 Found 1793 hits with Last Name = 'oeh' and Initial = 'j'
Found 1793 hits with Last Name = 'oeh' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044868
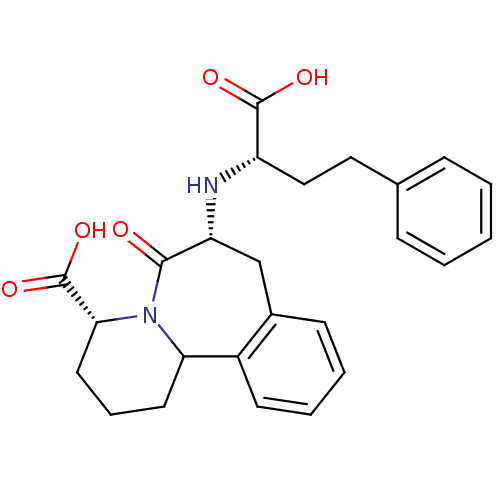
(7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...)Show SMILES OC(=O)[C@H](CCc1ccccc1)N[C@@H]1Cc2ccccc2C2CCC[C@@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19-,20+,21?,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50175518
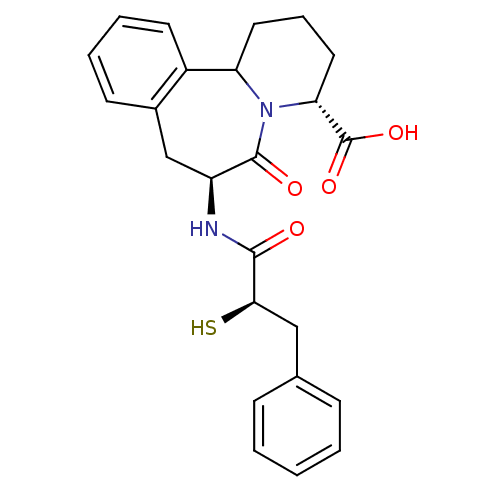
((3R,7S)-7-((R)-2-Mercapto-3-phenyl-propionylamino)...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18-,19?,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of neutral endopeptidase (NEP). |
Bioorg Med Chem Lett 6: 957-962 (1996)
Article DOI: 10.1016/0960-894X(96)00149-7
BindingDB Entry DOI: 10.7270/Q2BV7GMV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50044866
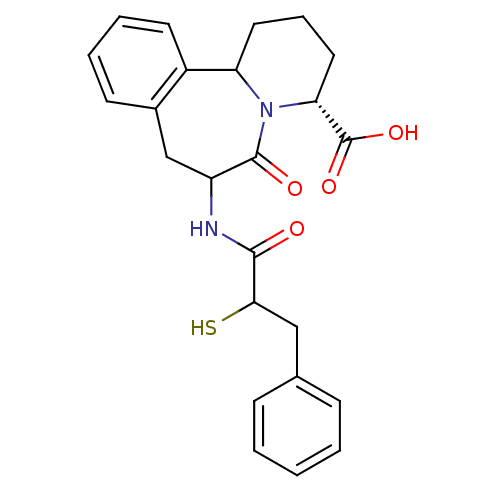
(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287694
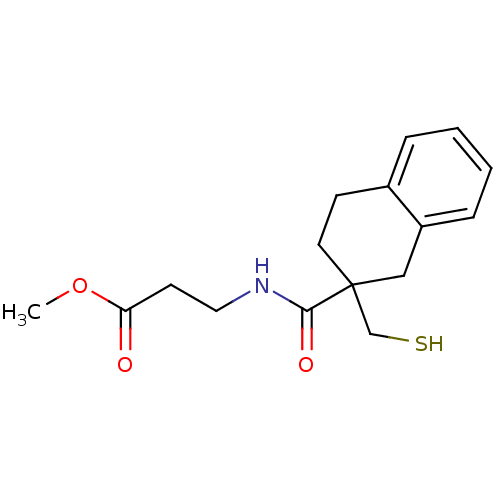
(3-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...)Show InChI InChI=1S/C16H21NO3S/c1-20-14(18)7-9-17-15(19)16(11-21)8-6-12-4-2-3-5-13(12)10-16/h2-5,21H,6-11H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287702
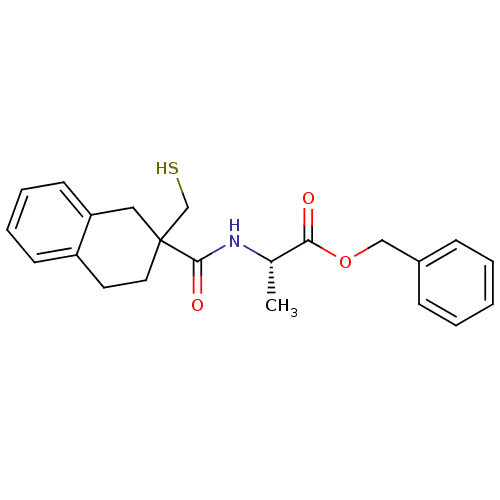
((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...)Show SMILES C[C@H](NC(=O)C1(CS)CCc2ccccc2C1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C22H25NO3S/c1-16(20(24)26-14-17-7-3-2-4-8-17)23-21(25)22(15-27)12-11-18-9-5-6-10-19(18)13-22/h2-10,16,27H,11-15H2,1H3,(H,23,25)/t16-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50002184

(CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C75H127N25O14/c1-7-45(6)61(71(113)96-54(25-17-35-88-75(84)85)72(114)100-36-18-26-58(100)70(112)95-51(22-12-14-32-77)65(107)97-55(37-43(2)3)67(109)92-50(62(79)104)21-11-13-31-76)99-66(108)53(24-16-34-87-74(82)83)93-64(106)52(23-15-33-86-73(80)81)94-68(110)56(38-44(4)5)98-69(111)57(40-46-19-9-8-10-20-46)91-60(103)42-89-59(102)41-90-63(105)49(78)39-47-27-29-48(101)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,101H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H2,79,104)(H,89,102)(H,90,105)(H,91,103)(H,92,109)(H,93,106)(H,94,110)(H,95,112)(H,96,113)(H,97,107)(H,98,111)(H,99,108)(H4,80,81,86)(H4,82,83,87)(H4,84,85,88)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-diprenorphine from mouse KOR expressed in HEK293 cell membranes by radioligand binding assay relative to control |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00158
BindingDB Entry DOI: 10.7270/Q21G0R4N |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287689
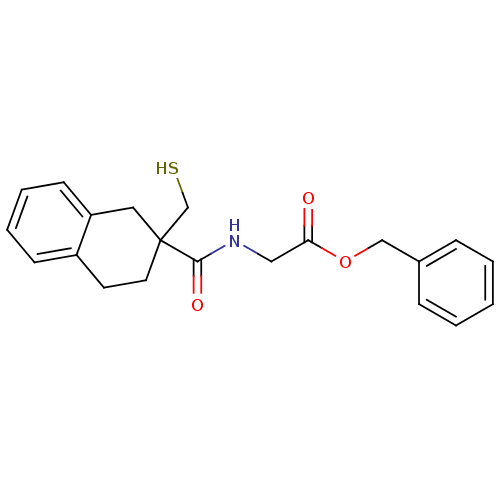
(CHEMBL304233 | [(2-Mercaptomethyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C21H23NO3S/c23-19(25-14-16-6-2-1-3-7-16)13-22-20(24)21(15-26)11-10-17-8-4-5-9-18(17)12-21/h1-9,26H,10-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50289194
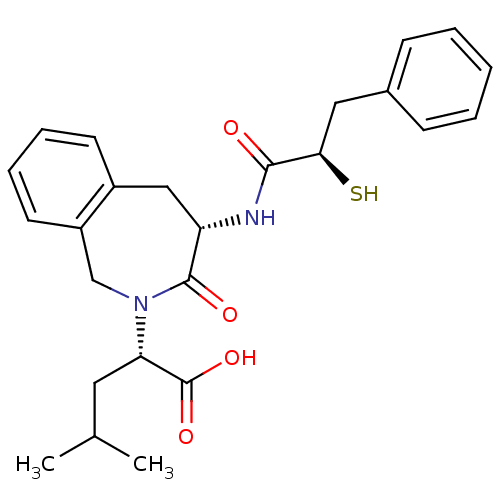
