

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460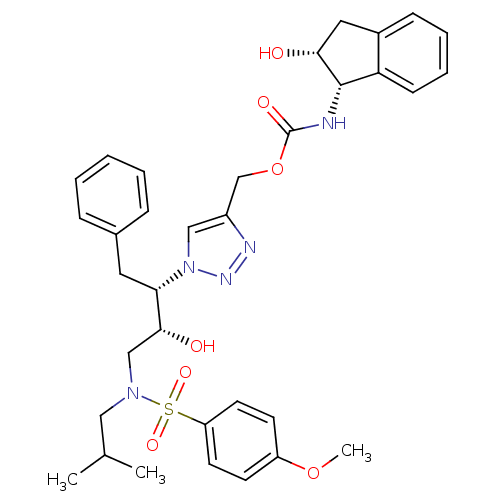 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 1.70 | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461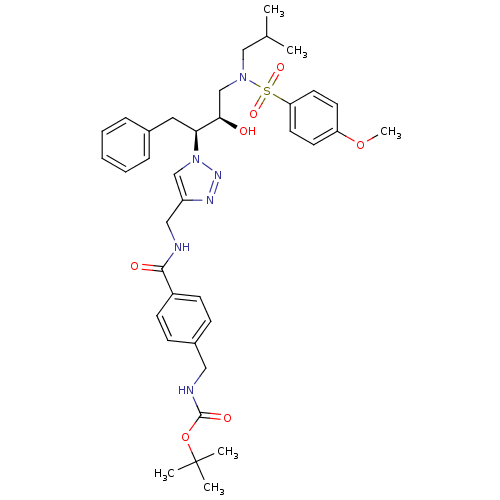 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 4 | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200943 (CHEMBL269769 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 9.70 | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 10 | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200945 (CHEMBL262751 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 13 | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200956 (CHEMBL262496 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200944 (CHEMBL407802 | cyclopentyl (2S,3S)-3-(4-((4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 22 | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200949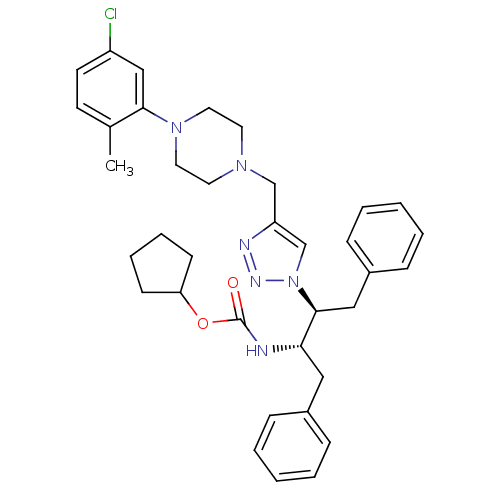 (CHEMBL262738 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200954 (CHEMBL386849 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-D...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84460 (HIV-1 PR Inhibitor, compound 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | MMDB Article PubMed | 27 | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200951 (CHEMBL267645 