

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50077669 ((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077702 (4-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077693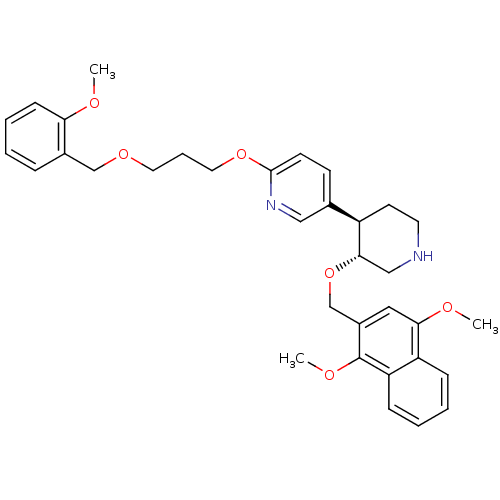 ((3'R,4'R)-3'-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699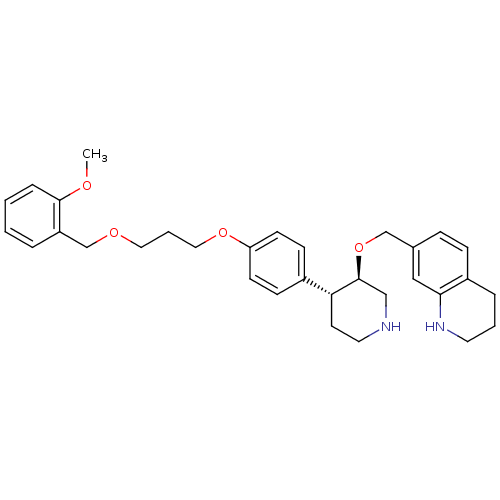 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077692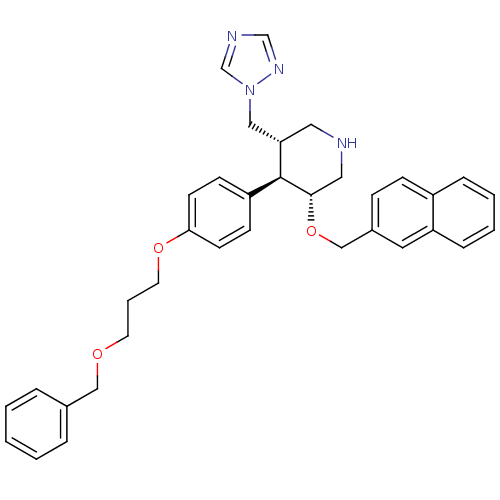 ((3R,4R,5S)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077688 ((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077703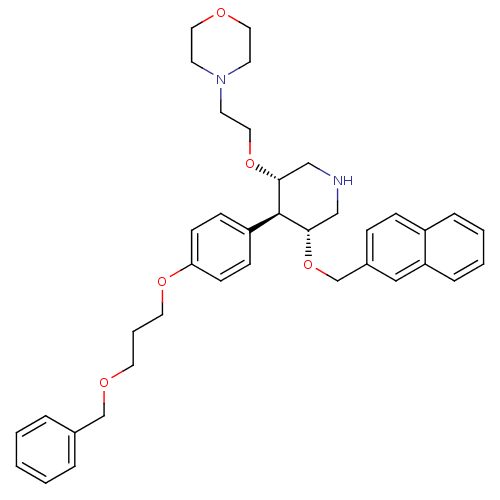 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077704 (CHEMBL31880 | Sulfuric acid mono-[(3S,4R,5R)-4-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077709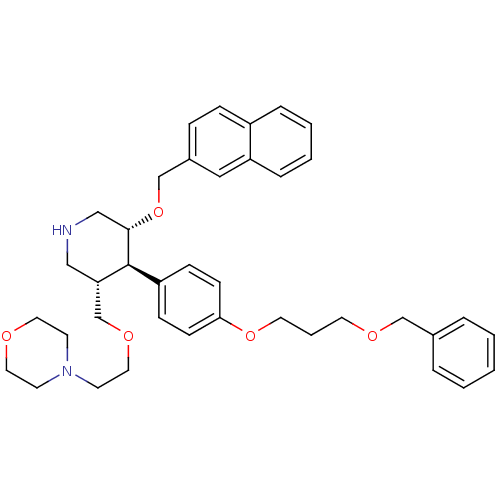 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077695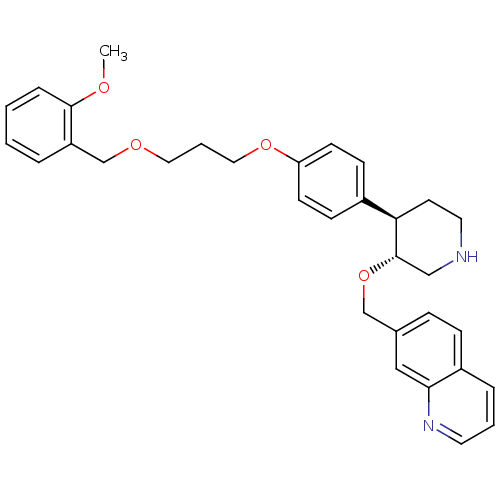 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077689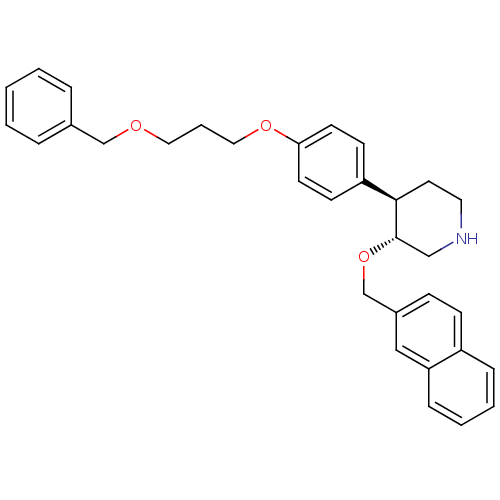 ((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077694 (7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077705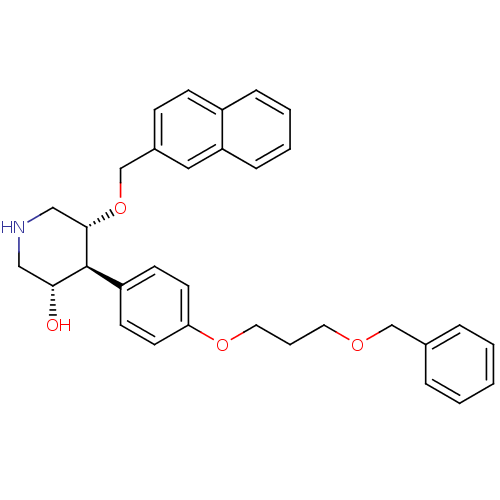 ((3S,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077697 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077689 ((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077707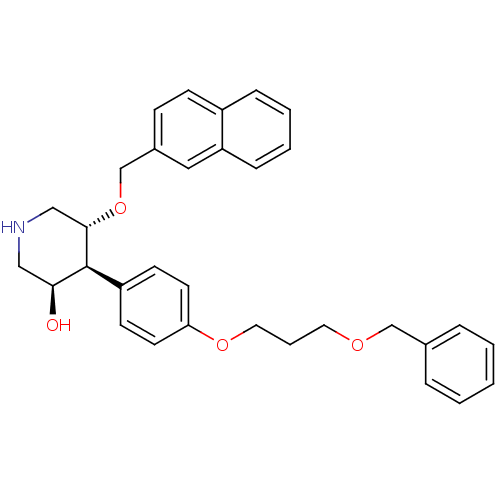 ((3R,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077698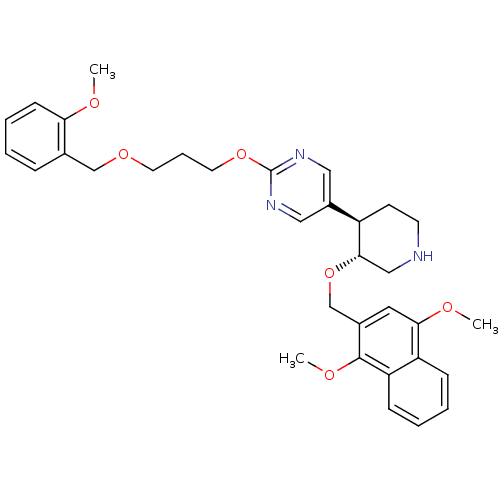 (5-[(3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077696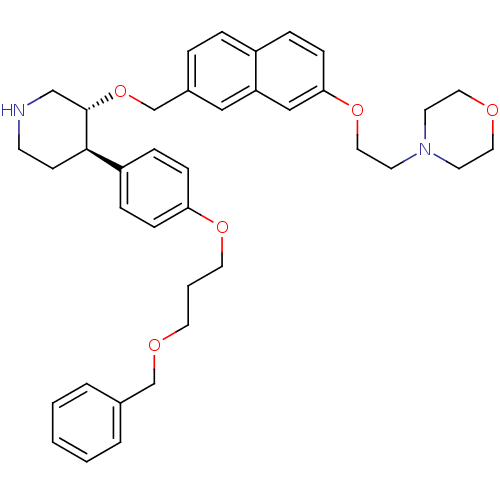 (4-[2-(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077706 (7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067060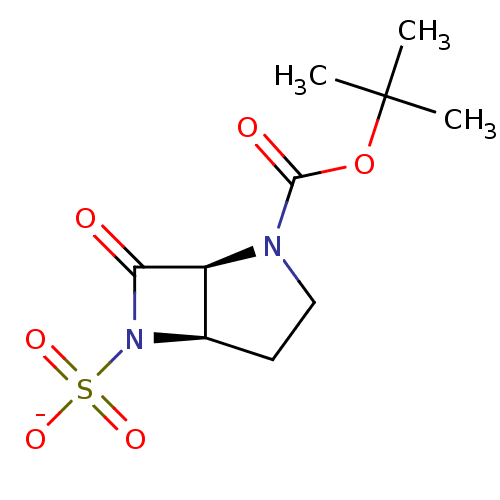 (CHEMBL128523 | Sodium; (1S,5R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077708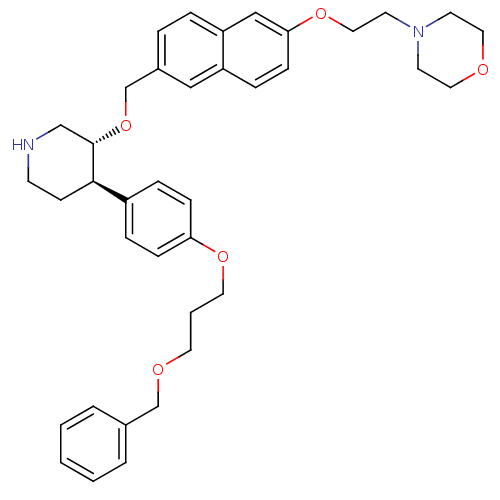 (4-[2-(6-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067058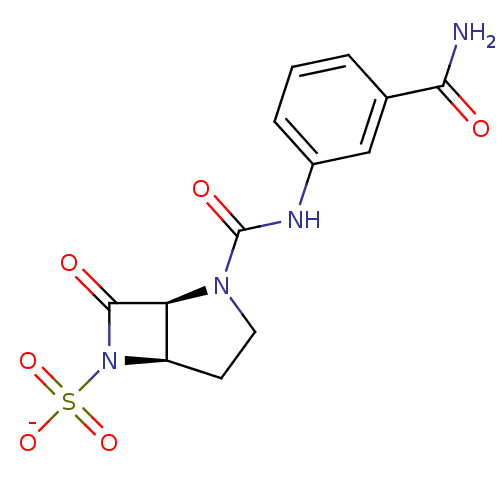 (CHEMBL338157 | Sodium; (1S,5R)-2-(3-carbamoyl-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067055 (CHEMBL338933 | Sodium; (1S,5R)-2-[2-(4-hydroxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077682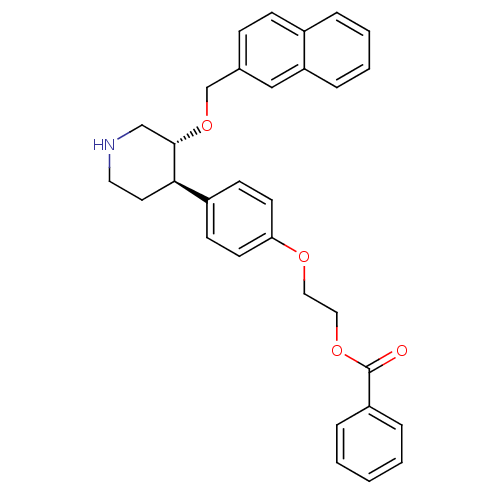 (Benzoic acid 2-{4-[(3R,4R)-3-(naphthalen-2-ylmetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523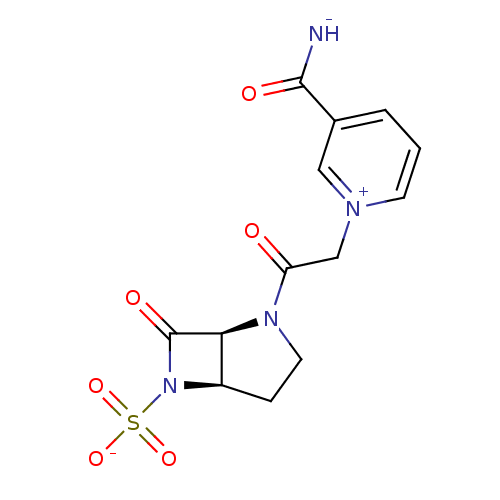 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067053 (CHEMBL127782 | Sodium; (1S,5R)-2-benzyloxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50067066 (CHEMBL340707 | Sodium; (1S,4R,5S)-2-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067050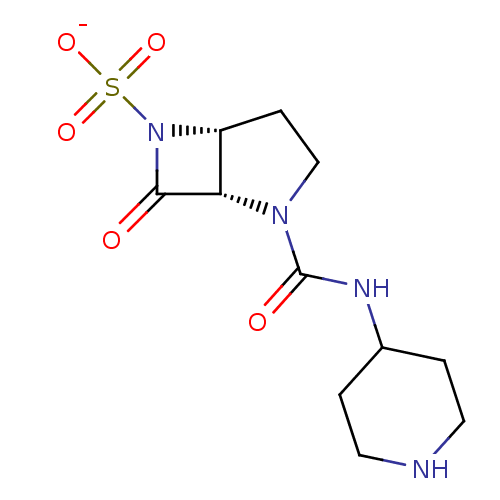 (CHEMBL336630 | Sodium; (1S,5R)-7-oxo-2-(piperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077671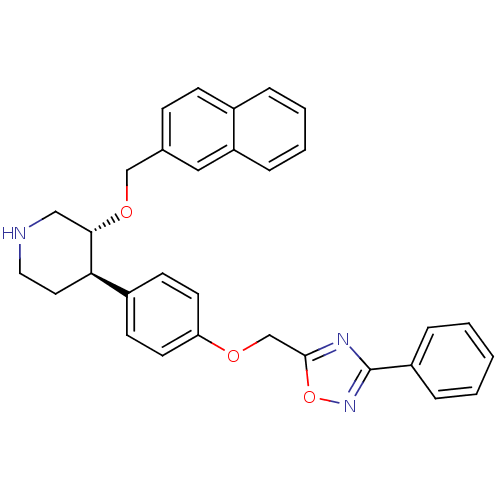 ((3R,4R)-3-(Naphthalen-2-ylmethoxy)-4-[4-(3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Staphylococcus aureus) | BDBM17871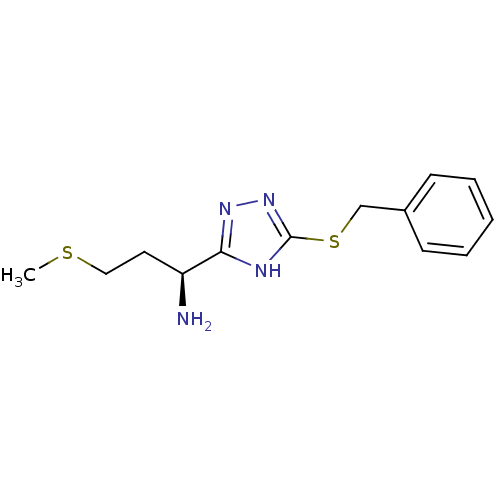 ((1S)-1-[5-(benzylsulfanyl)-4H-1,2,4-triazol-3-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Morphochem AG | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | J Mol Biol 332: 13-21 (2003) Article DOI: 10.1016/s0022-2836(03)00862-3 BindingDB Entry DOI: 10.7270/Q2C53J49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067051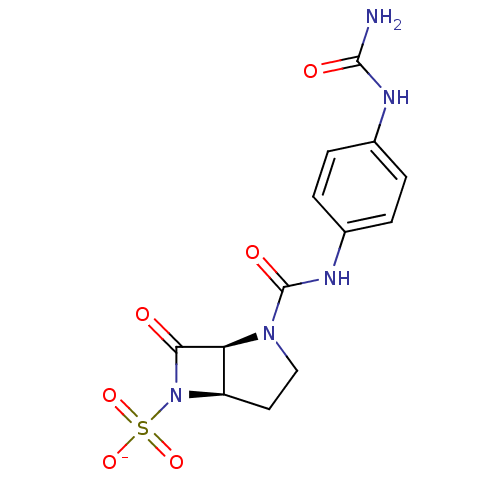 (CHEMBL130187 | Sodium; (1S,5R)-7-oxo-2-(4-ureido-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077690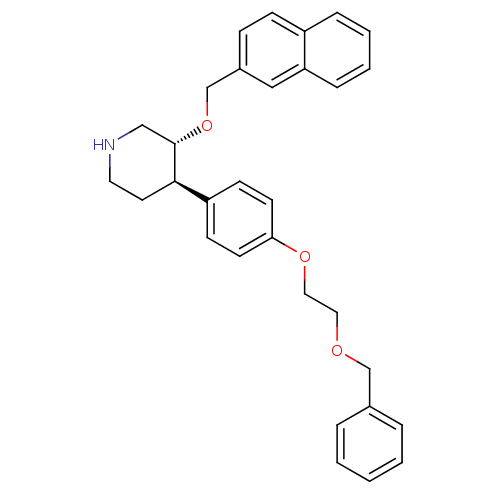 ((3R,4R)-4-[4-(2-Benzyloxy-ethoxy)-phenyl]-3-(napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067047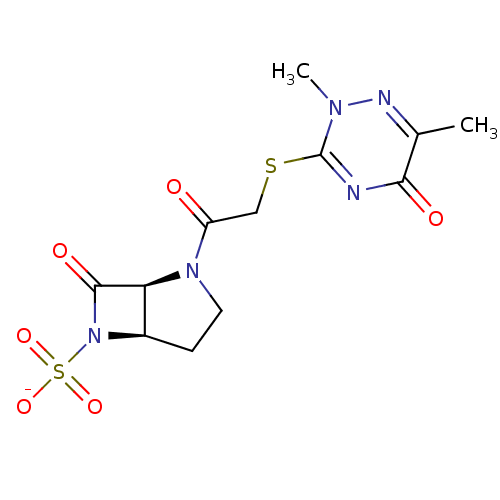 (CHEMBL341484 | Sodium; (1S,5R)-2-[2-(2,6-dimethyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077679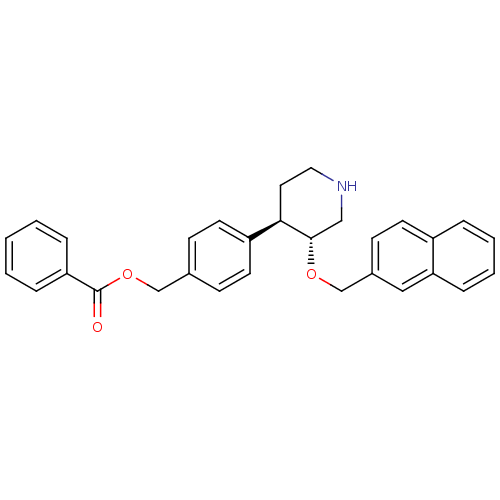 (Benzoic acid 4-[(3R,4R)-3-(naphthalen-2-ylmethoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077685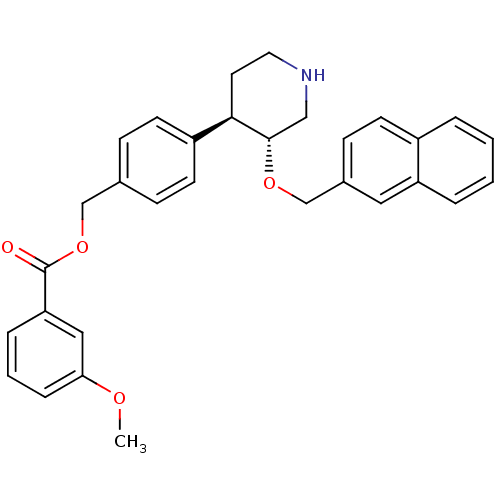 (3-Methoxy-benzoic acid 4-[(3R,4R)-3-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067065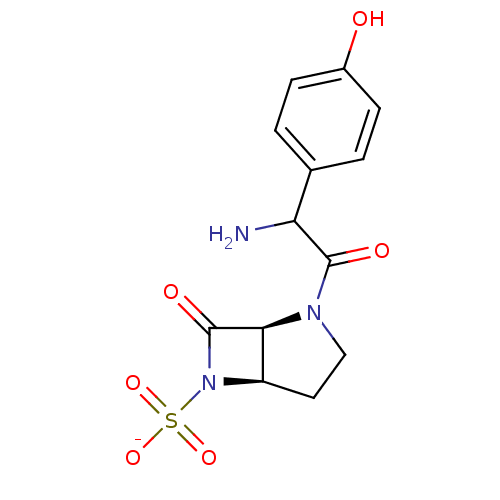 (CHEMBL130854 | Sodium; (1S,5R)-2-[2-amino-2-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077691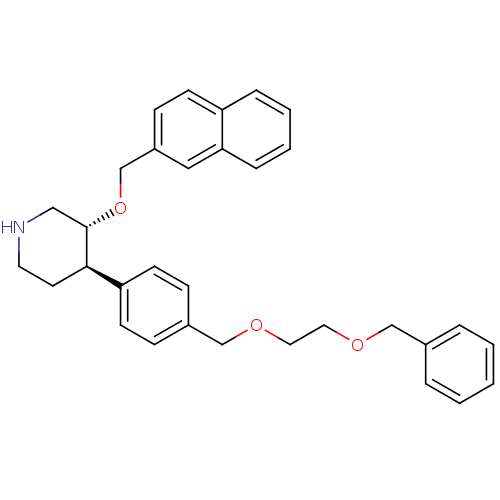 ((3R,4R)-4-[4-(2-Benzyloxy-ethoxymethyl)-phenyl]-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077667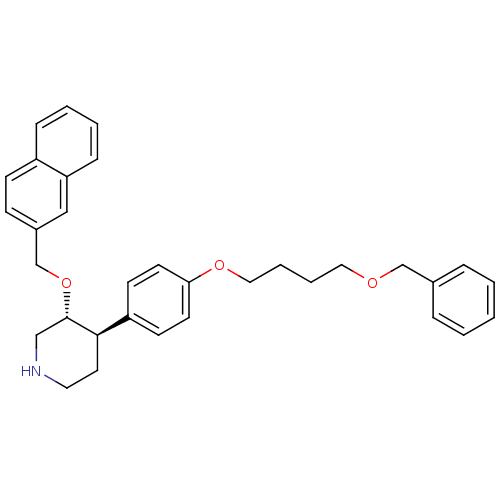 ((3R,4R)-4-[4-(4-Benzyloxy-butoxy)-phenyl]-3-(napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077693 ((3'R,4'R)-3'-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077697 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067057 (CHEMBL340937 | Sodium; (1S,5R)-2-((E)-3-1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077696 (4-[2-(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067062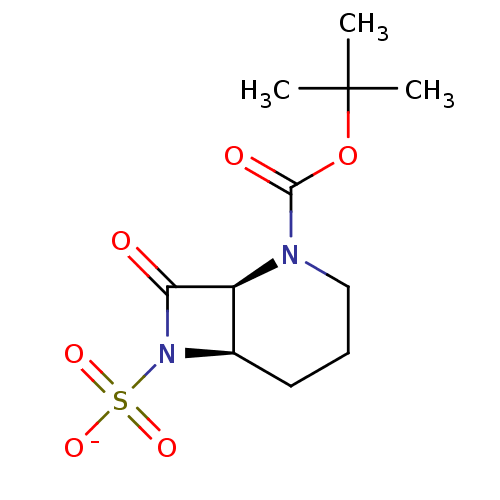 (CHEMBL415234 | Sodium; (1S,6R)-2-tert-butoxycarbon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50067056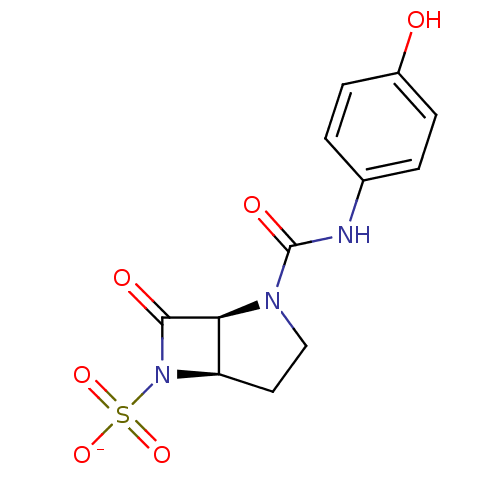 (CHEMBL338351 | Sodium; (1S,5R)-2-(4-hydroxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077709 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 134 total ) | Next | Last >> |