((S)-2-[(S)-4-((R)-2-Mercapto-3-phenyl-propionylami...)Show SMILES CC(C)C[C@H](N1Cc2ccccc2C[C@H](NC(=O)[C@H](S)Cc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C25H30N2O4S/c1-16(2)12-21(25(30)31)27-15-19-11-7-6-10-18(19)14-20(24(27)29)26-23(28)22(32)13-17-8-4-3-5-9-17/h3-11,16,20-22,32H,12-15H2,1-2H3,(H,26,28)(H,30,31)/t20-,21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of neutral endopeptidase (NEP). |
Bioorg Med Chem Lett 6: 957-962 (1996)
Article DOI: 10.1016/0960-894X(96)00149-7
BindingDB Entry DOI: 10.7270/Q2BV7GMV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035500
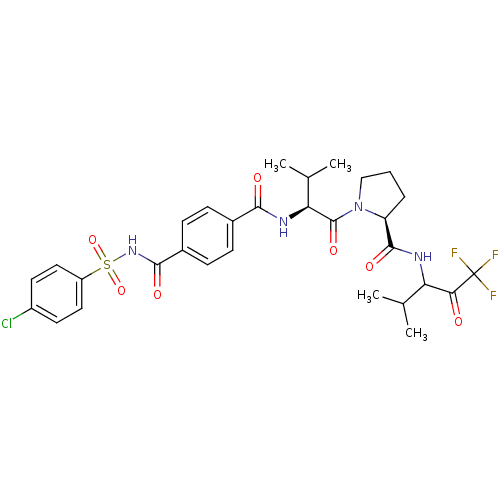
((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287700
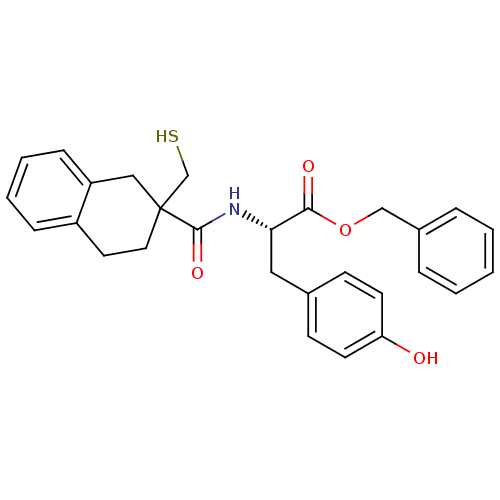
((S)-3-(4-Hydroxy-phenyl)-2-[(2-mercaptomethyl-1,2,...)Show SMILES Oc1ccc(C[C@H](NC(=O)C2(CS)CCc3ccccc3C2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C28H29NO4S/c30-24-12-10-20(11-13-24)16-25(26(31)33-18-21-6-2-1-3-7-21)29-27(32)28(19-34)15-14-22-8-4-5-9-23(22)17-28/h1-13,25,30,34H,14-19H2,(H,29,32)/t25-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786
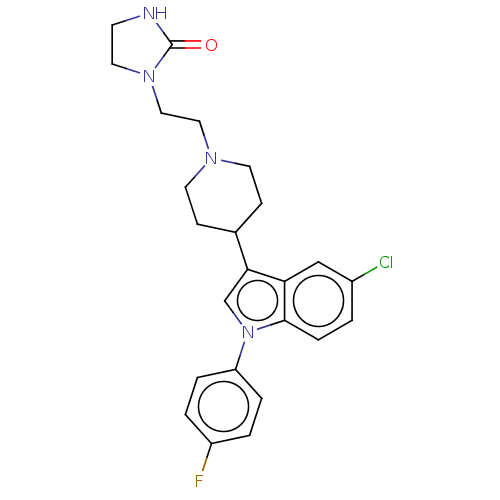
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287699
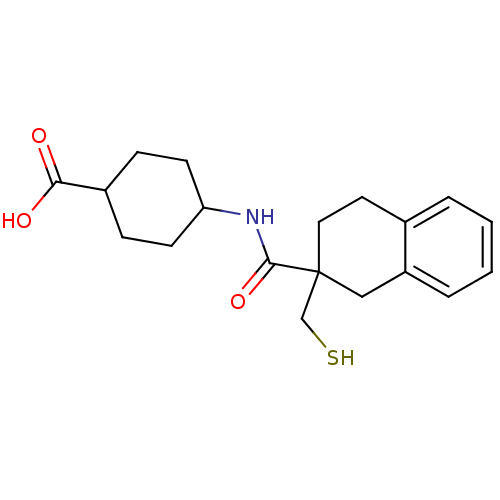
(4-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...)Show SMILES OC(=O)C1CCC(CC1)NC(=O)C1(CS)CCc2ccccc2C1 |(19.02,-7.43,;17.57,-6.91,;17.3,-5.39,;16.39,-7.89,;16.39,-9.43,;15.5,-8.45,;13.75,-8.17,;13.77,-6.63,;14.58,-7.58,;12.44,-8.97,;11.09,-8.22,;11.08,-6.68,;9.76,-8.99,;9.76,-10.53,;11.08,-11.32,;9.76,-7.44,;8.43,-6.67,;7.1,-7.45,;5.76,-6.68,;4.43,-7.45,;4.43,-8.99,;5.76,-9.76,;7.09,-8.99,;8.43,-9.76,)| Show InChI InChI=1S/C19H25NO3S/c21-17(22)14-5-7-16(8-6-14)20-18(23)19(12-24)10-9-13-3-1-2-4-15(13)11-19/h1-4,14,16,24H,5-12H2,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287690