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200953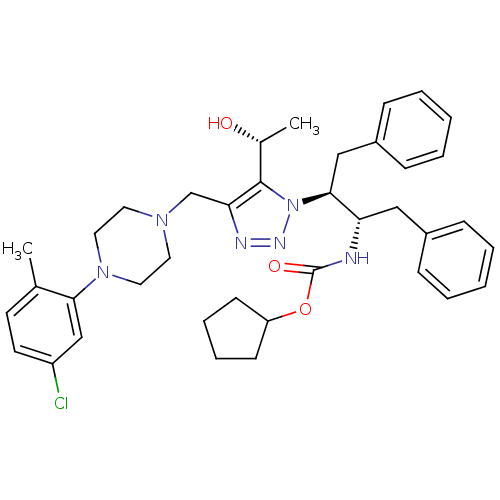 (CHEMBL405393 | cyclopentyl (2S,3S)-3-(4-((4-(5-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587,G537V,H558K,V571A] (Human immunodeficiency virus) | BDBM84461 (HIV-1 PR Inhibitor, compound 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 30 | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The inhibition activity against HIV-1 PR and three mutants (G48V, V82F, V82A)were done in 96 well microtiter plate and were assayed. Wells that show... | Chembiochem 4: 1246-8 (2003) Article DOI: 10.1002/cbic.200300724 BindingDB Entry DOI: 10.7270/Q23N21XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200948 (CHEMBL386847 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200958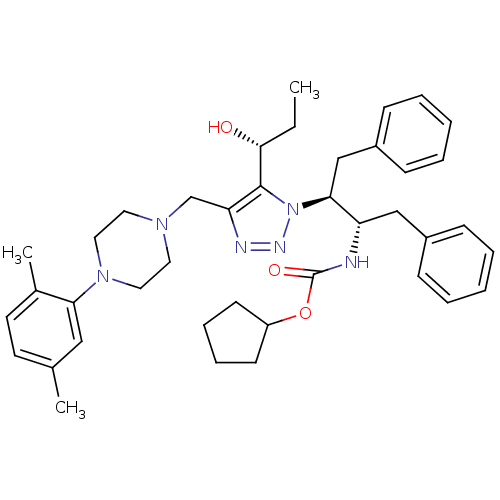 (CHEMBL428624 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22590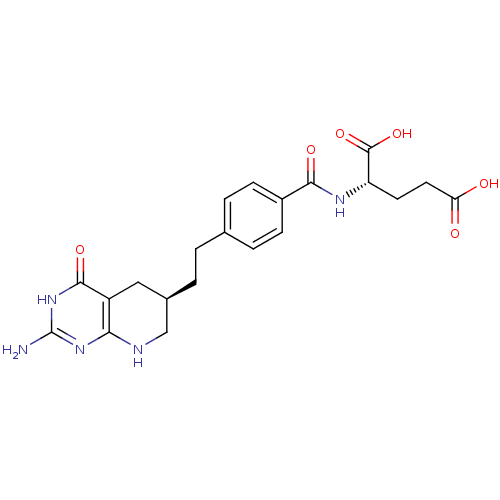 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 60 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200950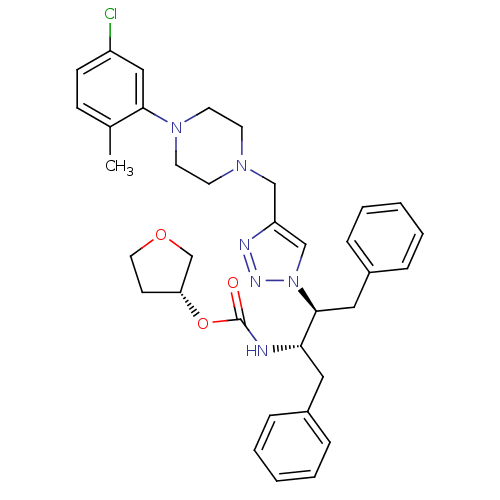 ((R)-tetrahydrofuran-3-yl (2S,3S)-3-(4-((4-(5-chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200955 (CHEMBL218359 | cyclopentyl (2S,3S)-5-methyl-1-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200946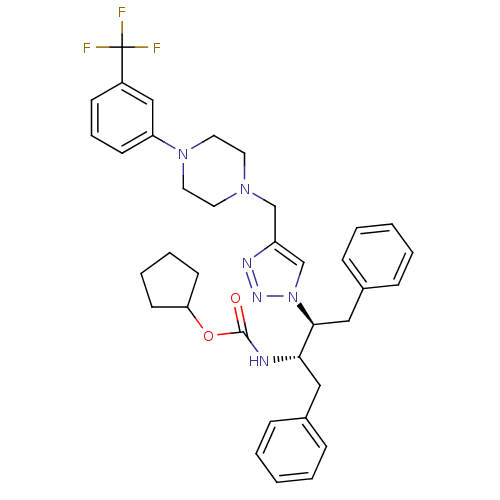 (CHEMBL385816 | cyclopentyl (2S,3S)-1,4-diphenyl-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosylglycinamide formyltransferase (Escherichia coli (strain K12)) | BDBM22590 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 100 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22585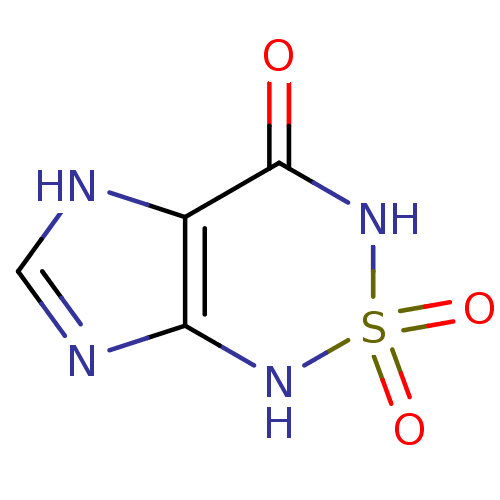 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22588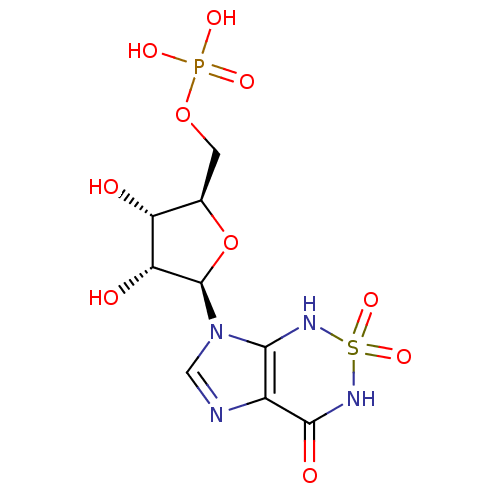 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158378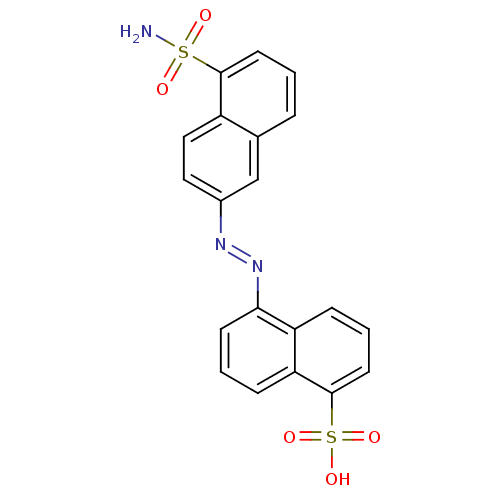 (5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22587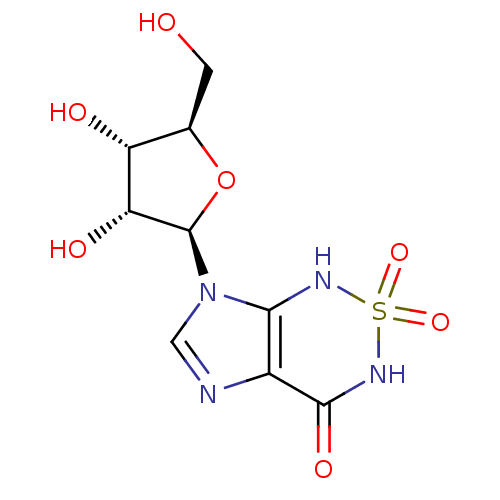 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 230 | -37.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay using the spectrophotometric method monitoring the appearance of IMP by measuring absorbance ... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200952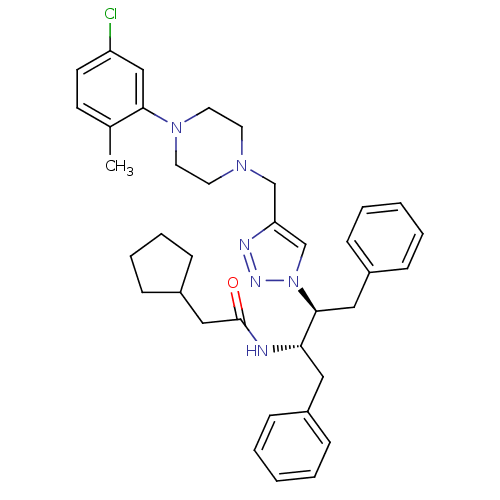 (CHEMBL407996 | N-((2S,3S)-3-(4-((4-(5-chloro-2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200947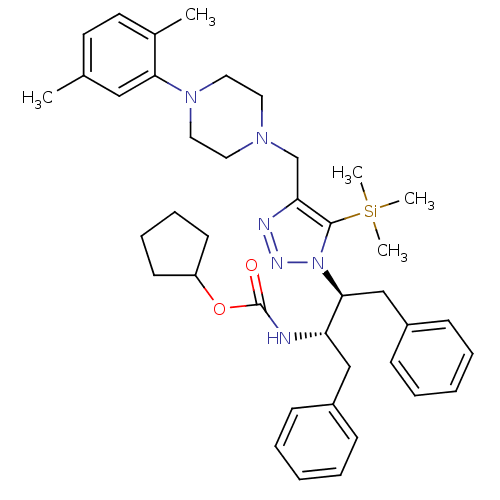 (CHEMBL405122 | cyclopentyl (2S,3S)-3-[4-([4-(2,5-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22588 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.80E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22578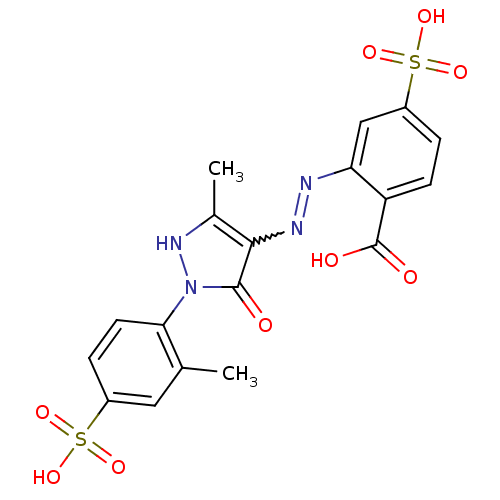 (2-[(E)-2-[5-hydroxy-3-methyl-1-(2-methyl-4-sulfoph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | 7.10E+3 | -29.1 | 1.16E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... | J Biol Chem 279: 50555-65 (2004) Article DOI: 10.1074/jbc.M406801200 BindingDB Entry DOI: 10.7270/Q2MC8X97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50200957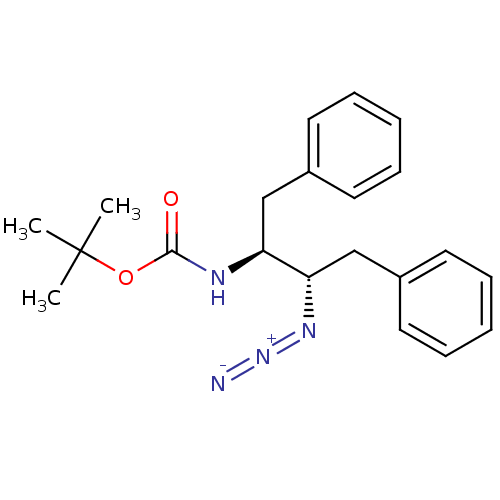 (CHEMBL216912 | [(1S,2S)-2-azido-1,2-dibenzylethyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 7697-710 (2006) Article DOI: 10.1021/jm060754+ BindingDB Entry DOI: 10.7270/Q2TH8MB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22588 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22585 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosylglycinamide formyltransferase (Escherichia coli (strain K12)) | BDBM22588 (Nucleotide, 3 | {[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22587 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22585 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance correspo... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosylglycinamide formyltransferase (Escherichia coli (strain K12)) | BDBM22585 (1H,3H,4H,7H-2,1,3,5,7-imidazo[4,5-c][1,2,6]thiadia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM22587 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosylglycinamide formyltransferase (Escherichia coli (strain K12)) | BDBM22587 (7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+5 | >-22.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 26 |
