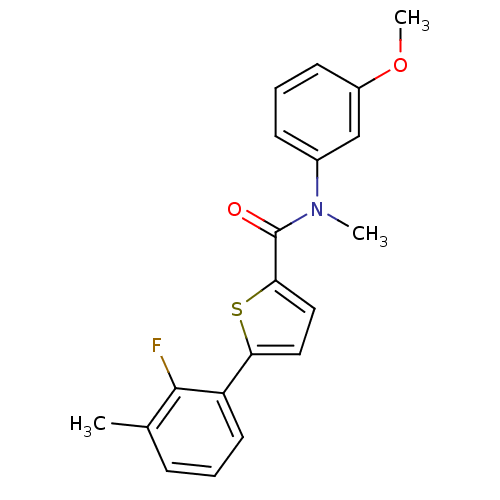 (CHEMBL2324365) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426588 (CHEMBL2324673) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50339805 ((3beta,18beta,20beta)-[3-(1,2,4-Oxadiazole-5(2H)-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [1,2-[3H]cortisone to cortisol after 10 mins by scint... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188390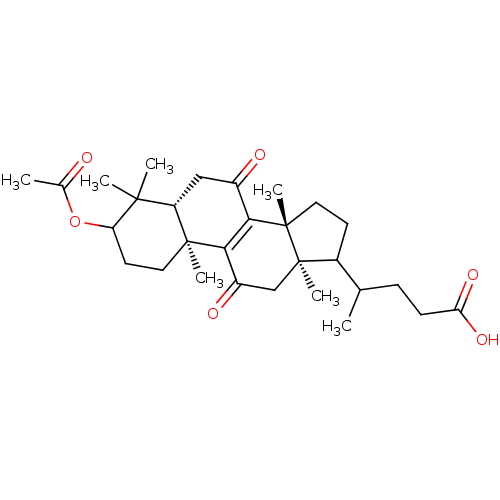 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426582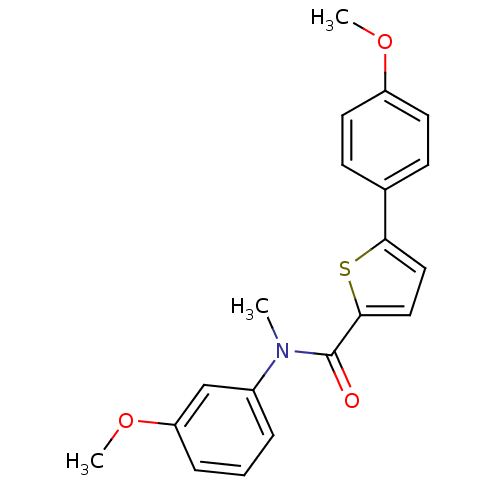 (CHEMBL2324678) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426593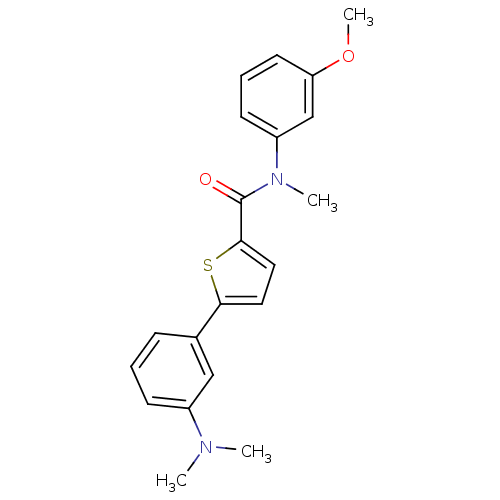 (CHEMBL2324691) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50241159 (CHEMBL373383 | N-butyl-6-((6R,8R,9S,13S,14S,17S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188387 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50426585 (CHEMBL2324366) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis | J Med Chem 56: 167-81 (2013) Article DOI: 10.1021/jm3014053 BindingDB Entry DOI: 10.7270/Q2ZK5J0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50339807 (CHEMBL1689277 | N-[(3beta,18beta,20beta)-3-(Acetox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [1,2,6,7-[3H]cortisol to cortisone after 10 mins by scintillation... | Bioorg Med Chem 19: 1866-80 (2011) Article DOI: 10.1016/j.bmc.2011.02.005 BindingDB Entry DOI: 10.7270/Q2VD6ZR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50450257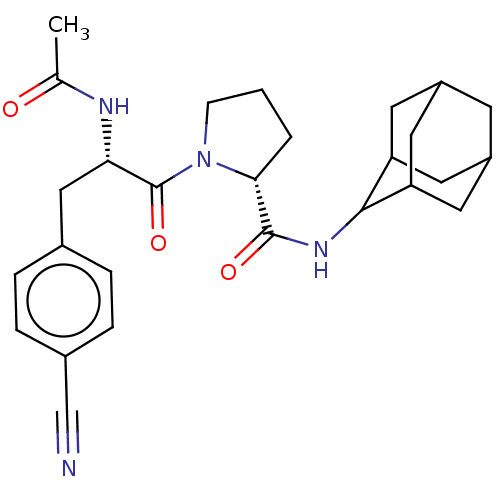 (CHEMBL4163055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
DSM Nutritional Products Ltd. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cell lysates using radiolabeled cortisone as substrate incubated for 10 mins by scintillation cou... | Bioorg Med Chem 26: 5128-5139 (2018) Article DOI: 10.1016/j.bmc.2018.09.009 BindingDB Entry DOI: 10.7270/Q2DJ5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188385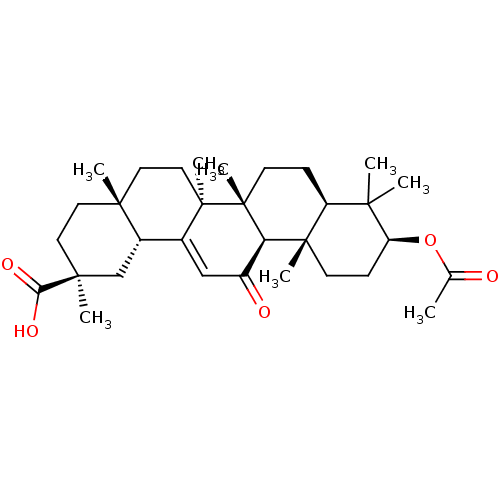 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 266 total ) | Next | Last >> |