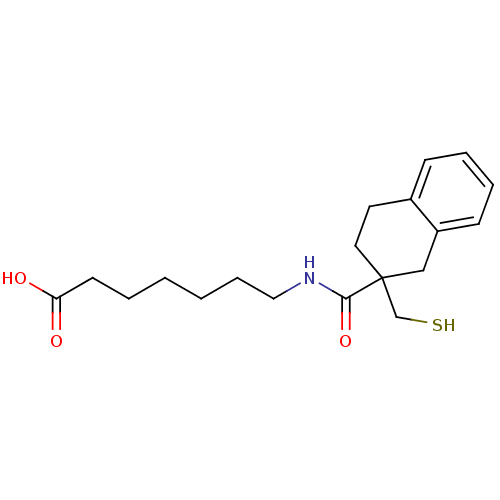
(7-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...)Show InChI InChI=1S/C19H27NO3S/c21-17(22)9-3-1-2-6-12-20-18(23)19(14-24)11-10-15-7-4-5-8-16(15)13-19/h4-5,7-8,24H,1-3,6,9-14H2,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50042149
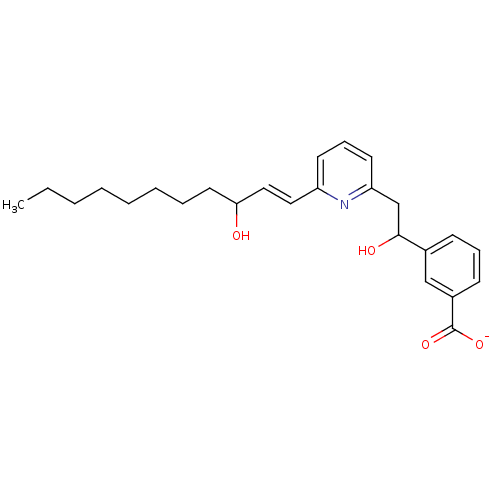
(CHEMBL112520 | Lithium; 3-{1-hydroxy-2-[6-(3-hydro...)Show SMILES CCCCCCCCC(O)\C=C\c1cccc(CC(O)c2cccc(c2)C([O-])=O)n1 Show InChI InChI=1S/C25H33NO4/c1-2-3-4-5-6-7-14-23(27)16-15-21-12-9-13-22(26-21)18-24(28)19-10-8-11-20(17-19)25(29)30/h8-13,15-17,23-24,27-28H,2-7,14,18H2,1H3,(H,29,30)/p-1/b16-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- LTB4 binding on human whole cells |
J Med Chem 36: 3308-20 (1993)
BindingDB Entry DOI: 10.7270/Q21R6R4P |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D4.2 in CHO cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400337
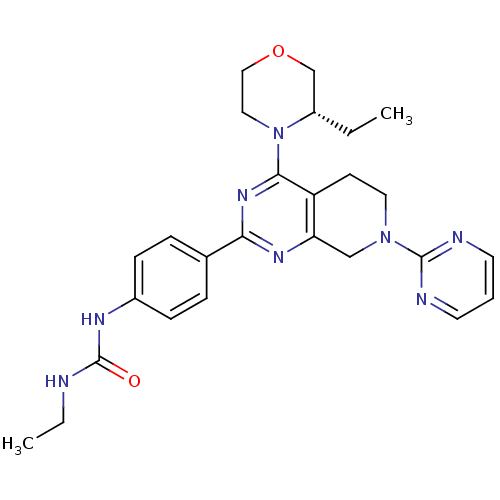
(CHEMBL2181523)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1CC)c1ncccn1 |r| Show InChI InChI=1S/C26H32N8O2/c1-3-20-17-36-15-14-34(20)24-21-10-13-33(25-28-11-5-12-29-25)16-22(21)31-23(32-24)18-6-8-19(9-7-18)30-26(35)27-4-2/h5-9,11-12,20H,3-4,10,13-17H2,1-2H3,(H2,27,30,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400335

(CHEMBL2181525)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1cccnn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)28-19-8-6-18(7-9-19)23-29-21-15-32(22-5-4-11-27-31-22)12-10-20(21)24(30-23)33-13-14-35-16-17(33)2/h4-9,11,17H,3,10,12-16H2,1-2H3,(H2,26,28,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400338