The Scripps Research Institute | Assay Description Assays were initiated by the addition of GAR to the reaction mixture containing GAR Tfase, test compounds, and cofactor. The assay monitors the defor... | J Biol Chem 282: 13033-46 (2007) Article DOI: 10.1074/jbc.M607293200 BindingDB Entry DOI: 10.7270/Q2GM85MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158378 (5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50480764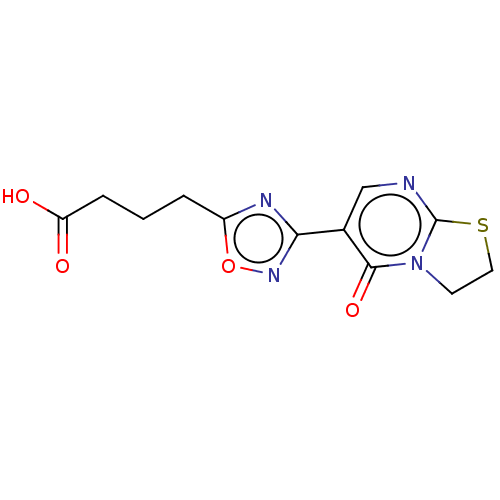 (CHEMBL562004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of ALR2 from Sprague-Dawley albino rat lens extract by spectrophotometrically | J Med Chem 52: 5578-81 (2009) Article DOI: 10.1021/jm901045w BindingDB Entry DOI: 10.7270/Q2HM5C8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22584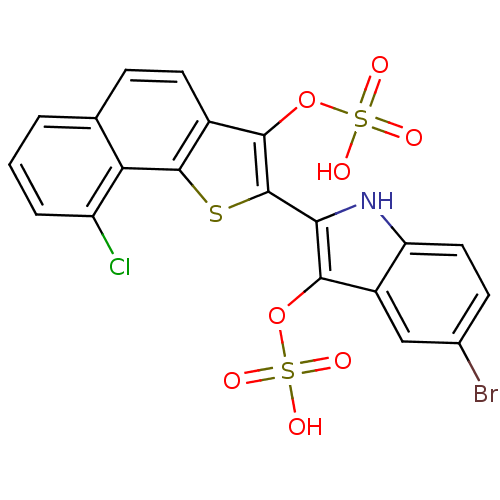 (5-bromo-2-[9-chloro-3-(sulfooxy)naphtho[1,2-b]thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... | J Biol Chem 279: 50555-65 (2004) Article DOI: 10.1074/jbc.M406801200 BindingDB Entry DOI: 10.7270/Q2MC8X97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22580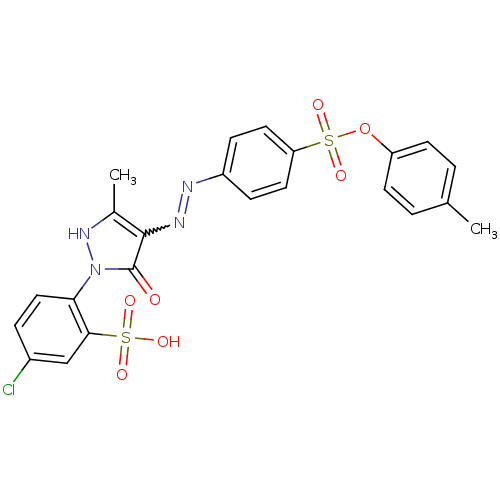 (47729-M | 5-chloro-2-{5-hydroxy-3-methyl-4-[(E)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22580 (47729-M | 5-chloro-2-{5-hydroxy-3-methyl-4-[(E)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute | Assay Description