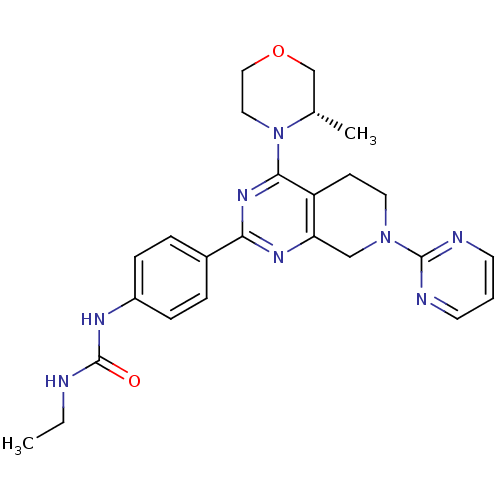
(CHEMBL2181522)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1ncccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)29-19-7-5-18(6-8-19)22-30-21-15-32(24-27-10-4-11-28-24)12-9-20(21)23(31-22)33-13-14-35-16-17(33)2/h4-8,10-11,17H,3,9,12-16H2,1-2H3,(H2,26,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50024096
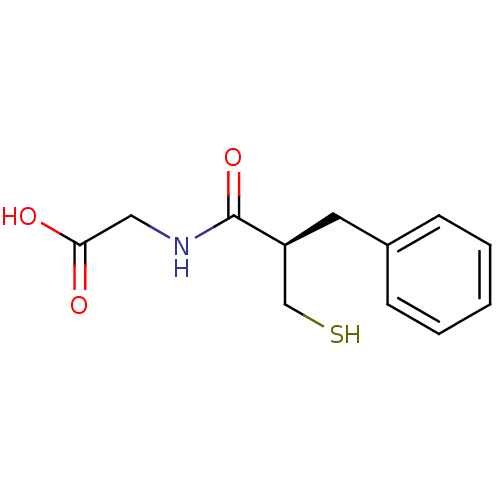
(((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400336
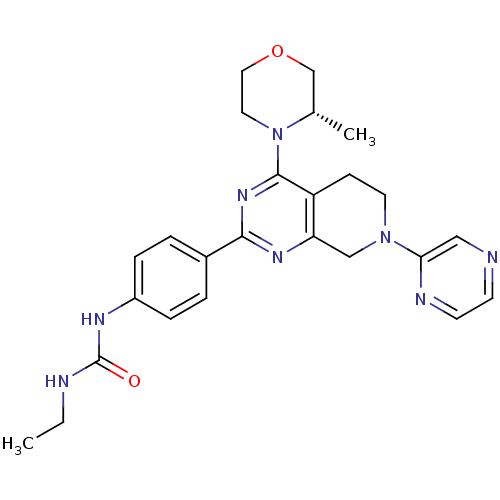
(CHEMBL2181524)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1cnccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-27-25(34)29-19-6-4-18(5-7-19)23-30-21-15-32(22-14-26-9-10-28-22)11-8-20(21)24(31-23)33-12-13-35-16-17(33)2/h4-7,9-10,14,17H,3,8,11-13,15-16H2,1-2H3,(H2,27,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50024102

(((S)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50287696
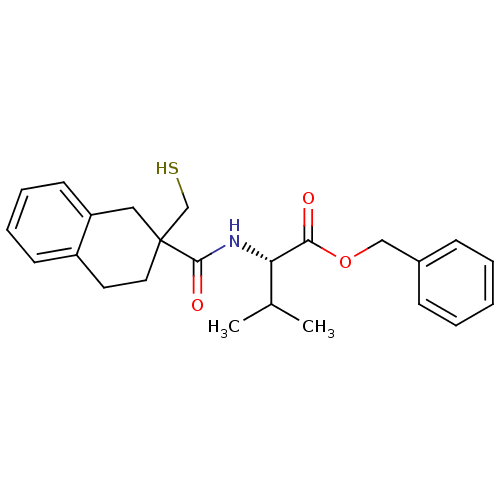
((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...)Show SMILES CC(C)[C@H](NC(=O)C1(CS)CCc2ccccc2C1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H29NO3S/c1-17(2)21(22(26)28-15-18-8-4-3-5-9-18)25-23(27)24(16-29)13-12-19-10-6-7-11-20(19)14-24/h3-11,17,21,29H,12-16H2,1-2H3,(H,25,27)/t21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase(NEP). |
Bioorg Med Chem Lett 6: 2053-2058 (1996)
Article DOI: 10.1016/0960-894X(96)00367-8
BindingDB Entry DOI: 10.7270/Q29G5MSW |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044867
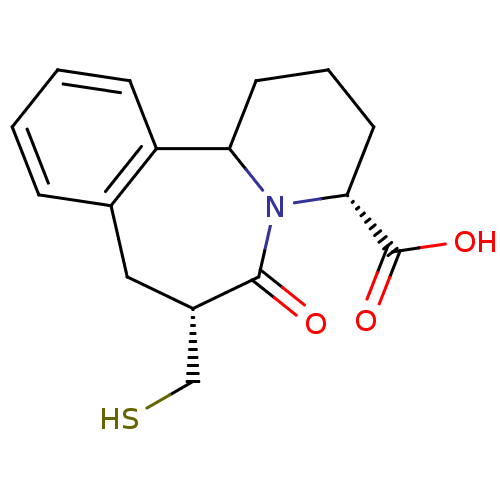
(7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@H](CS)Cc1ccccc21 Show InChI InChI=1S/C16H19NO3S/c18-15-11(9-21)8-10-4-1-2-5-12(10)13-6-3-7-14(16(19)20)17(13)15/h1-2,4-5,11,13-14,21H,3,6-9H2,(H,19,20)/t11-,13?,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50429069
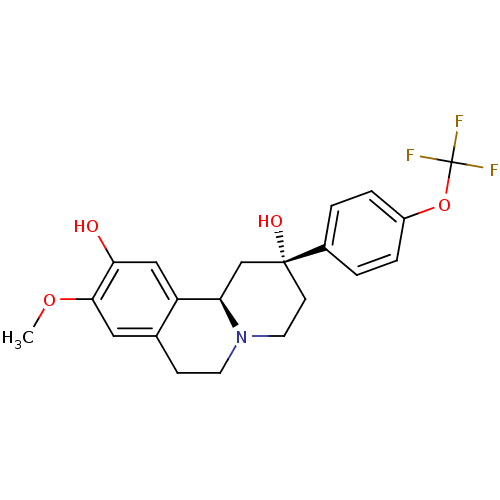
(CHEMBL2335736)Show SMILES COc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H22F3NO4/c1-28-19-10-13-6-8-25-9-7-20(27,12-17(25)16(13)11-18(19)26)14-2-4-15(5-3-14)29-21(22,23)24/h2-5,10-11,17,26-27H,6-9,12H2,1H3/t17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044869
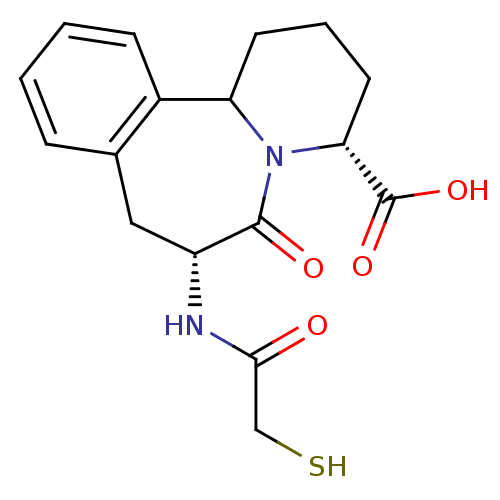
(7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)[C@@H](Cc1ccccc21)NC(=O)CS Show InChI InChI=1S/C17H20N2O4S/c20-15(9-24)18-12-8-10-4-1-2-5-11(10)13-6-3-7-14(17(22)23)19(13)16(12)21/h1-2,4-5,12-14,24H,3,6-9H2,(H,18,20)(H,22,23)/t12-,13?,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50024096

(((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50069667
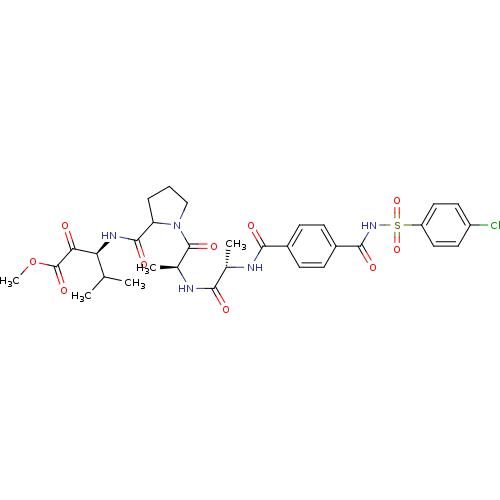
((S)-3-{[1-((S)-2-{(S)-2-[4-(4-Chloro-benzenesulfon...)Show SMILES COC(=O)C(=O)[C@@H](NC(=O)C1CCCN1C(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C Show InChI InChI=1S/C32H38ClN5O10S/c1-17(2)25(26(39)32(45)48-5)36-30(43)24-7-6-16-38(24)31(44)19(4)35-27(40)18(3)34-28(41)20-8-10-21(11-9-20)29(42)37-49(46,47)23-14-12-22(33)13-15-23/h8-15,17-19,24-25H,6-7,16H2,1-5H3,(H,34,41)(H,35,40)(H,36,43)(H,37,42)/t18-,19-,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cinc
Curated by ChEMBL
| Assay Description
Tested for rate of substrate hydrolysis in the presence of human neutrophil elastase |
Bioorg Med Chem Lett 8: 63-4 (1999)
BindingDB Entry DOI: 10.7270/Q2K936PK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035489
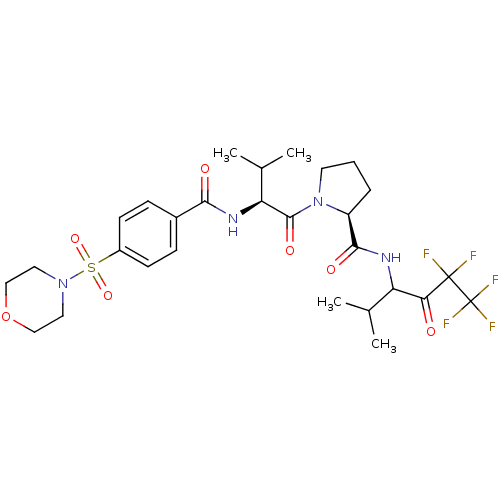
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-sulfonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)S(=O)(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C28H37F5N4O7S/c1-16(2)21(23(38)27(29,30)28(31,32)33)34-25(40)20-6-5-11-37(20)26(41)22(17(3)4)35-24(39)18-7-9-19(10-8-18)45(42,43)36-12-14-44-15-13-36/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,34,40)(H,35,39)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400349

(CHEMBL2181651)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CCN(Cc2c(n1)N1CCOC[C@@H]1C)c1ncccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)29-19-7-5-18(6-8-19)22-30-21-9-12-32(24-27-10-4-11-28-24)15-20(21)23(31-22)33-13-14-35-16-17(33)2/h4-8,10-11,17H,3,9,12-16H2,1-2H3,(H2,26,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400347

(CHEMBL2181653)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CCN(Cc2c(n1)N1CCOC[C@@H]1C)c1cnccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-27-25(34)29-19-6-4-18(5-7-19)23-30-21-8-11-32(22-14-26-9-10-28-22)15-20(21)24(31-23)33-12-13-35-16-17(33)2/h4-7,9-10,14,17H,3,8,11-13,15-16H2,1-2H3,(H2,27,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50042153
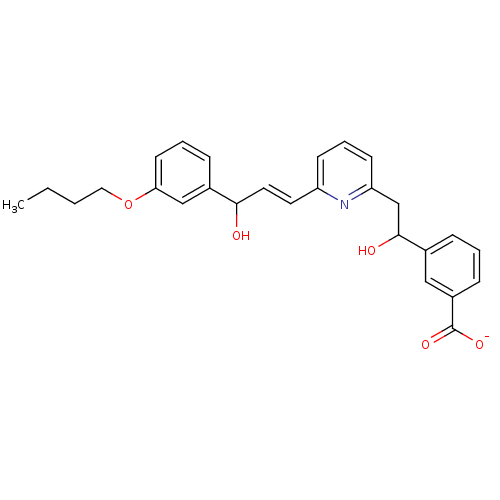
(CHEMBL113288 | Lithium; 3-(2-{7-[3-(3-butoxy-pheny...)Show SMILES CCCCOc1cccc(c1)C(O)\C=C\c1cccc(CC(O)c2cccc(c2)C([O-])=O)n1 Show InChI InChI=1S/C27H29NO5/c1-2-3-15-33-24-12-5-8-20(17-24)25(29)14-13-22-10-6-11-23(28-22)18-26(30)19-7-4-9-21(16-19)27(31)32/h4-14,16-17,25-26,29-30H,2-3,15,18H2,1H3,(H,31,32)/p-1/b14-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- LTB4 binding on human whole cells |
J Med Chem 36: 3308-20 (1993)
BindingDB Entry DOI: 10.7270/Q21R6R4P |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343759
(1-ethyl-3-(4-(6-(2-hydroxypropan-2-yl)-4-morpholin...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C22H27N5O3S/c1-4-23-21(28)24-15-7-5-14(6-8-15)19-25-16-13-17(22(2,3)29)31-18(16)20(26-19)27-9-11-30-12-10-27/h5-8,13,29H,4,9-12H2,1-3H3,(H2,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035528
(Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)N\C(C(C)C)=C(\OC(C)=O)C(F)(F)F Show InChI InChI=1S/C32H36ClF3N4O8S/c1-17(2)25(27(32(34,35)36)48-19(5)41)37-30(44)24-7-6-16-40(24)31(45)26(18(3)4)38-28(42)20-8-10-21(11-9-20)29(43)39-49(46,47)23-14-12-22(33)13-15-23/h8-15,17-18,24,26H,6-7,16H2,1-5H3,(H,37,44)(H,38,42)(H,39,43)/b27-25+/t24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035500

((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400359

(CHEMBL2181664)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(Cc2c(n1)N1CCOCC1)c1ncccn1 Show InChI InChI=1S/C23H26N8O2/c1-2-24-23(32)27-17-6-4-16(5-7-17)20-28-19-15-31(22-25-8-3-9-26-22)14-18(19)21(29-20)30-10-12-33-13-11-30/h3-9H,2,10-15H2,1H3,(H2,24,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data