The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... | J Biol Chem 279: 50555-65 (2004) Article DOI: 10.1074/jbc.M406801200 BindingDB Entry DOI: 10.7270/Q2MC8X97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50480755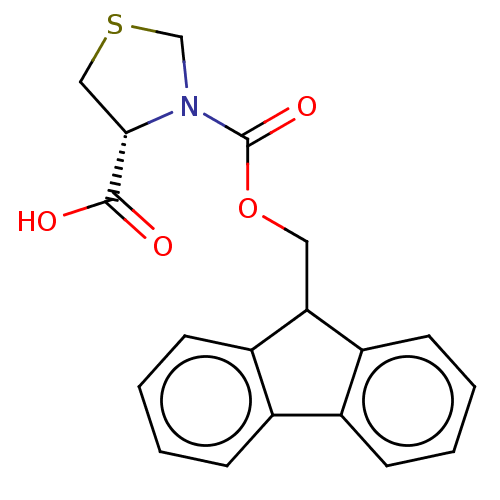 (CHEMBL565091 | SB-02066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli "Federico II" Curated by ChEMBL | Assay Description Inhibition of ALR2 from Sprague-Dawley albino rat lens extract by spectrophotometrically | J Med Chem 52: 5578-81 (2009) Article DOI: 10.1021/jm901045w BindingDB Entry DOI: 10.7270/Q2HM5C8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158388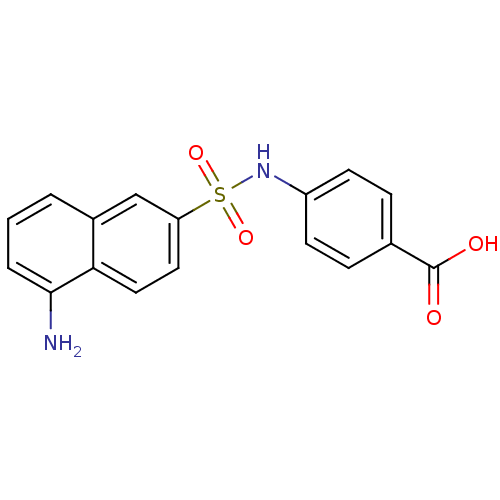 (4-(1-aminonaphthalene-6-sulfonamido)benzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158380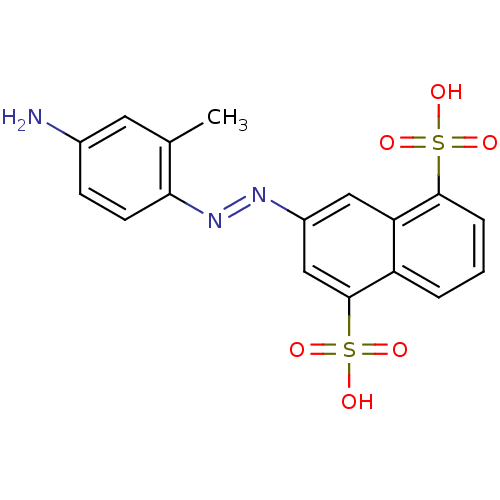 (3-(4-amino-2-methyl-phenylazo)-naphthalene-1,5-dis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158381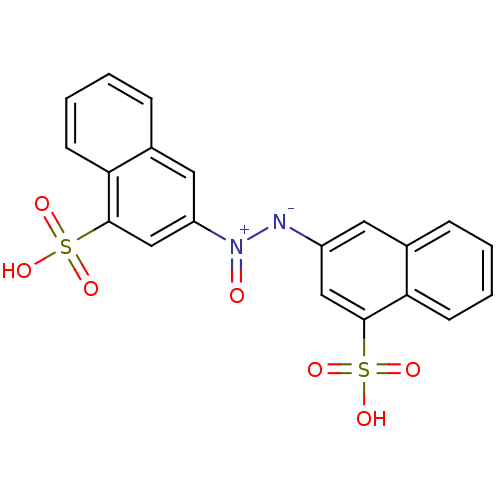 ((Z)-1,2-bis(4-sulfonaphthalen-2-yl)diazene oxide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50158387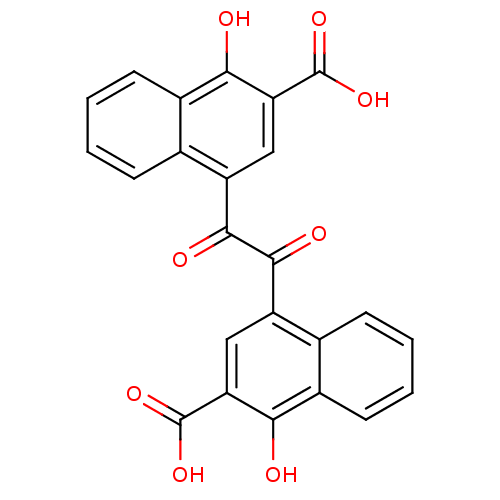 (4-[(3-carboxy-4-hydroxy-1-naphthyl)(oxo)acetyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human AICAR Tfase | J Med Chem 47: 6681-90 (2004) Article DOI: 10.1021/jm049504o BindingDB Entry DOI: 10.7270/Q2PZ589Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |