 Found 389 hits with Last Name = 'orsini' and Initial = 'm'
Found 389 hits with Last Name = 'orsini' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203781
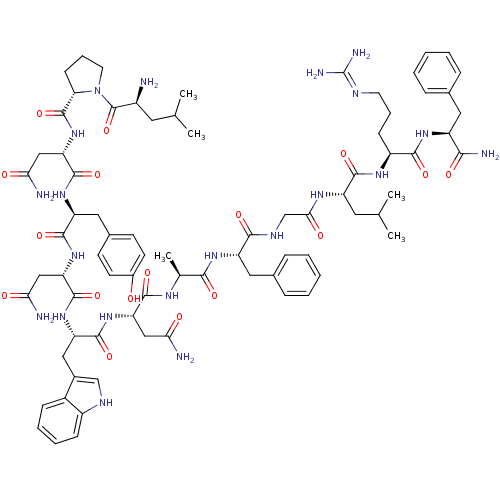
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,71.74,86.90,36.37,12.13,105.109,wD:66.70,24.24,94.98,16.16,44.45,(-10.05,-20.48,;-10.03,-22.02,;-11.35,-22.81,;-8.68,-22.76,;-8.68,-24.32,;-10.01,-25.09,;-7.34,-25.09,;-7.34,-26.63,;-6.01,-24.33,;-5.53,-22.87,;-3.99,-22.87,;-3.52,-24.33,;-4.77,-25.24,;-4.77,-26.78,;-6.11,-27.55,;-3.44,-27.55,;-3.44,-29.09,;-4.77,-29.86,;-4.78,-31.4,;-3.44,-32.17,;-6.11,-32.17,;-2.11,-29.86,;-2.11,-31.4,;-.77,-29.09,;.56,-29.86,;.56,-31.4,;1.89,-32.18,;1.88,-33.71,;3.22,-34.48,;4.55,-33.71,;5.89,-34.48,;4.55,-32.17,;3.23,-31.41,;1.89,-29.1,;1.9,-27.56,;3.23,-29.87,;4.56,-29.1,;4.56,-27.56,;5.9,-26.79,;7.23,-27.56,;5.9,-25.25,;5.89,-29.87,;5.89,-31.41,;7.23,-29.1,;8.56,-29.87,;8.56,-31.41,;9.89,-32.18,;11.14,-31.19,;12.38,-32.18,;11.9,-33.65,;12.66,-34.97,;11.9,-36.29,;10.36,-36.29,;9.6,-34.96,;10.37,-33.64,;9.9,-29.1,;9.9,-27.56,;11.23,-29.88,;12.56,-29.11,;12.57,-27.57,;13.9,-26.8,;15.23,-27.57,;13.9,-25.26,;13.9,-29.88,;13.9,-31.42,;15.23,-29.11,;16.56,-29.88,;16.56,-31.42,;17.9,-29.11,;17.9,-27.57,;19.23,-29.88,;20.57,-29.12,;20.57,-27.58,;21.9,-26.81,;23.23,-27.58,;24.57,-26.82,;24.57,-25.27,;23.23,-24.5,;21.9,-25.27,;21.9,-29.89,;21.9,-31.43,;23.23,-29.12,;24.57,-29.89,;25.9,-29.12,;25.9,-27.58,;27.23,-29.89,;28.57,-29.12,;28.57,-27.58,;29.9,-26.82,;29.91,-25.28,;31.24,-27.59,;29.9,-29.9,;29.9,-31.44,;31.24,-29.13,;32.57,-29.9,;32.57,-31.44,;33.9,-32.21,;33.9,-33.75,;35.23,-34.52,;35.23,-36.06,;36.56,-36.83,;33.9,-36.83,;33.9,-29.13,;33.91,-27.59,;35.24,-29.9,;36.57,-29.13,;36.57,-27.59,;37.91,-26.82,;39.24,-27.6,;40.57,-26.84,;40.57,-25.3,;39.23,-24.52,;37.9,-25.3,;37.9,-29.9,;39.24,-29.13,;37.9,-31.44,)| Show InChI InChI=1S/C78H107N21O17/c1-41(2)30-50(79)77(116)99-29-15-23-61(99)76(115)98-60(38-64(82)103)75(114)94-56(34-46-24-26-48(100)27-25-46)72(111)97-59(37-63(81)102)74(113)95-57(35-47-39-87-51-21-13-12-20-49(47)51)73(112)96-58(36-62(80)101)70(109)89-43(5)67(106)93-55(33-45-18-10-7-11-19-45)68(107)88-40-65(104)90-54(31-42(3)4)71(110)91-52(22-14-28-86-78(84)85)69(108)92-53(66(83)105)32-44-16-8-6-9-17-44/h6-13,16-21,24-27,39,41-43,50,52-61,87,100H,14-15,22-23,28-38,40,79H2,1-5H3,(H2,80,101)(H2,81,102)(H2,82,103)(H2,83,105)(H,88,107)(H,89,109)(H,90,104)(H,91,110)(H,92,108)(H,93,106)(H,94,114)(H,95,113)(H,96,112)(H,97,111)(H,98,115)(H4,84,85,86)/t43-,50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203803
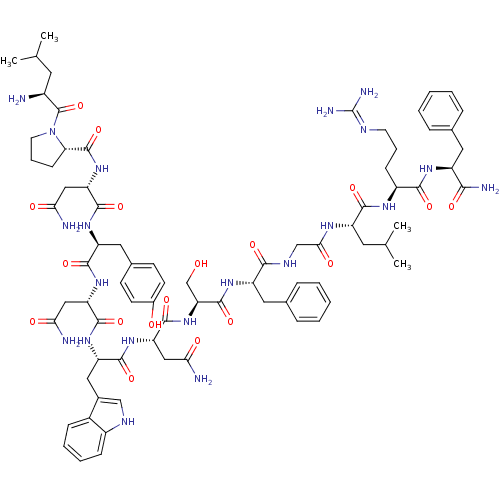
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-13.2,3.34,;-13.16,1.8,;-14.49,1.01,;-11.81,1.05,;-11.81,-.5,;-13.14,-1.27,;-10.47,-1.27,;-10.47,-2.81,;-9.14,-.51,;-8.66,.95,;-7.12,.95,;-6.65,-.51,;-7.9,-1.42,;-7.9,-2.96,;-9.24,-3.73,;-6.57,-3.73,;-6.57,-5.27,;-7.91,-6.04,;-7.91,-7.58,;-6.57,-8.35,;-9.24,-8.35,;-5.24,-6.04,;-5.24,-7.58,;-3.9,-5.27,;-2.57,-6.04,;-2.57,-7.59,;-1.24,-8.36,;-1.25,-9.89,;.08,-10.66,;1.42,-9.9,;2.75,-10.67,;1.42,-8.35,;.09,-7.59,;-1.24,-5.28,;-1.24,-3.74,;.1,-6.05,;1.43,-5.28,;1.43,-3.74,;2.77,-2.97,;4.1,-3.74,;2.77,-1.43,;2.76,-6.05,;2.76,-7.59,;4.1,-5.28,;5.43,-6.05,;5.43,-7.59,;6.76,-8.36,;8.01,-7.37,;9.25,-8.36,;8.77,-9.83,;9.53,-11.15,;8.77,-12.47,;7.23,-12.47,;6.47,-11.14,;7.24,-9.82,;6.77,-5.28,;6.77,-3.74,;8.1,-6.06,;9.43,-5.29,;9.43,-3.75,;10.77,-2.98,;12.1,-3.75,;10.77,-1.44,;10.77,-6.06,;10.76,-7.6,;12.1,-5.29,;13.43,-6.06,;13.43,-7.6,;14.76,-8.37,;14.77,-5.29,;14.77,-3.75,;16.1,-6.06,;17.44,-5.3,;17.44,-3.76,;18.77,-2.99,;20.1,-3.76,;21.44,-3,;21.44,-1.45,;20.1,-.68,;18.77,-1.46,;18.77,-6.07,;18.77,-7.61,;20.1,-5.3,;21.44,-6.07,;22.77,-5.3,;22.77,-3.76,;24.1,-6.07,;25.44,-5.3,;25.44,-3.76,;26.77,-3,;26.78,-1.46,;28.11,-3.77,;26.77,-6.08,;26.77,-7.62,;28.1,-5.31,;29.44,-6.08,;29.44,-7.62,;30.77,-8.39,;30.77,-9.93,;32.1,-10.7,;32.1,-12.24,;33.43,-13.01,;30.76,-13.01,;30.77,-5.31,;30.77,-3.77,;32.1,-6.08,;33.44,-5.31,;33.44,-3.77,;34.78,-3,;36.1,-3.79,;37.44,-3.02,;37.44,-1.48,;36.1,-.7,;34.77,-1.48,;34.77,-6.08,;36.11,-5.32,;34.77,-7.62,)| Show InChI InChI=1S/C78H107N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h5-12,15-20,23-26,38,41-42,49,51-61,87,100-101H,13-14,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203803

((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-13.2,3.34,;-13.16,1.8,;-14.49,1.01,;-11.81,1.05,;-11.81,-.5,;-13.14,-1.27,;-10.47,-1.27,;-10.47,-2.81,;-9.14,-.51,;-8.66,.95,;-7.12,.95,;-6.65,-.51,;-7.9,-1.42,;-7.9,-2.96,;-9.24,-3.73,;-6.57,-3.73,;-6.57,-5.27,;-7.91,-6.04,;-7.91,-7.58,;-6.57,-8.35,;-9.24,-8.35,;-5.24,-6.04,;-5.24,-7.58,;-3.9,-5.27,;-2.57,-6.04,;-2.57,-7.59,;-1.24,-8.36,;-1.25,-9.89,;.08,-10.66,;1.42,-9.9,;2.75,-10.67,;1.42,-8.35,;.09,-7.59,;-1.24,-5.28,;-1.24,-3.74,;.1,-6.05,;1.43,-5.28,;1.43,-3.74,;2.77,-2.97,;4.1,-3.74,;2.77,-1.43,;2.76,-6.05,;2.76,-7.59,;4.1,-5.28,;5.43,-6.05,;5.43,-7.59,;6.76,-8.36,;8.01,-7.37,;9.25,-8.36,;8.77,-9.83,;9.53,-11.15,;8.77,-12.47,;7.23,-12.47,;6.47,-11.14,;7.24,-9.82,;6.77,-5.28,;6.77,-3.74,;8.1,-6.06,;9.43,-5.29,;9.43,-3.75,;10.77,-2.98,;12.1,-3.75,;10.77,-1.44,;10.77,-6.06,;10.76,-7.6,;12.1,-5.29,;13.43,-6.06,;13.43,-7.6,;14.76,-8.37,;14.77,-5.29,;14.77,-3.75,;16.1,-6.06,;17.44,-5.3,;17.44,-3.76,;18.77,-2.99,;20.1,-3.76,;21.44,-3,;21.44,-1.45,;20.1,-.68,;18.77,-1.46,;18.77,-6.07,;18.77,-7.61,;20.1,-5.3,;21.44,-6.07,;22.77,-5.3,;22.77,-3.76,;24.1,-6.07,;25.44,-5.3,;25.44,-3.76,;26.77,-3,;26.78,-1.46,;28.11,-3.77,;26.77,-6.08,;26.77,-7.62,;28.1,-5.31,;29.44,-6.08,;29.44,-7.62,;30.77,-8.39,;30.77,-9.93,;32.1,-10.7,;32.1,-12.24,;33.43,-13.01,;30.76,-13.01,;30.77,-5.31,;30.77,-3.77,;32.1,-6.08,;33.44,-5.31,;33.44,-3.77,;34.78,-3,;36.1,-3.79,;37.44,-3.02,;37.44,-1.48,;36.1,-.7,;34.77,-1.48,;34.77,-6.08,;36.11,-5.32,;34.77,-7.62,)| Show InChI InChI=1S/C78H107N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h5-12,15-20,23-26,38,41-42,49,51-61,87,100-101H,13-14,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203782
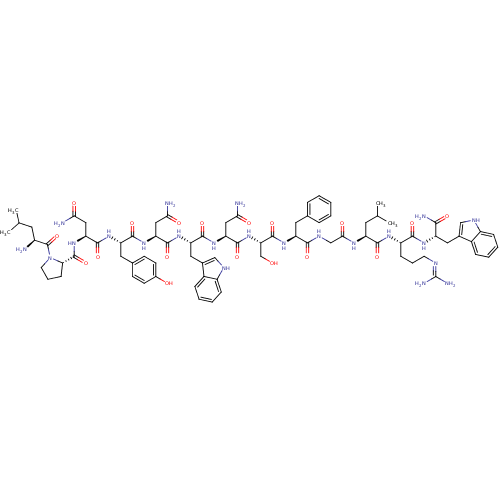
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |wU:72.75,87.91,36.37,12.13,106.110,58.61,4.4,wD:66.69,24.24,95.99,16.16,44.45,(-6.95,-18.11,;-6.92,-19.65,;-8.24,-20.44,;-5.57,-20.4,;-5.57,-21.96,;-6.9,-22.73,;-4.23,-22.73,;-4.23,-24.27,;-2.9,-21.96,;-2.42,-20.5,;-.89,-20.5,;-.41,-21.96,;-1.66,-22.87,;-1.66,-24.41,;-3,-25.18,;-.33,-25.18,;-.33,-26.72,;-1.66,-27.49,;-1.66,-29.03,;-.33,-29.8,;-3,-29.8,;1,-27.49,;1,-29.03,;2.34,-26.72,;3.67,-27.5,;3.67,-29.04,;5,-29.81,;5,-31.34,;6.32,-32.11,;7.67,-31.35,;9,-32.12,;7.66,-29.8,;6.34,-29.04,;5.01,-26.73,;5.01,-25.19,;6.34,-27.5,;7.67,-26.73,;7.67,-25.19,;9.01,-24.42,;10.34,-25.19,;9.01,-22.88,;9.01,-27.5,;9,-29.04,;10.34,-26.73,;11.67,-27.5,;11.67,-29.04,;13,-29.81,;14.26,-28.82,;15.49,-29.81,;15.01,-31.28,;15.77,-32.6,;15.01,-33.92,;13.47,-33.92,;12.71,-32.59,;13.48,-31.27,;13.01,-26.74,;13.01,-25.2,;14.34,-27.51,;15.67,-26.74,;15.68,-25.2,;17.01,-24.43,;18.35,-25.2,;17.01,-22.89,;17,-27.51,;17,-29.05,;18.34,-26.74,;19.67,-27.51,;19.67,-29.05,;21.01,-29.83,;21.01,-26.75,;21.01,-25.21,;22.34,-27.52,;23.67,-26.75,;23.67,-25.21,;25.01,-24.44,;26.34,-25.21,;27.68,-24.44,;27.68,-22.9,;26.33,-22.13,;25.01,-22.9,;25.01,-27.52,;25.01,-29.06,;26.34,-26.75,;27.68,-27.51,;29.01,-26.75,;29.01,-25.21,;30.34,-27.52,;31.68,-26.75,;31.68,-25.21,;33.01,-24.44,;33.01,-22.9,;34.35,-25.21,;33.01,-27.52,;33.01,-29.06,;34.35,-26.75,;35.68,-27.52,;35.68,-29.06,;37.01,-29.84,;37.01,-31.38,;38.34,-32.15,;38.34,-33.69,;39.67,-34.46,;37.01,-34.46,;37.02,-26.76,;37.02,-25.22,;38.35,-27.53,;39.68,-26.76,;39.68,-25.22,;41.02,-24.45,;42.48,-24.95,;43.41,-23.72,;42.52,-22.45,;42.87,-20.96,;41.75,-19.9,;40.27,-20.36,;39.93,-21.85,;41.05,-22.9,;41.02,-27.53,;42.35,-26.76,;41.01,-29.07,)| Show InChI InChI=1S/C80H108N22O18/c1-41(2)28-50(81)79(120)102-27-13-21-63(102)78(119)100-61(36-66(84)107)75(116)96-57(31-44-22-24-47(104)25-23-44)72(113)98-59(34-64(82)105)74(115)97-58(33-46-38-90-52-19-11-9-17-49(46)52)73(114)99-60(35-65(83)106)76(117)101-62(40-103)77(118)95-56(30-43-14-6-5-7-15-43)69(110)91-39-67(108)92-55(29-42(3)4)71(112)93-53(20-12-26-88-80(86)87)70(111)94-54(68(85)109)32-45-37-89-51-18-10-8-16-48(45)51/h5-11,14-19,22-25,37-38,41-42,50,53-63,89-90,103-104H,12-13,20-21,26-36,39-40,81H2,1-4H3,(H2,82,105)(H2,83,106)(H2,84,107)(H2,85,109)(H,91,110)(H,92,108)(H,93,112)(H,94,111)(H,95,118)(H,96,116)(H,97,115)(H,98,113)(H,99,114)(H,100,119)(H,101,117)(H4,86,87,88)/t50-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
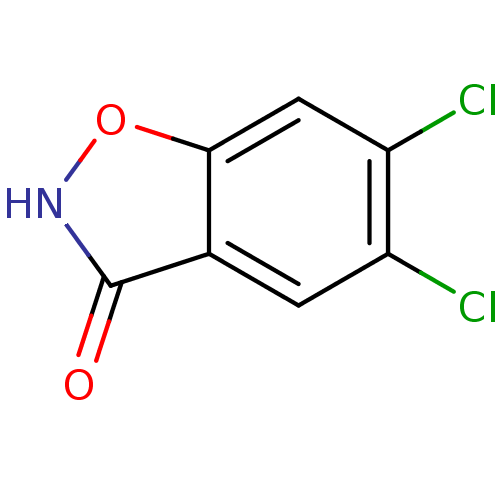
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203794
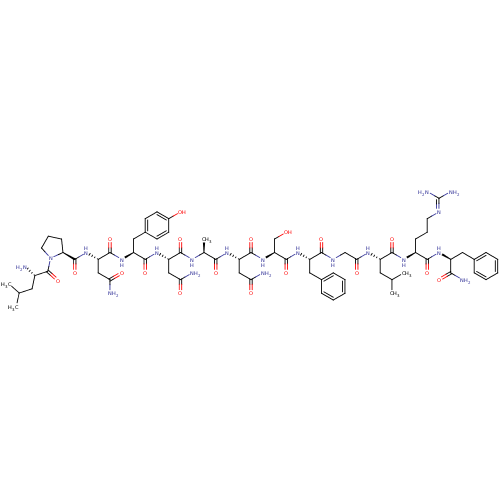
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O Show InChI InChI=1S/C70H102N20O18/c1-36(2)26-43(71)69(108)90-25-13-19-53(90)68(107)88-51(33-56(74)95)65(104)86-48(30-41-20-22-42(92)23-21-41)64(103)87-49(31-54(72)93)62(101)80-38(5)59(98)84-50(32-55(73)94)66(105)89-52(35-91)67(106)85-47(29-40-16-10-7-11-17-40)60(99)79-34-57(96)81-46(27-37(3)4)63(102)82-44(18-12-24-78-70(76)77)61(100)83-45(58(75)97)28-39-14-8-6-9-15-39/h6-11,14-17,20-23,36-38,43-53,91-92H,12-13,18-19,24-35,71H2,1-5H3,(H2,72,93)(H2,73,94)(H2,74,95)(H2,75,97)(H,79,99)(H,80,101)(H,81,96)(H,82,102)(H,83,100)(H,84,98)(H,85,106)(H,86,104)(H,87,103)(H,88,107)(H,89,105)(H4,76,77,78)/t38-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203811
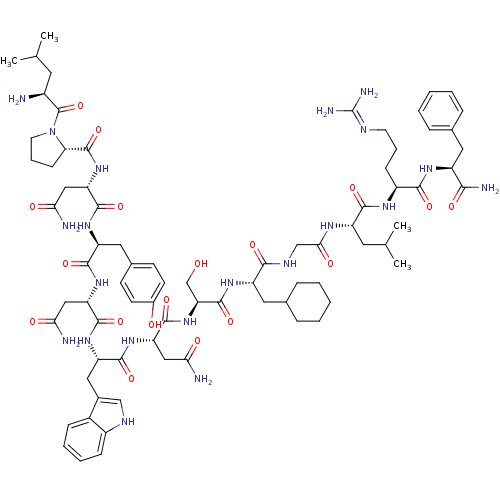
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;27.56,-26.37,;28.9,-25.61,;28.91,-24.06,;27.58,-23.28,;26.23,-24.05,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C78H113N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h5,7-8,11-12,15-16,19-20,23-26,38,41-42,44,49,51-61,87,100-101H,6,9-10,13-14,17-18,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185799
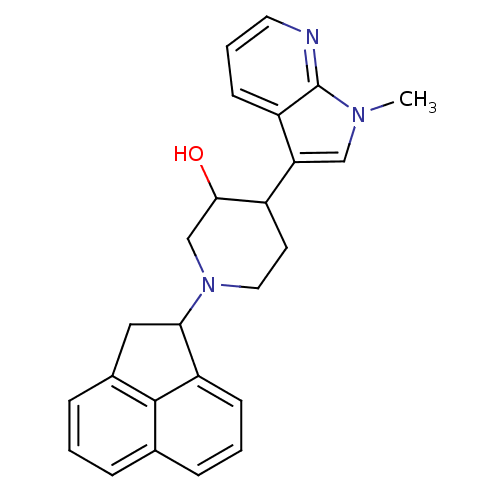
(1-(1,2-dihydroacenaphthylen-1-yl)-4-(1-methyl-1H-p...)Show SMILES Cn1cc(C2CCN(CC2O)C2Cc3cccc4cccc2c34)c2cccnc12 Show InChI InChI=1S/C25H25N3O/c1-27-14-21(19-9-4-11-26-25(19)27)18-10-12-28(15-23(18)29)22-13-17-7-2-5-16-6-3-8-20(22)24(16)17/h2-9,11,14,18,22-23,29H,10,12-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
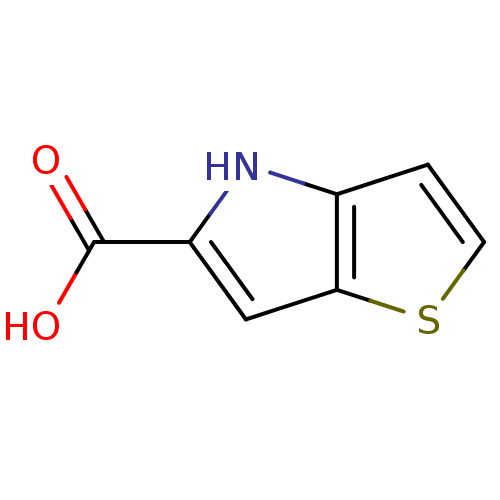
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185800
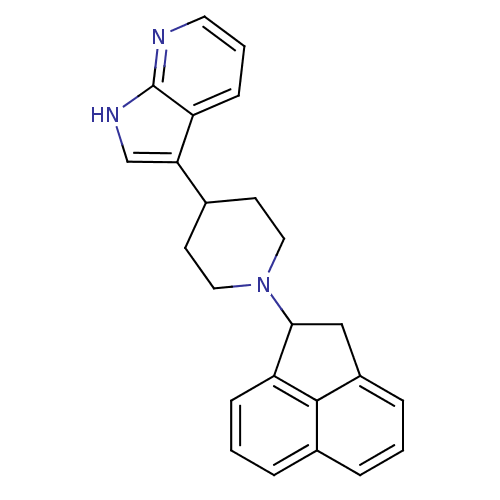
(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...)Show SMILES C1C(N2CCC(CC2)c2c[nH]c3ncccc23)c2cccc3cccc1c23 Show InChI InChI=1S/C24H23N3/c1-4-17-5-2-7-20-22(14-18(6-1)23(17)20)27-12-9-16(10-13-27)21-15-26-24-19(21)8-3-11-25-24/h1-8,11,15-16,22H,9-10,12-14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203802
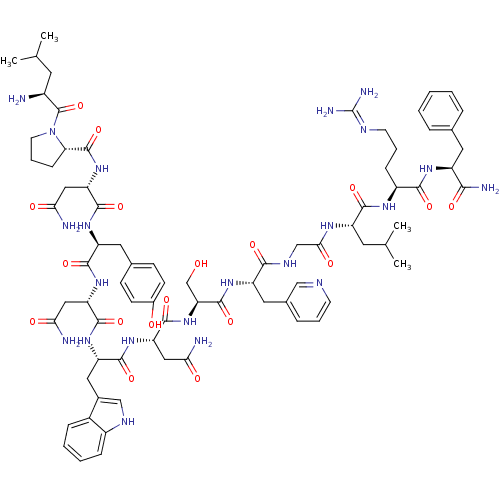
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cccnc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;27.57,-26.36,;28.91,-25.6,;28.91,-24.05,;27.57,-23.27,;26.24,-24.05,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C77H106N22O18/c1-40(2)27-48(78)76(117)99-26-12-19-60(99)75(116)97-58(35-63(81)104)72(113)93-54(30-43-20-22-46(101)23-21-43)69(110)95-56(33-61(79)102)71(112)94-55(32-45-37-87-49-17-9-8-16-47(45)49)70(111)96-57(34-62(80)103)73(114)98-59(39-100)74(115)92-53(31-44-15-10-24-85-36-44)66(107)88-38-64(105)89-52(28-41(3)4)68(109)90-50(18-11-25-86-77(83)84)67(108)91-51(65(82)106)29-42-13-6-5-7-14-42/h5-10,13-17,20-24,36-37,40-41,48,50-60,87,100-101H,11-12,18-19,25-35,38-39,78H2,1-4H3,(H2,79,102)(H2,80,103)(H2,81,104)(H2,82,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,83,84,86)/t48-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203814
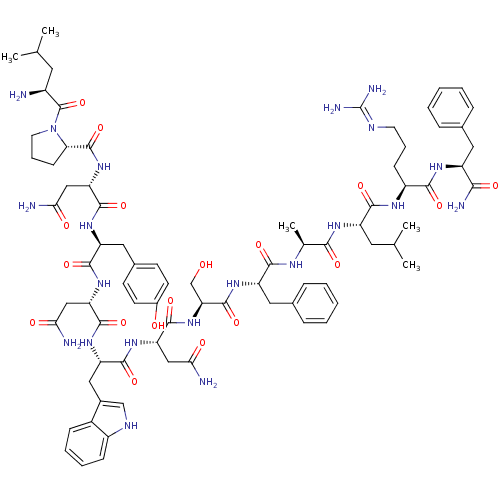
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,88.92,36.37,12.13,107.111,wD:66.69,24.24,96.100,16.16,44.45,83.88,(-10.05,-20.79,;-10.03,-22.33,;-11.35,-23.13,;-8.68,-23.08,;-8.68,-24.64,;-10.01,-25.41,;-7.34,-25.41,;-7.34,-26.95,;-6.01,-24.64,;-5.53,-23.18,;-3.99,-23.18,;-3.52,-24.65,;-4.77,-25.55,;-4.77,-27.09,;-6.11,-27.86,;-3.44,-27.87,;-3.44,-29.41,;-4.77,-30.17,;-4.78,-31.71,;-3.44,-32.49,;-6.11,-32.48,;-2.11,-30.18,;-2.11,-31.72,;-.77,-29.41,;.56,-30.18,;.56,-31.72,;1.89,-32.49,;1.88,-34.03,;3.22,-34.8,;4.55,-34.03,;5.89,-34.8,;4.55,-32.48,;3.23,-31.72,;1.89,-29.41,;1.9,-27.87,;3.23,-30.18,;4.56,-29.41,;4.56,-27.87,;5.9,-27.11,;7.23,-27.88,;5.9,-25.57,;5.89,-30.19,;5.89,-31.73,;7.23,-29.42,;8.56,-30.19,;8.56,-31.73,;9.89,-32.5,;11.14,-31.51,;12.38,-32.5,;11.9,-33.96,;12.66,-35.28,;11.9,-36.61,;10.36,-36.61,;9.6,-35.28,;10.37,-33.96,;9.9,-29.42,;9.9,-27.88,;11.23,-30.19,;12.56,-29.42,;12.57,-27.88,;13.9,-27.11,;15.23,-27.89,;13.9,-25.57,;13.9,-30.19,;13.9,-31.73,;15.23,-29.43,;16.56,-30.2,;16.56,-31.74,;15.23,-32.51,;17.9,-29.43,;17.9,-27.89,;19.23,-30.2,;20.57,-29.43,;20.57,-27.89,;21.9,-27.12,;23.23,-27.9,;24.57,-27.13,;24.57,-25.59,;23.23,-24.82,;21.9,-25.59,;21.9,-30.2,;21.9,-31.74,;23.23,-29.43,;24.57,-30.21,;24.56,-31.75,;25.9,-29.44,;25.9,-27.9,;27.23,-30.21,;28.57,-29.44,;28.57,-27.9,;29.9,-27.13,;29.91,-25.59,;31.24,-27.9,;29.9,-30.21,;29.9,-31.75,;31.24,-29.44,;32.57,-30.21,;32.57,-31.75,;33.9,-32.53,;33.9,-34.07,;35.23,-34.84,;35.23,-36.38,;36.56,-37.15,;33.9,-37.15,;33.9,-29.45,;33.91,-27.91,;35.24,-30.22,;36.57,-29.45,;36.57,-27.91,;37.91,-27.14,;39.24,-27.92,;40.57,-27.15,;40.57,-25.61,;39.23,-24.84,;37.9,-25.61,;37.9,-30.22,;39.24,-29.45,;37.9,-31.76,)| Show InChI InChI=1S/C79H109N21O18/c1-41(2)30-50(80)78(118)100-29-15-23-62(100)77(117)98-60(38-65(83)105)74(114)93-56(34-46-24-26-48(102)27-25-46)71(111)96-58(36-63(81)103)73(113)95-57(35-47-39-88-51-21-13-12-20-49(47)51)72(112)97-59(37-64(82)104)75(115)99-61(40-101)76(116)94-55(33-45-18-10-7-11-19-45)69(109)89-43(5)67(107)92-54(31-42(3)4)70(110)90-52(22-14-28-87-79(85)86)68(108)91-53(66(84)106)32-44-16-8-6-9-17-44/h6-13,16-21,24-27,39,41-43,50,52-62,88,101-102H,14-15,22-23,28-38,40,80H2,1-5H3,(H2,81,103)(H2,82,104)(H2,83,105)(H2,84,106)(H,89,109)(H,90,110)(H,91,108)(H,92,107)(H,93,114)(H,94,116)(H,95,113)(H,96,111)(H,97,112)(H,98,117)(H,99,115)(H4,85,86,87)/t43-,50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
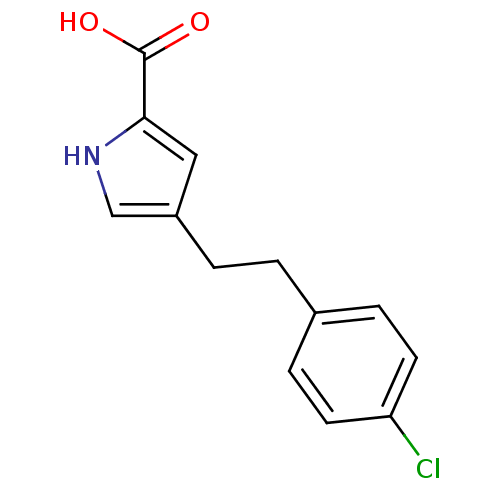
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203813
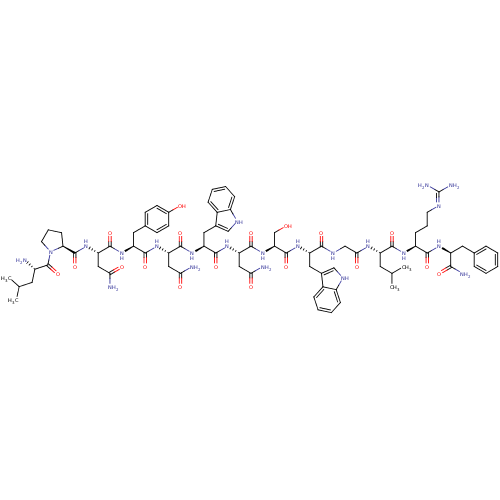
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,90.95,36.37,12.13,109.114,wD:66.69,24.24,98.103,16.16,44.45,(-6.38,1.31,;-6.36,-.23,;-7.68,-1.02,;-5.01,-.97,;-5.01,-2.53,;-6.34,-3.3,;-3.67,-3.3,;-3.67,-4.84,;-2.34,-2.53,;-1.86,-1.07,;-.32,-1.08,;.15,-2.54,;-1.1,-3.45,;-1.1,-4.99,;-2.44,-5.76,;.23,-5.76,;.23,-7.3,;-1.1,-8.07,;-1.11,-9.61,;.23,-10.38,;-2.44,-10.38,;1.56,-8.07,;1.56,-9.61,;2.9,-7.3,;4.23,-8.07,;4.23,-9.61,;5.56,-10.38,;5.55,-11.92,;6.89,-12.69,;8.22,-11.92,;9.56,-12.69,;8.22,-10.38,;6.9,-9.62,;5.57,-7.3,;5.57,-5.76,;6.9,-8.08,;8.23,-7.31,;8.23,-5.77,;9.57,-5,;10.9,-5.77,;9.57,-3.46,;9.57,-8.08,;9.56,-9.62,;10.9,-7.31,;12.23,-8.08,;12.23,-9.62,;13.56,-10.39,;14.81,-9.4,;16.05,-10.39,;15.57,-11.86,;16.33,-13.18,;15.57,-14.5,;14.03,-14.5,;13.27,-13.17,;14.04,-11.85,;13.57,-7.31,;13.57,-5.77,;14.9,-8.08,;16.23,-7.32,;16.24,-5.78,;17.57,-5.01,;18.9,-5.78,;17.57,-3.47,;17.57,-8.09,;17.57,-9.63,;18.9,-7.32,;20.23,-8.09,;20.23,-9.63,;21.57,-10.4,;21.57,-7.32,;21.57,-5.78,;22.9,-8.09,;24.24,-7.32,;24.24,-5.78,;25.57,-5.02,;27.05,-5.45,;27.92,-4.18,;26.98,-2.97,;27.27,-1.46,;26.1,-.46,;24.65,-.98,;24.37,-2.48,;25.53,-3.47,;25.57,-8.1,;25.57,-9.64,;26.9,-7.33,;28.24,-8.1,;29.57,-7.33,;29.57,-5.79,;30.9,-8.1,;32.24,-7.33,;32.24,-5.79,;33.58,-5.02,;33.58,-3.48,;34.91,-5.8,;33.57,-8.1,;33.57,-9.64,;34.91,-7.34,;36.24,-8.11,;36.24,-9.65,;37.57,-10.42,;37.57,-11.96,;38.9,-12.73,;38.9,-14.27,;40.23,-15.04,;37.57,-15.04,;37.57,-7.34,;37.58,-5.8,;38.91,-8.11,;40.24,-7.34,;40.24,-5.8,;41.58,-5.03,;42.91,-5.81,;44.24,-5.05,;44.24,-3.5,;42.9,-2.73,;41.57,-3.5,;41.57,-8.11,;42.91,-7.34,;41.57,-9.65,)| Show InChI InChI=1S/C80H108N22O18/c1-41(2)28-50(81)79(120)102-27-13-21-63(102)78(119)100-61(36-66(84)107)75(116)95-56(31-44-22-24-47(104)25-23-44)72(113)98-59(34-64(82)105)74(115)97-58(33-46-38-90-52-19-11-9-17-49(46)52)73(114)99-60(35-65(83)106)76(117)101-62(40-103)77(118)96-57(32-45-37-89-51-18-10-8-16-48(45)51)69(110)91-39-67(108)92-55(29-42(3)4)71(112)93-53(20-12-26-88-80(86)87)70(111)94-54(68(85)109)30-43-14-6-5-7-15-43/h5-11,14-19,22-25,37-38,41-42,50,53-63,89-90,103-104H,12-13,20-21,26-36,39-40,81H2,1-4H3,(H2,82,105)(H2,83,106)(H2,84,107)(H2,85,109)(H,91,110)(H,92,108)(H,93,112)(H,94,111)(H,95,116)(H,96,118)(H,97,115)(H,98,113)(H,99,114)(H,100,119)(H,101,117)(H4,86,87,88)/t50-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203816
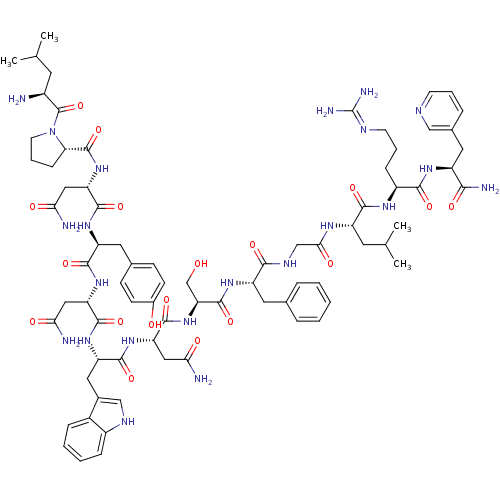
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1cccnc1)C(N)=O |wU:4.4,72.75,87.91,36.37,12.13,106.110,58.61,wD:66.69,24.24,95.99,16.16,44.45,(-20.28,-19.15,;-20.26,-20.69,;-21.58,-21.47,;-18.91,-21.43,;-18.9,-22.99,;-20.24,-23.76,;-17.57,-23.76,;-17.57,-25.3,;-16.25,-22.99,;-15.77,-21.53,;-14.22,-21.54,;-13.74,-22.99,;-14.99,-23.9,;-14.99,-25.44,;-16.34,-26.21,;-13.67,-26.22,;-13.67,-27.76,;-15,-28.52,;-15,-30.06,;-13.68,-30.84,;-16.33,-30.83,;-12.34,-28.53,;-12.34,-30.07,;-10.99,-27.76,;-9.66,-28.53,;-9.66,-30.07,;-8.33,-30.84,;-8.33,-32.38,;-7.01,-33.15,;-5.66,-32.38,;-4.33,-33.15,;-5.67,-30.83,;-6.99,-30.07,;-8.32,-27.76,;-8.32,-26.22,;-6.99,-28.53,;-5.66,-27.77,;-5.66,-26.23,;-4.32,-25.46,;-2.99,-26.23,;-4.32,-23.92,;-4.32,-28.54,;-4.33,-30.08,;-2.99,-27.77,;-1.65,-28.54,;-1.66,-30.08,;-.33,-30.85,;.93,-29.86,;2.16,-30.85,;1.68,-32.32,;2.44,-33.63,;1.68,-34.96,;.14,-34.96,;-.62,-33.63,;.15,-32.31,;-.32,-27.77,;-.32,-26.23,;1.01,-28.54,;2.35,-27.77,;2.35,-26.23,;3.68,-25.47,;5.02,-26.24,;3.68,-23.93,;3.67,-28.55,;3.67,-30.09,;5.01,-27.78,;6.34,-28.55,;6.34,-30.09,;7.68,-30.86,;7.68,-27.78,;7.68,-26.24,;9.01,-28.55,;10.34,-27.78,;10.35,-26.24,;11.68,-25.48,;13.01,-26.24,;14.35,-25.48,;14.35,-23.93,;13.01,-23.16,;11.68,-23.94,;11.68,-28.56,;11.68,-30.1,;13.01,-27.79,;14.35,-28.55,;15.68,-27.78,;15.68,-26.24,;17.01,-28.55,;18.35,-27.78,;18.35,-26.24,;19.68,-25.48,;19.68,-23.94,;21.02,-26.25,;19.68,-28.56,;19.68,-30.1,;21.02,-27.79,;22.35,-28.56,;22.35,-30.1,;23.68,-30.87,;23.68,-32.41,;25.01,-33.18,;25.01,-34.72,;26.34,-35.49,;23.68,-35.49,;23.69,-27.79,;23.69,-26.25,;25.02,-28.56,;26.35,-27.79,;26.35,-26.25,;27.69,-25.48,;27.68,-23.95,;29.01,-23.18,;30.35,-23.95,;30.35,-25.49,;29.01,-26.26,;27.69,-28.57,;29.02,-27.8,;27.68,-30.11,)| Show InChI InChI=1S/C77H106N22O18/c1-40(2)27-48(78)76(117)99-26-12-19-60(99)75(116)97-58(35-63(81)104)72(113)93-54(30-43-20-22-46(101)23-21-43)69(110)95-56(33-61(79)102)71(112)94-55(32-45-37-87-49-17-9-8-16-47(45)49)70(111)96-57(34-62(80)103)73(114)98-59(39-100)74(115)92-53(29-42-13-6-5-7-14-42)66(107)88-38-64(105)89-52(28-41(3)4)68(109)90-50(18-11-25-86-77(83)84)67(108)91-51(65(82)106)31-44-15-10-24-85-36-44/h5-10,13-17,20-24,36-37,40-41,48,50-60,87,100-101H,11-12,18-19,25-35,38-39,78H2,1-4H3,(H2,79,102)(H2,80,103)(H2,81,104)(H2,82,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,83,84,86)/t48-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203791
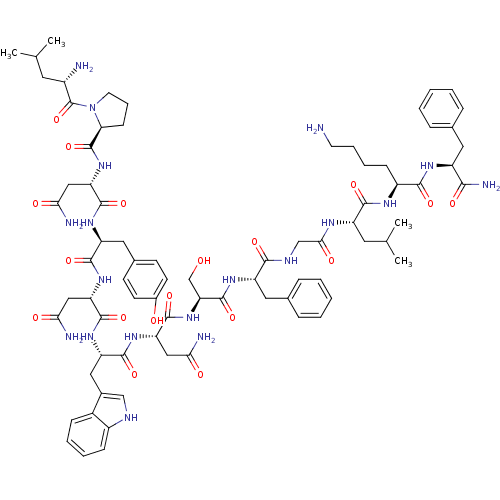
((2S)-N-[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C78H107N19O18/c1-42(2)30-50(80)78(115)97-29-15-23-62(97)77(114)95-60(38-65(83)102)74(111)91-56(34-46-24-26-48(99)27-25-46)71(108)93-58(36-63(81)100)73(110)92-57(35-47-39-85-51-21-12-11-20-49(47)51)72(109)94-59(37-64(82)101)75(112)96-61(41-98)76(113)90-55(33-45-18-9-6-10-19-45)68(105)86-40-66(103)87-54(31-43(3)4)70(107)88-52(22-13-14-28-79)69(106)89-53(67(84)104)32-44-16-7-5-8-17-44/h5-12,16-21,24-27,39,42-43,50,52-62,85,98-99H,13-15,22-23,28-38,40-41,79-80H2,1-4H3,(H2,81,100)(H2,82,101)(H2,83,102)(H2,84,104)(H,86,105)(H,87,103)(H,88,107)(H,89,106)(H,90,113)(H,91,111)(H,92,110)(H,93,108)(H,94,109)(H,95,114)(H,96,112)/t50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185796
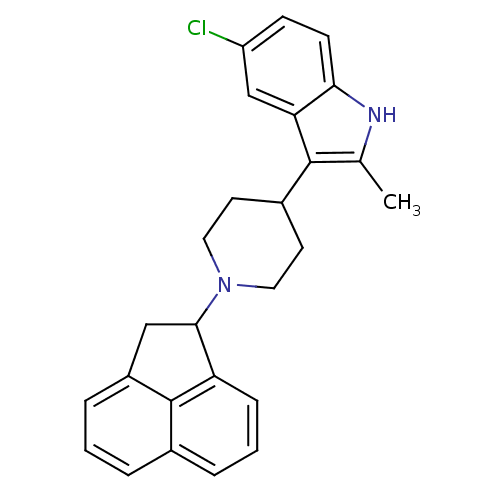
(5-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...)Show SMILES Cc1[nH]c2ccc(Cl)cc2c1C1CCN(CC1)C1Cc2cccc3cccc1c23 Show InChI InChI=1S/C26H25ClN2/c1-16-25(22-15-20(27)8-9-23(22)28-16)18-10-12-29(13-11-18)24-14-19-6-2-4-17-5-3-7-21(24)26(17)19/h2-9,15,18,24,28H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203800
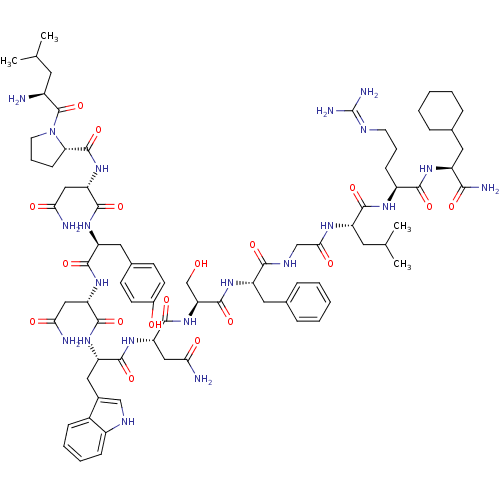
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1CCCCC1)C(N)=O |wU:4.4,72.75,87.91,36.37,12.13,106.110,58.61,wD:66.69,24.24,95.99,16.16,44.45,(-20.28,-19.15,;-20.26,-20.69,;-21.58,-21.47,;-18.91,-21.43,;-18.9,-22.99,;-20.24,-23.76,;-17.57,-23.76,;-17.57,-25.3,;-16.25,-22.99,;-15.77,-21.53,;-14.22,-21.54,;-13.74,-22.99,;-14.99,-23.9,;-14.99,-25.44,;-16.34,-26.21,;-13.67,-26.22,;-13.67,-27.76,;-15,-28.52,;-15,-30.06,;-13.68,-30.84,;-16.33,-30.83,;-12.34,-28.53,;-12.34,-30.07,;-10.99,-27.76,;-9.66,-28.53,;-9.66,-30.07,;-8.33,-30.84,;-8.33,-32.38,;-7.01,-33.15,;-5.66,-32.38,;-4.33,-33.15,;-5.67,-30.83,;-6.99,-30.07,;-8.32,-27.76,;-8.32,-26.22,;-6.99,-28.53,;-5.66,-27.77,;-5.66,-26.23,;-4.32,-25.46,;-2.99,-26.23,;-4.32,-23.92,;-4.32,-28.54,;-4.33,-30.08,;-2.99,-27.77,;-1.65,-28.54,;-1.66,-30.08,;-.33,-30.85,;.93,-29.86,;2.16,-30.85,;1.68,-32.32,;2.44,-33.63,;1.68,-34.96,;.14,-34.96,;-.62,-33.63,;.15,-32.31,;-.32,-27.77,;-.32,-26.23,;1.01,-28.54,;2.35,-27.77,;2.35,-26.23,;3.68,-25.47,;5.02,-26.24,;3.68,-23.93,;3.67,-28.55,;3.67,-30.09,;5.01,-27.78,;6.34,-28.55,;6.34,-30.09,;7.68,-30.86,;7.68,-27.78,;7.68,-26.24,;9.01,-28.55,;10.34,-27.78,;10.35,-26.24,;11.68,-25.48,;13.01,-26.24,;14.35,-25.48,;14.35,-23.93,;13.01,-23.16,;11.68,-23.94,;11.68,-28.56,;11.68,-30.1,;13.01,-27.79,;14.35,-28.55,;15.68,-27.78,;15.68,-26.24,;17.01,-28.55,;18.35,-27.78,;18.35,-26.24,;19.68,-25.48,;19.68,-23.94,;21.02,-26.25,;19.68,-28.56,;19.68,-30.1,;21.02,-27.79,;22.35,-28.56,;22.35,-30.1,;23.68,-30.87,;23.68,-32.41,;25.01,-33.18,;25.01,-34.72,;26.34,-35.49,;23.68,-35.49,;23.69,-27.79,;23.69,-26.25,;25.02,-28.56,;26.35,-27.79,;26.35,-26.25,;27.68,-25.49,;29.01,-26.26,;30.34,-25.49,;30.35,-23.95,;29.01,-23.18,;27.68,-23.95,;27.69,-28.57,;29.02,-27.8,;27.68,-30.11,)| Show InChI InChI=1S/C78H113N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h6,9-12,17-20,23-26,38,41-43,49,51-61,87,100-101H,5,7-8,13-16,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
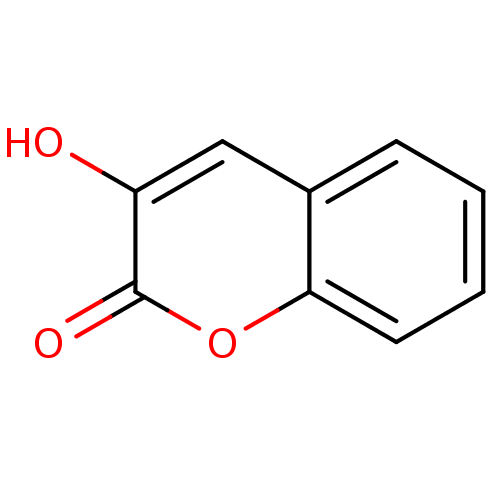
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203779
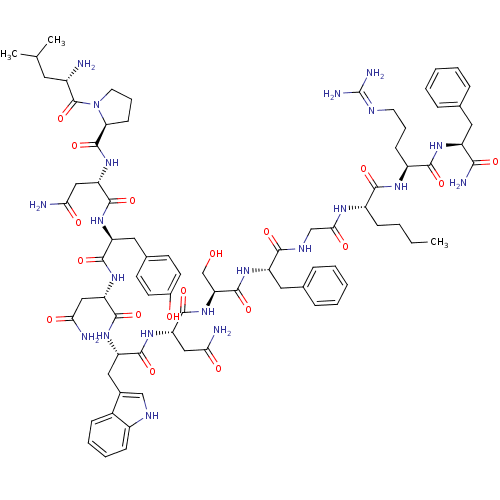
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CCCC[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:29.34,86.91,12.20,4.4,51.58,79.82,106.110,wD:23.26,59.71,95.99,71.79,37.50,(27.31,-23.46,;25.97,-24.23,;25.97,-25.77,;24.64,-26.54,;24.64,-28.08,;23.3,-28.84,;21.97,-28.07,;21.97,-26.53,;20.64,-28.84,;19.3,-28.08,;17.97,-28.85,;17.97,-30.39,;16.63,-28.07,;16.63,-26.53,;17.97,-25.77,;19.3,-26.53,;20.64,-25.77,;20.64,-24.23,;19.29,-23.45,;17.97,-24.23,;15.3,-28.84,;13.97,-28.07,;13.97,-26.53,;12.63,-28.84,;12.63,-30.38,;13.97,-31.15,;11.3,-28.07,;9.96,-28.84,;9.96,-30.38,;8.63,-28.07,;8.64,-26.53,;9.97,-25.76,;11.3,-26.53,;9.97,-24.22,;7.3,-28.83,;5.96,-28.06,;5.97,-26.52,;4.63,-28.83,;4.63,-30.37,;5.95,-31.14,;7.22,-30.15,;8.45,-31.14,;7.97,-32.61,;8.73,-33.92,;7.97,-35.25,;6.43,-35.25,;5.67,-33.92,;6.44,-32.6,;3.3,-28.06,;1.96,-28.83,;1.96,-30.37,;.63,-28.06,;.63,-26.52,;1.97,-25.75,;3.3,-26.52,;1.97,-24.21,;-.7,-28.82,;-2.03,-28.05,;-2.03,-26.51,;-3.37,-28.82,;-3.38,-30.36,;-2.04,-31.13,;-2.05,-32.67,;-.72,-33.44,;.62,-32.67,;1.95,-33.44,;.62,-31.12,;-.71,-30.36,;-4.7,-28.05,;-6.04,-28.82,;-6.04,-30.36,;-7.37,-28.05,;-8.7,-28.82,;-8.7,-30.36,;-7.38,-31.13,;-10.04,-31.12,;-7.37,-26.51,;-8.7,-25.74,;-10.04,-26.5,;-8.7,-24.2,;-7.45,-23.28,;-7.93,-21.83,;-9.46,-21.83,;-9.94,-23.29,;-11.27,-24.05,;-11.27,-25.59,;-12.61,-23.28,;-13.95,-24.05,;-12.62,-21.72,;-13.97,-20.98,;-13.99,-19.44,;-15.3,-21.76,;25.97,-28.85,;25.97,-30.39,;27.31,-28.08,;28.64,-28.85,;28.63,-30.39,;29.97,-31.16,;29.97,-32.7,;31.3,-33.47,;31.3,-35.01,;32.63,-35.79,;29.97,-35.78,;29.98,-28.08,;29.98,-26.54,;31.31,-28.85,;32.64,-28.09,;32.64,-26.55,;33.98,-25.78,;35.3,-26.56,;36.64,-25.79,;36.64,-24.25,;35.3,-23.48,;33.97,-24.25,;33.97,-28.86,;35.31,-28.09,;33.97,-30.4,)| Show InChI InChI=1S/C78H107N21O18/c1-4-5-21-51(68(108)90-52(23-14-29-86-78(84)85)69(109)91-53(66(83)106)32-43-16-8-6-9-17-43)89-65(105)40-88-67(107)54(33-44-18-10-7-11-19-44)92-75(115)60(41-100)98-74(114)58(37-63(81)103)96-71(111)56(35-46-39-87-50-22-13-12-20-48(46)50)94-72(112)57(36-62(80)102)95-70(110)55(34-45-25-27-47(101)28-26-45)93-73(113)59(38-64(82)104)97-76(116)61-24-15-30-99(61)77(117)49(79)31-42(2)3/h6-13,16-20,22,25-28,39,42,49,51-61,87,100-101H,4-5,14-15,21,23-24,29-38,40-41,79H2,1-3H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,108)(H,91,109)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203789
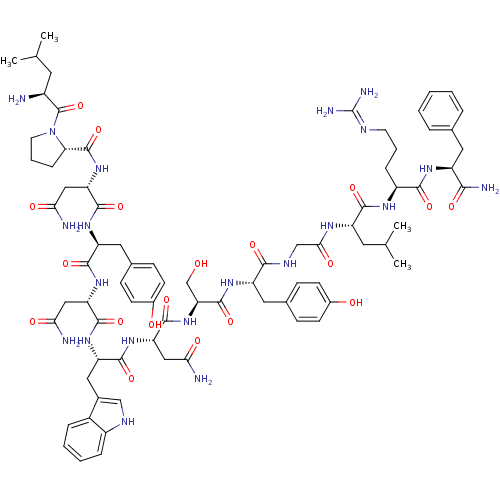
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,88.92,36.37,12.13,107.111,wD:66.69,24.24,96.100,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;27.56,-26.36,;28.9,-25.59,;28.91,-24.05,;30.24,-23.27,;27.56,-23.27,;26.23,-24.05,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C78H107N21O19/c1-40(2)28-49(79)77(118)99-27-11-17-61(99)76(117)97-59(36-64(82)105)73(114)93-55(32-44-20-24-47(102)25-21-44)70(111)95-57(34-62(80)103)72(113)94-56(33-45-37-87-50-15-9-8-14-48(45)50)71(112)96-58(35-63(81)104)74(115)98-60(39-100)75(116)92-54(31-43-18-22-46(101)23-19-43)67(108)88-38-65(106)89-53(29-41(3)4)69(110)90-51(16-10-26-86-78(84)85)68(109)91-52(66(83)107)30-42-12-6-5-7-13-42/h5-9,12-15,18-25,37,40-41,49,51-61,87,100-102H,10-11,16-17,26-36,38-39,79H2,1-4H3,(H2,80,103)(H2,81,104)(H2,82,105)(H2,83,107)(H,88,108)(H,89,106)(H,90,110)(H,91,109)(H,92,116)(H,93,114)(H,94,113)(H,95,111)(H,96,112)(H,97,117)(H,98,115)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50327695
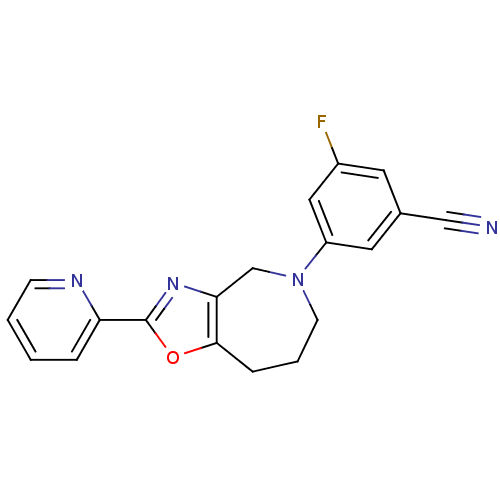
(3-Fluoro-5-(2-(pyridin-2-yl)-7,8-dihydro-4H-oxazol...)Show InChI InChI=1S/C19H15FN4O/c20-14-8-13(11-21)9-15(10-14)24-7-3-5-18-17(12-24)23-19(25-18)16-4-1-2-6-22-16/h1-2,4,6,8-10H,3,5,7,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from mGluR5 in rat cortical membranes |
J Med Chem 53: 7107-18 (2010)
Article DOI: 10.1021/jm100736h
BindingDB Entry DOI: 10.7270/Q20G3KCJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50327688
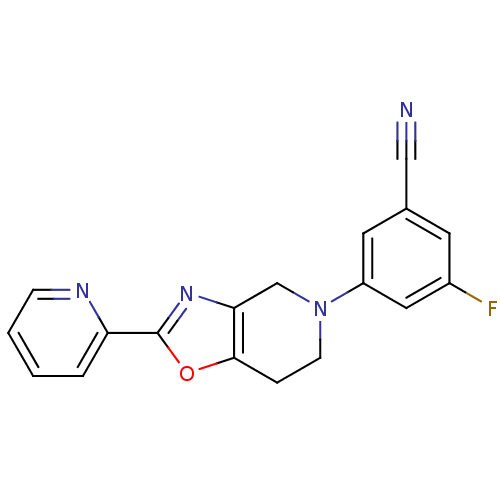
(3-Fluoro-5-(2-(pyridin-2-yl)-6,7-dihydrooxazolo[4,...)Show InChI InChI=1S/C18H13FN4O/c19-13-7-12(10-20)8-14(9-13)23-6-4-17-16(11-23)22-18(24-17)15-3-1-2-5-21-15/h1-3,5,7-9H,4,6,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from mGluR5 in rat cortical membranes |
J Med Chem 53: 7107-18 (2010)
Article DOI: 10.1021/jm100736h
BindingDB Entry DOI: 10.7270/Q20G3KCJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185805
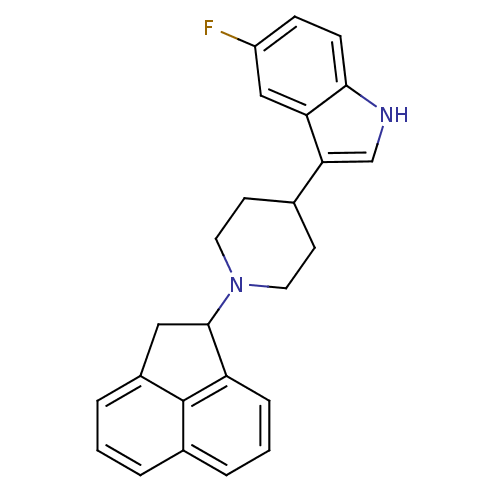
(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...)Show SMILES Fc1ccc2[nH]cc(C3CCN(CC3)C3Cc4cccc5cccc3c45)c2c1 Show InChI InChI=1S/C25H23FN2/c26-19-7-8-23-21(14-19)22(15-27-23)16-9-11-28(12-10-16)24-13-18-5-1-3-17-4-2-6-20(24)25(17)18/h1-8,14-16,24,27H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-nociceptin from ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203810
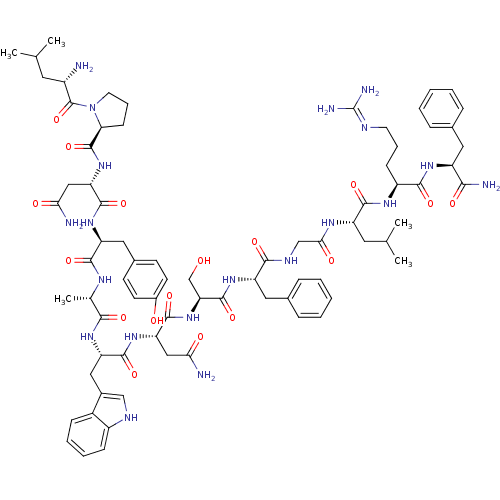
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:55.58,4.4,69.72,84.88,12.13,36.38,103.107,wD:63.66,24.24,92.96,41.42,16.16,(-6.31,-18.18,;-6.29,-19.72,;-7.61,-20.51,;-4.94,-20.46,;-4.94,-22.02,;-6.27,-22.79,;-3.6,-22.79,;-3.6,-24.33,;-2.27,-22.02,;-1.79,-20.56,;-.25,-20.57,;.22,-22.03,;-1.03,-22.94,;-1.03,-24.48,;-2.37,-25.25,;.3,-25.25,;.3,-26.79,;-1.04,-27.56,;-1.04,-29.1,;.3,-29.87,;-2.37,-29.87,;1.63,-27.56,;1.63,-29.1,;2.97,-26.79,;4.3,-27.56,;4.3,-29.1,;5.63,-29.87,;5.62,-31.41,;6.95,-32.18,;8.29,-31.41,;9.62,-32.18,;8.29,-29.87,;6.96,-29.11,;5.63,-26.79,;5.64,-25.25,;6.97,-27.57,;8.3,-26.8,;8.3,-25.26,;9.63,-27.57,;9.63,-29.11,;10.97,-26.8,;12.3,-27.57,;12.3,-29.11,;13.63,-29.88,;14.88,-28.89,;16.12,-29.88,;15.64,-31.35,;16.4,-32.67,;15.64,-33.99,;14.1,-33.99,;13.34,-32.66,;14.11,-31.34,;13.64,-26.8,;13.64,-25.26,;14.97,-27.57,;16.3,-26.81,;16.3,-25.27,;17.64,-24.5,;18.97,-25.27,;17.64,-22.96,;17.64,-27.58,;17.63,-29.12,;18.97,-26.81,;20.3,-27.58,;20.3,-29.12,;21.63,-29.89,;21.64,-26.81,;21.64,-25.27,;22.97,-27.58,;24.31,-26.81,;24.31,-25.27,;25.64,-24.51,;26.97,-25.28,;28.31,-24.51,;28.31,-22.97,;26.97,-22.2,;25.64,-22.97,;25.64,-27.59,;25.64,-29.13,;26.97,-26.82,;28.31,-27.59,;29.64,-26.82,;29.64,-25.28,;30.97,-27.59,;32.31,-26.82,;32.31,-25.28,;33.64,-24.51,;33.65,-22.97,;34.98,-25.28,;33.64,-27.59,;33.64,-29.13,;34.97,-26.82,;36.31,-27.6,;36.31,-29.14,;37.64,-29.91,;37.64,-31.45,;38.97,-32.22,;38.97,-33.76,;40.3,-34.53,;37.63,-34.53,;37.64,-26.83,;37.64,-25.29,;38.98,-27.6,;40.31,-26.83,;40.31,-25.29,;41.65,-24.52,;42.97,-25.3,;44.31,-24.54,;44.31,-22.99,;42.97,-22.22,;41.64,-22.99,;41.64,-27.6,;42.98,-26.83,;41.64,-29.14,)| Show InChI InChI=1S/C77H106N20O17/c1-41(2)30-50(78)76(114)97-29-15-23-61(97)75(113)95-59(37-63(80)101)72(110)93-56(34-46-24-26-48(99)27-25-46)69(107)87-43(5)66(104)91-57(35-47-38-85-51-21-13-12-20-49(47)51)71(109)94-58(36-62(79)100)73(111)96-60(40-98)74(112)92-55(33-45-18-10-7-11-19-45)67(105)86-39-64(102)88-54(31-42(3)4)70(108)89-52(22-14-28-84-77(82)83)68(106)90-53(65(81)103)32-44-16-8-6-9-17-44/h6-13,16-21,24-27,38,41-43,50,52-61,85,98-99H,14-15,22-23,28-37,39-40,78H2,1-5H3,(H2,79,100)(H2,80,101)(H2,81,103)(H,86,105)(H,87,107)(H,88,102)(H,89,108)(H,90,106)(H,91,104)(H,92,112)(H,93,110)(H,94,109)(H,95,113)(H,96,111)(H4,82,83,84)/t43-,50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203780
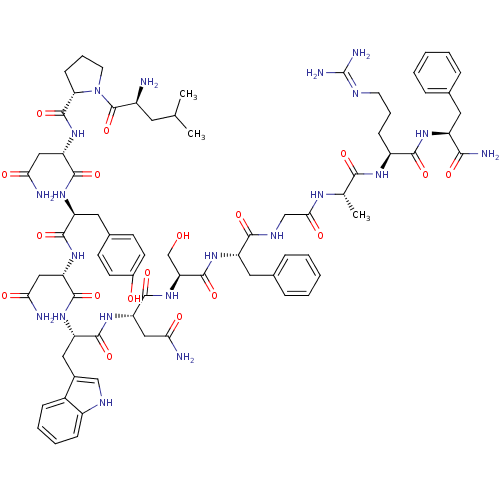
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.92,36.37,12.13,103.107,wD:66.69,24.24,92.96,16.16,44.45,(-9.16,-19.13,;-9.14,-20.67,;-10.46,-21.46,;-7.79,-21.41,;-7.79,-22.97,;-9.12,-23.74,;-6.45,-23.74,;-6.45,-25.28,;-5.12,-22.97,;-4.64,-21.51,;-3.1,-21.52,;-2.63,-22.98,;-3.88,-23.89,;-3.88,-25.43,;-5.22,-26.19,;-2.55,-26.2,;-2.55,-27.74,;-3.88,-28.51,;-3.89,-30.05,;-2.55,-30.82,;-5.22,-30.82,;-1.22,-28.51,;-1.22,-30.05,;.12,-27.74,;1.45,-28.51,;1.45,-30.05,;2.78,-30.82,;2.77,-32.36,;4.11,-33.13,;5.44,-32.36,;6.78,-33.13,;5.44,-30.81,;4.12,-30.06,;2.78,-27.74,;2.79,-26.2,;4.12,-28.51,;5.45,-27.75,;5.45,-26.21,;6.79,-25.44,;8.12,-26.21,;6.79,-23.9,;6.78,-28.52,;6.78,-30.06,;8.12,-27.75,;9.45,-28.52,;9.45,-30.06,;10.78,-30.83,;12.03,-29.84,;13.27,-30.83,;12.79,-32.3,;13.55,-33.62,;12.79,-34.94,;11.25,-34.94,;10.49,-33.61,;11.26,-32.29,;10.79,-27.75,;10.79,-26.21,;12.12,-28.52,;13.45,-27.75,;13.46,-26.21,;14.79,-25.45,;16.12,-26.22,;14.79,-23.91,;14.79,-28.53,;14.79,-30.07,;16.12,-27.76,;17.45,-28.53,;17.45,-30.07,;16.12,-30.84,;18.79,-27.76,;18.79,-26.22,;20.12,-28.53,;21.46,-27.76,;21.46,-26.22,;22.79,-25.45,;24.12,-26.23,;25.46,-25.46,;25.46,-23.92,;24.12,-23.15,;22.79,-23.92,;22.79,-28.53,;22.79,-30.07,;24.12,-27.77,;25.46,-28.54,;26.79,-27.77,;26.79,-26.23,;28.12,-28.54,;29.46,-27.77,;29.46,-26.23,;30.79,-28.54,;30.79,-30.08,;32.13,-27.77,;33.46,-28.55,;33.46,-30.09,;34.79,-30.86,;34.79,-32.4,;36.12,-33.17,;36.12,-34.71,;37.45,-35.48,;34.79,-35.48,;34.79,-27.78,;34.79,-26.24,;36.13,-28.55,;37.46,-27.78,;37.46,-26.24,;38.8,-25.47,;40.13,-26.25,;41.46,-25.49,;41.46,-23.94,;40.12,-23.17,;38.79,-23.94,;38.79,-28.55,;40.13,-27.78,;38.79,-30.09,)| Show InChI InChI=1S/C75H101N21O18/c1-39(2)28-47(76)74(114)96-27-13-21-58(96)73(113)94-56(35-61(79)101)70(110)90-52(31-43-22-24-45(98)25-23-43)67(107)92-54(33-59(77)99)69(109)91-53(32-44-36-84-48-19-11-10-18-46(44)48)68(108)93-55(34-60(78)100)71(111)95-57(38-97)72(112)89-51(30-42-16-8-5-9-17-42)65(105)85-37-62(102)86-40(3)64(104)87-49(20-12-26-83-75(81)82)66(106)88-50(63(80)103)29-41-14-6-4-7-15-41/h4-11,14-19,22-25,36,39-40,47,49-58,84,97-98H,12-13,20-21,26-35,37-38,76H2,1-3H3,(H2,77,99)(H2,78,100)(H2,79,101)(H2,80,103)(H,85,105)(H,86,102)(H,87,104)(H,88,106)(H,89,112)(H,90,110)(H,91,109)(H,92,107)(H,93,108)(H,94,113)(H,95,111)(H4,81,82,83)/t40-,47-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203787

((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:4.4,58.62,69.72,84.88,36.37,12.13,103.107,wD:63.66,24.24,92.96,16.16,44.45,(-9.93,-20.42,;-9.9,-21.96,;-11.22,-22.75,;-8.55,-22.7,;-8.55,-24.26,;-9.88,-25.03,;-7.21,-25.03,;-7.21,-26.57,;-5.88,-24.27,;-5.4,-22.81,;-3.86,-22.81,;-3.39,-24.27,;-4.64,-25.18,;-4.64,-26.72,;-5.98,-27.49,;-3.31,-27.49,;-3.31,-29.03,;-4.65,-29.8,;-4.65,-31.34,;-3.32,-32.11,;-5.98,-32.11,;-1.98,-29.8,;-1.98,-31.34,;-.64,-29.03,;.69,-29.8,;.69,-31.34,;2.02,-32.12,;2.01,-33.65,;3.34,-34.42,;4.68,-33.65,;6.01,-34.42,;4.68,-32.11,;3.35,-31.35,;2.02,-29.04,;2.02,-27.5,;3.36,-29.81,;4.69,-29.04,;4.69,-27.5,;6.03,-26.73,;7.36,-27.5,;6.03,-25.19,;6.02,-29.81,;6.02,-31.35,;7.36,-29.04,;8.69,-29.81,;8.69,-31.35,;10.02,-32.12,;11.27,-31.13,;12.51,-32.12,;12.03,-33.59,;12.79,-34.91,;12.03,-36.23,;10.49,-36.23,;9.73,-34.9,;10.5,-33.58,;10.03,-29.04,;10.03,-27.5,;11.36,-29.82,;12.69,-29.05,;12.69,-27.51,;14.03,-29.82,;14.02,-31.36,;15.36,-29.05,;16.69,-29.82,;16.69,-31.36,;18.02,-32.13,;18.03,-29.05,;18.03,-27.51,;19.36,-29.82,;20.69,-29.06,;20.7,-27.52,;22.03,-26.75,;23.36,-27.52,;24.7,-26.76,;24.7,-25.21,;23.36,-24.44,;22.03,-25.21,;22.03,-29.83,;22.03,-31.37,;23.36,-29.06,;24.7,-29.83,;26.03,-29.06,;26.03,-27.52,;27.36,-29.83,;28.7,-29.06,;28.7,-27.52,;30.03,-26.76,;30.03,-25.22,;31.37,-27.53,;30.03,-29.84,;30.03,-31.38,;31.36,-29.07,;32.7,-29.84,;32.7,-31.38,;34.03,-32.15,;34.03,-33.69,;35.36,-34.46,;35.36,-36,;36.69,-36.77,;34.02,-36.77,;34.03,-29.07,;34.03,-27.53,;35.36,-29.84,;36.7,-29.07,;36.7,-27.53,;38.04,-26.76,;39.36,-27.54,;40.7,-26.78,;40.7,-25.24,;39.36,-24.46,;38.03,-25.24,;38.03,-29.84,;39.37,-29.08,;38.03,-31.38,)| Show InChI InChI=1S/C77H106N20O17/c1-41(2)30-50(78)76(114)97-29-15-23-61(97)75(113)95-59(37-63(80)101)73(111)92-56(34-46-24-26-48(99)27-25-46)71(109)94-58(36-62(79)100)72(110)93-57(35-47-38-85-51-21-13-12-20-49(47)51)69(107)87-43(5)66(104)96-60(40-98)74(112)91-55(33-45-18-10-7-11-19-45)67(105)86-39-64(102)88-54(31-42(3)4)70(108)89-52(22-14-28-84-77(82)83)68(106)90-53(65(81)103)32-44-16-8-6-9-17-44/h6-13,16-21,24-27,38,41-43,50,52-61,85,98-99H,14-15,22-23,28-37,39-40,78H2,1-5H3,(H2,79,100)(H2,80,101)(H2,81,103)(H,86,105)(H,87,107)(H,88,102)(H,89,108)(H,90,106)(H,91,112)(H,92,111)(H,93,110)(H,94,109)(H,95,113)(H,96,104)(H4,82,83,84)/t43-,50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203799
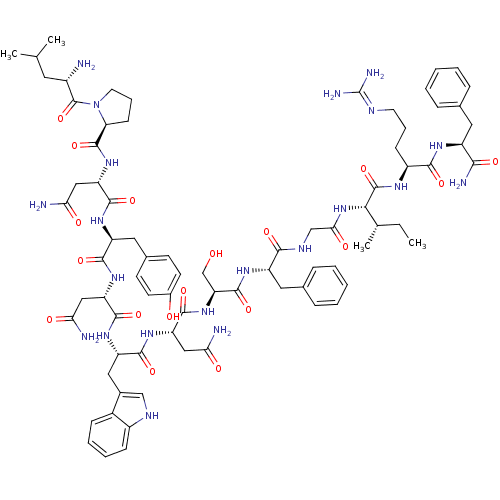
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:29.34,86.91,12.20,4.4,51.58,79.82,106.110,2.2,wD:23.26,59.71,95.99,71.79,37.50,(27.31,-26.54,;25.97,-25.77,;24.64,-26.54,;23.31,-25.76,;24.64,-28.08,;23.3,-28.84,;21.97,-28.07,;21.97,-26.53,;20.64,-28.84,;19.3,-28.08,;17.97,-28.85,;17.97,-30.39,;16.63,-28.07,;16.63,-26.53,;17.97,-25.77,;19.3,-26.53,;20.64,-25.77,;20.64,-24.23,;19.29,-23.45,;17.97,-24.23,;15.3,-28.84,;13.97,-28.07,;13.97,-26.53,;12.63,-28.84,;12.63,-30.38,;13.97,-31.15,;11.3,-28.07,;9.96,-28.84,;9.96,-30.38,;8.63,-28.07,;8.64,-26.53,;9.97,-25.76,;11.3,-26.53,;9.97,-24.22,;7.3,-28.83,;5.96,-28.06,;5.97,-26.52,;4.63,-28.83,;4.63,-30.37,;5.95,-31.14,;7.22,-30.15,;8.45,-31.14,;7.97,-32.61,;8.73,-33.92,;7.97,-35.25,;6.43,-35.25,;5.67,-33.92,;6.44,-32.6,;3.3,-28.06,;1.96,-28.83,;1.96,-30.37,;.63,-28.06,;.63,-26.52,;1.97,-25.75,;3.3,-26.52,;1.97,-24.21,;-.7,-28.82,;-2.03,-28.05,;-2.03,-26.51,;-3.37,-28.82,;-3.38,-30.36,;-2.04,-31.13,;-2.05,-32.67,;-.72,-33.44,;.62,-32.67,;1.95,-33.44,;.62,-31.12,;-.71,-30.36,;-4.7,-28.05,;-6.04,-28.82,;-6.04,-30.36,;-7.37,-28.05,;-8.7,-28.82,;-8.7,-30.36,;-7.38,-31.13,;-10.04,-31.12,;-7.37,-26.51,;-8.7,-25.74,;-10.04,-26.5,;-8.7,-24.2,;-7.45,-23.28,;-7.93,-21.83,;-9.46,-21.83,;-9.94,-23.29,;-11.27,-24.05,;-11.27,-25.59,;-12.61,-23.28,;-13.95,-24.05,;-12.62,-21.72,;-13.97,-20.98,;-13.99,-19.44,;-15.3,-21.76,;25.97,-28.85,;25.97,-30.39,;27.31,-28.08,;28.64,-28.85,;28.63,-30.39,;29.97,-31.16,;29.97,-32.7,;31.3,-33.47,;31.3,-35.01,;32.63,-35.79,;29.97,-35.78,;29.98,-28.08,;29.98,-26.54,;31.31,-28.85,;32.64,-28.09,;32.64,-26.55,;33.98,-25.78,;35.3,-26.56,;36.64,-25.79,;36.64,-24.25,;35.3,-23.48,;33.97,-24.25,;33.97,-28.86,;35.31,-28.09,;33.97,-30.4,)| Show InChI InChI=1S/C78H107N21O18/c1-5-42(4)65(76(116)89-51(22-14-28-86-78(84)85)68(108)90-52(66(83)106)31-43-16-8-6-9-17-43)98-64(105)39-88-67(107)53(32-44-18-10-7-11-19-44)91-74(114)59(40-100)97-73(113)57(36-62(81)103)95-70(110)55(34-46-38-87-50-21-13-12-20-48(46)50)93-71(111)56(35-61(80)102)94-69(109)54(33-45-24-26-47(101)27-25-45)92-72(112)58(37-63(82)104)96-75(115)60-23-15-29-99(60)77(117)49(79)30-41(2)3/h6-13,16-21,24-27,38,41-42,49,51-60,65,87,100-101H,5,14-15,22-23,28-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,116)(H,90,108)(H,91,114)(H,92,112)(H,93,111)(H,94,109)(H,95,110)(H,96,115)(H,97,113)(H,98,105)(H4,84,85,86)/t42-,49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185813

(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1CCN(CC1)C1Cc2cccc3cccc1c23 Show InChI InChI=1S/C25H23FN2/c26-19-7-8-20-22(15-27-23(20)14-19)16-9-11-28(12-10-16)24-13-18-5-1-3-17-4-2-6-21(24)25(17)18/h1-8,14-16,24,27H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185811
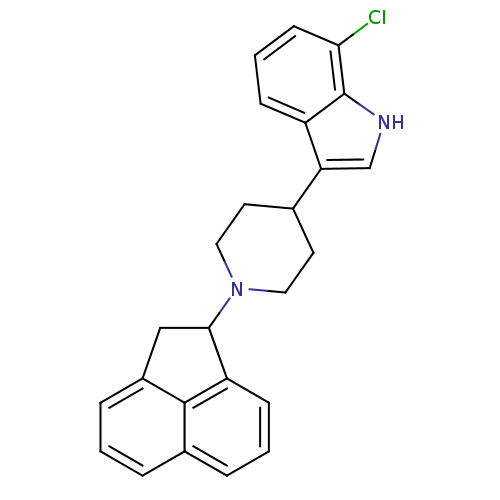
(7-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...)Show SMILES Clc1cccc2c(c[nH]c12)C1CCN(CC1)C1Cc2cccc3cccc1c23 Show InChI InChI=1S/C25H23ClN2/c26-22-9-3-7-19-21(15-27-25(19)22)16-10-12-28(13-11-16)23-14-18-6-1-4-17-5-2-8-20(23)24(17)18/h1-9,15-16,23,27H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-nociceptin from ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185788
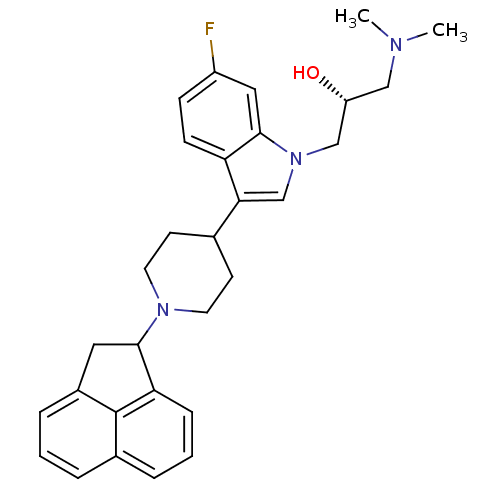
((2R)-1-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperi...)Show SMILES CN(C)C[C@@H](O)Cn1cc(C2CCN(CC2)C2Cc3cccc4cccc2c34)c2ccc(F)cc12 Show InChI InChI=1S/C30H34FN3O/c1-32(2)17-24(35)18-34-19-27(25-10-9-23(31)16-29(25)34)20-11-13-33(14-12-20)28-15-22-7-3-5-21-6-4-8-26(28)30(21)22/h3-10,16,19-20,24,28,35H,11-15,17-18H2,1-2H3/t24-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203797
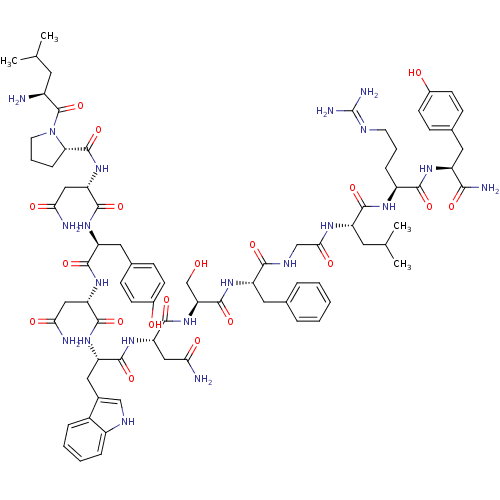
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:72.75,87.91,36.37,12.13,106.110,58.61,4.4,wD:66.69,24.24,95.99,16.16,44.45,(-4.64,-18.24,;-4.61,-19.78,;-5.93,-20.57,;-3.26,-20.52,;-3.26,-22.09,;-4.59,-22.86,;-1.92,-22.86,;-1.92,-24.4,;-.59,-22.09,;-.11,-20.63,;1.42,-20.63,;1.9,-22.09,;.65,-23,;.65,-24.54,;-.69,-25.31,;1.98,-25.31,;1.98,-26.85,;.65,-27.62,;.65,-29.16,;1.98,-29.93,;-.69,-29.93,;3.31,-27.62,;3.31,-29.16,;4.65,-26.85,;5.98,-27.62,;5.98,-29.16,;7.31,-29.94,;7.31,-31.47,;8.63,-32.24,;9.98,-31.47,;11.31,-32.24,;9.97,-29.93,;8.65,-29.17,;7.32,-26.86,;7.32,-25.32,;8.65,-27.63,;9.98,-26.86,;9.98,-25.32,;11.32,-24.55,;12.65,-25.32,;11.32,-23.01,;11.32,-27.63,;11.31,-29.17,;12.65,-26.86,;13.98,-27.63,;13.98,-29.17,;15.31,-29.94,;16.57,-28.95,;17.8,-29.94,;17.32,-31.41,;18.08,-32.72,;17.32,-34.05,;15.78,-34.05,;15.02,-32.72,;15.79,-31.4,;15.32,-26.86,;15.32,-25.32,;16.65,-27.64,;17.98,-26.87,;17.99,-25.33,;19.32,-24.56,;20.66,-25.33,;19.32,-23.02,;19.31,-27.64,;19.31,-29.18,;20.65,-26.87,;21.98,-27.64,;21.98,-29.18,;23.32,-29.95,;23.32,-26.87,;23.32,-25.33,;24.65,-27.65,;25.98,-26.88,;25.98,-25.34,;27.32,-24.57,;28.65,-25.34,;29.99,-24.57,;29.99,-23.03,;28.64,-22.26,;27.32,-23.03,;27.32,-27.65,;27.32,-29.19,;28.65,-26.88,;29.99,-27.64,;31.32,-26.87,;31.32,-25.33,;32.65,-27.65,;33.99,-26.88,;33.99,-25.34,;35.32,-24.57,;35.32,-23.03,;36.66,-25.34,;35.32,-27.65,;35.32,-29.19,;36.66,-26.88,;37.99,-27.65,;37.99,-29.19,;39.32,-29.96,;39.32,-31.5,;40.65,-32.28,;40.65,-33.82,;41.98,-34.59,;39.32,-34.58,;39.33,-26.88,;39.33,-25.34,;40.66,-27.66,;41.99,-26.89,;41.99,-25.35,;43.32,-24.58,;44.65,-25.35,;45.98,-24.58,;45.99,-23.04,;47.32,-22.27,;44.65,-22.27,;43.32,-23.04,;43.33,-27.66,;44.66,-26.89,;43.32,-29.2,)| Show InChI InChI=1S/C78H107N21O19/c1-40(2)28-49(79)77(118)99-27-11-17-61(99)76(117)97-59(36-64(82)105)73(114)93-55(32-44-20-24-47(102)25-21-44)70(111)95-57(34-62(80)103)72(113)94-56(33-45-37-87-50-15-9-8-14-48(45)50)71(112)96-58(35-63(81)104)74(115)98-60(39-100)75(116)92-54(31-42-12-6-5-7-13-42)67(108)88-38-65(106)89-53(29-41(3)4)69(110)90-51(16-10-26-86-78(84)85)68(109)91-52(66(83)107)30-43-18-22-46(101)23-19-43/h5-9,12-15,18-25,37,40-41,49,51-61,87,100-102H,10-11,16-17,26-36,38-39,79H2,1-4H3,(H2,80,103)(H2,81,104)(H2,82,105)(H2,83,107)(H,88,108)(H,89,106)(H,90,110)(H,91,109)(H,92,116)(H,93,114)(H,94,113)(H,95,111)(H,96,112)(H,97,117)(H,98,115)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203797

((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |wU:72.75,87.91,36.37,12.13,106.110,58.61,4.4,wD:66.69,24.24,95.99,16.16,44.45,(-4.64,-18.24,;-4.61,-19.78,;-5.93,-20.57,;-3.26,-20.52,;-3.26,-22.09,;-4.59,-22.86,;-1.92,-22.86,;-1.92,-24.4,;-.59,-22.09,;-.11,-20.63,;1.42,-20.63,;1.9,-22.09,;.65,-23,;.65,-24.54,;-.69,-25.31,;1.98,-25.31,;1.98,-26.85,;.65,-27.62,;.65,-29.16,;1.98,-29.93,;-.69,-29.93,;3.31,-27.62,;3.31,-29.16,;4.65,-26.85,;5.98,-27.62,;5.98,-29.16,;7.31,-29.94,;7.31,-31.47,;8.63,-32.24,;9.98,-31.47,;11.31,-32.24,;9.97,-29.93,;8.65,-29.17,;7.32,-26.86,;7.32,-25.32,;8.65,-27.63,;9.98,-26.86,;9.98,-25.32,;11.32,-24.55,;12.65,-25.32,;11.32,-23.01,;11.32,-27.63,;11.31,-29.17,;12.65,-26.86,;13.98,-27.63,;13.98,-29.17,;15.31,-29.94,;16.57,-28.95,;17.8,-29.94,;17.32,-31.41,;18.08,-32.72,;17.32,-34.05,;15.78,-34.05,;15.02,-32.72,;15.79,-31.4,;15.32,-26.86,;15.32,-25.32,;16.65,-27.64,;17.98,-26.87,;17.99,-25.33,;19.32,-24.56,;20.66,-25.33,;19.32,-23.02,;19.31,-27.64,;19.31,-29.18,;20.65,-26.87,;21.98,-27.64,;21.98,-29.18,;23.32,-29.95,;23.32,-26.87,;23.32,-25.33,;24.65,-27.65,;25.98,-26.88,;25.98,-25.34,;27.32,-24.57,;28.65,-25.34,;29.99,-24.57,;29.99,-23.03,;28.64,-22.26,;27.32,-23.03,;27.32,-27.65,;27.32,-29.19,;28.65,-26.88,;29.99,-27.64,;31.32,-26.87,;31.32,-25.33,;32.65,-27.65,;33.99,-26.88,;33.99,-25.34,;35.32,-24.57,;35.32,-23.03,;36.66,-25.34,;35.32,-27.65,;35.32,-29.19,;36.66,-26.88,;37.99,-27.65,;37.99,-29.19,;39.32,-29.96,;39.32,-31.5,;40.65,-32.28,;40.65,-33.82,;41.98,-34.59,;39.32,-34.58,;39.33,-26.88,;39.33,-25.34,;40.66,-27.66,;41.99,-26.89,;41.99,-25.35,;43.32,-24.58,;44.65,-25.35,;45.98,-24.58,;45.99,-23.04,;47.32,-22.27,;44.65,-22.27,;43.32,-23.04,;43.33,-27.66,;44.66,-26.89,;43.32,-29.2,)| Show InChI InChI=1S/C78H107N21O19/c1-40(2)28-49(79)77(118)99-27-11-17-61(99)76(117)97-59(36-64(82)105)73(114)93-55(32-44-20-24-47(102)25-21-44)70(111)95-57(34-62(80)103)72(113)94-56(33-45-37-87-50-15-9-8-14-48(45)50)71(112)96-58(35-63(81)104)74(115)98-60(39-100)75(116)92-54(31-42-12-6-5-7-13-42)67(108)88-38-65(106)89-53(29-41(3)4)69(110)90-51(16-10-26-86-78(84)85)68(109)91-52(66(83)107)30-43-18-22-46(101)23-19-43/h5-9,12-15,18-25,37,40-41,49,51-61,87,100-102H,10-11,16-17,26-36,38-39,79H2,1-4H3,(H2,80,103)(H2,81,104)(H2,82,105)(H2,83,107)(H,88,108)(H,89,106)(H,90,110)(H,91,109)(H,92,116)(H,93,114)(H,94,113)(H,95,111)(H,96,112)(H,97,117)(H,98,115)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185803

(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...)Show SMILES Cn1cc(C2CCN(CC2)C2Cc3cccc4cccc2c34)c2cccnc12 Show InChI InChI=1S/C25H25N3/c1-27-16-22(20-9-4-12-26-25(20)27)17-10-13-28(14-11-17)23-15-19-7-2-5-18-6-3-8-21(23)24(18)19/h2-9,12,16-17,23H,10-11,13-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203790
((2S)-2-[(2S)-2-[(2S)-2-{[(2S)-1-[(2S)-2-amino-4-me...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C75H100N18O18/c1-39(2)27-48(76)75(111)93-26-14-21-59(93)74(110)91-57(35-62(79)98)71(107)87-53(31-44-22-24-46(95)25-23-44)68(104)89-55(33-60(77)96)70(106)88-54(32-45-36-81-49-20-13-12-19-47(45)49)69(105)90-56(34-61(78)97)72(108)92-58(38-94)73(109)86-52(30-43-17-10-7-11-18-43)66(102)82-37-63(99)84-51(28-40(3)4)67(103)83-41(5)65(101)85-50(64(80)100)29-42-15-8-6-9-16-42/h6-13,15-20,22-25,36,39-41,48,50-59,81,94-95H,14,21,26-35,37-38,76H2,1-5H3,(H2,77,96)(H2,78,97)(H2,79,98)(H2,80,100)(H,82,102)(H,83,103)(H,84,99)(H,85,101)(H,86,109)(H,87,107)(H,88,106)(H,89,104)(H,90,105)(H,91,110)(H,92,108)/t41-,48-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203798
((2S)-2-[(2S)-2-[(2S)-2-{[(2S)-1-[(2S)-2-amino-4-me...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C78H106N20O19/c1-41(2)29-49(79)77(116)98-28-14-22-61(98)76(115)96-59(37-64(82)103)73(112)92-55(33-45-23-25-47(100)26-24-45)70(109)94-57(35-62(80)101)72(111)93-56(34-46-38-86-50-20-12-11-19-48(46)50)71(110)95-58(36-63(81)102)74(113)97-60(40-99)75(114)91-54(32-44-17-9-6-10-18-44)67(106)87-39-65(104)88-53(30-42(3)4)69(108)89-51(21-13-27-85-78(84)117)68(107)90-52(66(83)105)31-43-15-7-5-8-16-43/h5-12,15-20,23-26,38,41-42,49,51-61,86,99-100H,13-14,21-22,27-37,39-40,79H2,1-4H3,(H2,80,101)(H2,81,102)(H2,82,103)(H2,83,105)(H,87,106)(H,88,104)(H,89,108)(H,90,107)(H,91,114)(H,92,112)(H,93,111)(H,94,109)(H,95,110)(H,96,115)(H,97,113)(H3,84,85,117)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185812
(2-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4...)Show SMILES OCCn1cc(C2CCN(CC2)C2Cc3cccc4cccc2c34)c2cccnc12 Show InChI InChI=1S/C26H27N3O/c30-15-14-29-17-23(21-8-3-11-27-26(21)29)18-9-12-28(13-10-18)24-16-20-6-1-4-19-5-2-7-22(24)25(19)20/h1-8,11,17-18,24,30H,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203807
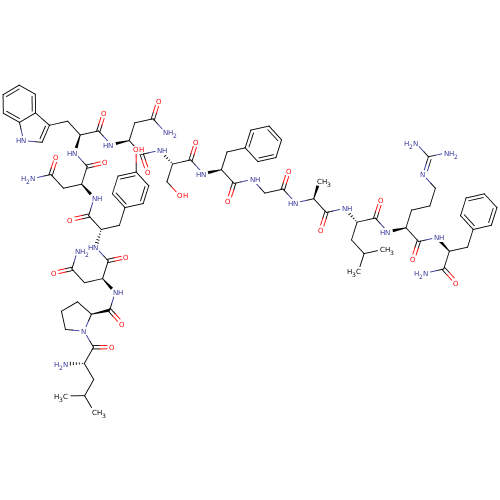
(CHEMBL374077 | LPNYNWNSFGLRF-NH2)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:87.92,16.16,72.75,58.61,100.104,36.37,wD:12.13,4.4,66.69,24.24,44.45,92.96,111.115,(-21.16,-38.2,;-20.07,-37.12,;-20.46,-35.63,;-18.58,-37.52,;-17.5,-36.43,;-17.89,-34.94,;-16,-36.83,;-14.92,-35.74,;-15.69,-38.34,;-16.71,-39.48,;-15.94,-40.8,;-14.44,-40.48,;-14.28,-38.96,;-12.95,-38.19,;-12.95,-36.65,;-11.48,-38.64,;-10.03,-38.11,;-10.03,-36.57,;-8.7,-35.79,;-7.37,-36.57,;-8.7,-34.26,;-8.7,-38.88,;-8.7,-40.42,;-7.47,-37.95,;-6.33,-38.98,;-6.33,-40.52,;-5,-41.29,;-3.68,-40.52,;-2.36,-41.29,;-2.36,-42.83,;-1.02,-43.6,;-3.68,-43.59,;-5,-42.82,;-5,-38.21,;-5,-36.67,;-3.67,-38.98,;-2.34,-38.21,;-2.34,-36.67,;-1.01,-35.89,;.44,-36.34,;-1.01,-34.36,;-1.01,-38.98,;-1.01,-40.52,;.22,-38.05,;1.36,-39.09,;1.36,-40.62,;2.69,-41.39,;4.17,-40.77,;5.27,-41.85,;4.37,-43.11,;4.86,-44.46,;3.82,-45.49,;2.39,-45.18,;1.95,-43.77,;2.96,-42.75,;2.69,-38.31,;2.69,-36.77,;4.02,-39.09,;5.36,-38.31,;5.36,-36.77,;6.69,-36,;8.02,-36.77,;6.69,-34.46,;6.69,-39.09,;6.69,-40.62,;7.91,-38.15,;9.06,-39.19,;9.06,-40.72,;10.5,-41.17,;10.39,-38.41,;10.39,-36.87,;11.86,-38.87,;13.3,-38.33,;13.3,-36.79,;14.63,-36.02,;15.97,-36.79,;17.3,-36.01,;17.3,-34.47,;15.95,-33.69,;14.63,-34.49,;14.63,-39.11,;14.63,-40.64,;15.86,-38.18,;17,-39.21,;18.33,-38.44,;18.33,-36.89,;19.8,-38.89,;21.25,-38.36,;21.25,-36.82,;22.59,-39.13,;22.59,-40.66,;23.81,-38.2,;24.96,-39.23,;24.96,-40.77,;26.29,-41.54,;26.29,-43.08,;27.62,-40.77,;26.29,-38.46,;26.29,-36.92,;27.62,-39.23,;28.95,-38.46,;28.95,-36.92,;30.28,-36.15,;30.28,-34.61,;31.61,-33.84,;31.61,-32.3,;30.28,-31.53,;32.94,-31.53,;30.28,-39.23,;30.28,-40.77,;31.61,-38.46,;32.94,-39.23,;32.94,-40.77,;34.27,-41.54,;35.61,-40.77,;36.93,-41.54,;36.93,-43.07,;35.6,-43.84,;34.27,-43.07,;34.27,-38.46,;35.61,-39.23,;34.27,-36.92,)| Show InChI InChI=1S/C81H112N22O19/c1-42(2)30-51(82)80(122)103-29-15-23-63(103)79(121)101-61(38-66(85)108)76(118)97-57(34-47-24-26-49(105)27-25-47)73(115)99-59(36-64(83)106)75(117)98-58(35-48-39-90-52-21-13-12-20-50(48)52)74(116)100-60(37-65(84)107)77(119)102-62(41-104)78(120)96-56(33-46-18-10-7-11-19-46)70(112)91-40-67(109)92-44(5)69(111)95-55(31-43(3)4)72(114)93-53(22-14-28-89-81(87)88)71(113)94-54(68(86)110)32-45-16-8-6-9-17-45/h6-13,16-21,24-27,39,42-44,51,53-63,90,104-105H,14-15,22-23,28-38,40-41,82H2,1-5H3,(H2,83,106)(H2,84,107)(H2,85,108)(H2,86,110)(H,91,112)(H,92,109)(H,93,114)(H,94,113)(H,95,111)(H,96,120)(H,97,118)(H,98,117)(H,99,115)(H,100,116)(H,101,121)(H,102,119)(H4,87,88,89)/t44-,51-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203786
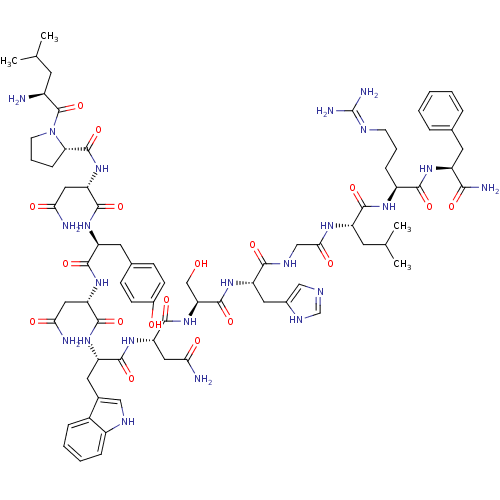
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,86.90,36.37,12.13,105.109,wD:66.69,24.24,94.98,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;27.7,-26.07,;28.62,-24.82,;27.72,-23.57,;26.24,-24.03,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C75H105N23O18/c1-38(2)24-46(76)74(116)98-23-11-17-58(98)73(115)96-56(32-61(79)103)70(112)91-51(27-41-18-20-44(100)21-19-41)67(109)94-54(30-59(77)101)69(111)92-52(28-42-33-85-47-15-9-8-14-45(42)47)68(110)95-55(31-60(78)102)71(113)97-57(36-99)72(114)93-53(29-43-34-83-37-87-43)64(106)86-35-62(104)88-50(25-39(3)4)66(108)89-48(16-10-22-84-75(81)82)65(107)90-49(63(80)105)26-40-12-6-5-7-13-40/h5-9,12-15,18-21,33-34,37-39,46,48-58,85,99-100H,10-11,16-17,22-32,35-36,76H2,1-4H3,(H2,77,101)(H2,78,102)(H2,79,103)(H2,80,105)(H,83,87)(H,86,106)(H,88,104)(H,89,108)(H,90,107)(H,91,112)(H,92,111)(H,93,114)(H,94,109)(H,95,110)(H,96,115)(H,97,113)(H4,81,82,84)/t46-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203795
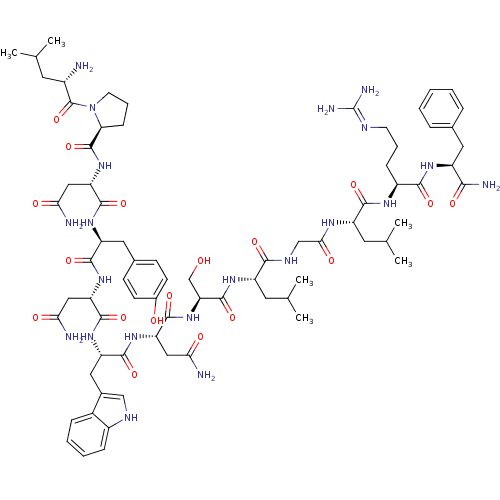
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,84.87,36.37,12.13,103.106,wD:66.69,24.24,92.95,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;27.56,-26.36,;26.23,-24.05,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C75H109N21O18/c1-38(2)26-46(76)74(114)96-25-13-19-58(96)73(113)94-56(34-61(79)101)70(110)90-52(30-42-20-22-44(98)23-21-42)67(107)92-54(32-59(77)99)69(109)91-53(31-43-35-84-47-17-11-10-16-45(43)47)68(108)93-55(33-60(78)100)71(111)95-57(37-97)72(112)89-50(27-39(3)4)64(104)85-36-62(102)86-51(28-40(5)6)66(106)87-48(18-12-24-83-75(81)82)65(105)88-49(63(80)103)29-41-14-8-7-9-15-41/h7-11,14-17,20-23,35,38-40,46,48-58,84,97-98H,12-13,18-19,24-34,36-37,76H2,1-6H3,(H2,77,99)(H2,78,100)(H2,79,101)(H2,80,103)(H,85,104)(H,86,102)(H,87,106)(H,88,105)(H,89,112)(H,90,110)(H,91,109)(H,92,107)(H,93,108)(H,94,113)(H,95,111)(H4,81,82,83)/t46-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50185786
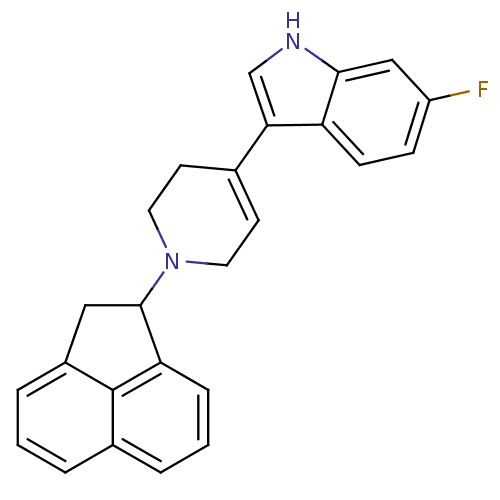
(3-(1-(1,2-dihydroacenaphthylen-1-yl)-1,2,3,6-tetra...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CC1)C1Cc2cccc3cccc1c23 |t:12| Show InChI InChI=1S/C25H21FN2/c26-19-7-8-20-22(15-27-23(20)14-19)16-9-11-28(12-10-16)24-13-18-5-1-3-17-4-2-6-21(24)25(17)18/h1-9,14-15,24,27H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203817

((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-20.28,-19.15,;-20.26,-20.69,;-21.58,-21.47,;-18.91,-21.43,;-18.9,-22.99,;-20.24,-23.76,;-17.57,-23.76,;-17.57,-25.3,;-16.25,-22.99,;-15.77,-21.53,;-14.22,-21.54,;-13.74,-22.99,;-14.99,-23.9,;-14.99,-25.44,;-16.34,-26.21,;-13.67,-26.22,;-13.67,-27.76,;-15,-28.52,;-15,-30.06,;-13.68,-30.84,;-16.33,-30.83,;-12.34,-28.53,;-12.34,-30.07,;-10.99,-27.76,;-9.66,-28.53,;-9.66,-30.07,;-8.33,-30.84,;-8.33,-32.38,;-7.01,-33.15,;-5.66,-32.38,;-4.33,-33.15,;-5.67,-30.83,;-6.99,-30.07,;-8.32,-27.76,;-8.32,-26.22,;-6.99,-28.53,;-5.66,-27.77,;-5.66,-26.23,;-4.32,-25.46,;-2.99,-26.23,;-4.32,-23.92,;-4.32,-28.54,;-4.33,-30.08,;-2.99,-27.77,;-1.65,-28.54,;-1.66,-30.08,;-.33,-30.85,;.93,-29.86,;2.16,-30.85,;1.68,-32.32,;2.44,-33.63,;1.68,-34.96,;.14,-34.96,;-.62,-33.63,;.15,-32.31,;-.32,-27.77,;-.32,-26.23,;1.01,-28.54,;2.35,-27.77,;2.35,-26.23,;3.68,-25.47,;5.02,-26.24,;3.68,-23.93,;3.67,-28.55,;3.67,-30.09,;5.01,-27.78,;6.34,-28.55,;6.34,-30.09,;7.68,-30.86,;7.68,-27.78,;7.68,-26.24,;9.01,-28.55,;10.34,-27.78,;10.35,-26.24,;11.68,-25.48,;13.01,-26.24,;14.35,-25.48,;14.35,-23.93,;13.01,-23.16,;11.68,-23.94,;11.68,-28.56,;11.68,-30.1,;13.01,-27.79,;14.35,-28.55,;15.68,-27.78,;15.68,-26.24,;17.01,-28.55,;18.35,-27.78,;18.35,-26.24,;19.68,-25.48,;19.68,-23.94,;21.02,-26.25,;19.68,-28.56,;19.68,-30.1,;21.02,-27.79,;22.35,-28.56,;22.35,-30.1,;23.68,-30.87,;23.68,-32.41,;25.01,-33.18,;25.01,-34.72,;26.34,-35.49,;23.68,-35.49,;23.69,-27.79,;23.69,-26.25,;25.02,-28.56,;26.35,-27.79,;26.35,-26.25,;27.69,-25.48,;29.15,-25.94,;30.05,-24.69,;29.14,-23.45,;27.67,-23.94,;27.69,-28.57,;29.02,-27.8,;27.68,-30.11,)| Show InChI InChI=1S/C75H105N23O18/c1-38(2)24-46(76)74(116)98-23-11-17-58(98)73(115)96-56(32-61(79)103)70(112)92-52(27-41-18-20-44(100)21-19-41)67(109)94-54(30-59(77)101)69(111)93-53(28-42-33-85-47-15-9-8-14-45(42)47)68(110)95-55(31-60(78)102)71(113)97-57(36-99)72(114)91-51(26-40-12-6-5-7-13-40)64(106)86-35-62(104)88-50(25-39(3)4)66(108)89-48(16-10-22-84-75(81)82)65(107)90-49(63(80)105)29-43-34-83-37-87-43/h5-9,12-15,18-21,33-34,37-39,46,48-58,85,99-100H,10-11,16-17,22-32,35-36,76H2,1-4H3,(H2,77,101)(H2,78,102)(H2,79,103)(H2,80,105)(H,83,87)(H,86,106)(H,88,104)(H,89,108)(H,90,107)(H,91,114)(H,92,112)(H,93,111)(H,94,109)(H,95,110)(H,96,115)(H,97,113)(H4,81,82,84)/t46-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50185799

(1-(1,2-dihydroacenaphthylen-1-yl)-4-(1-methyl-1H-p...)Show SMILES Cn1cc(C2CCN(CC2O)C2Cc3cccc4cccc2c34)c2cccnc12 Show InChI InChI=1S/C25H25N3O/c1-27-14-21(19-9-4-11-26-25(19)27)18-10-12-28(15-23(18)29)22-13-17-7-2-5-16-6-3-8-20(22)24(16)17/h2-9,11,14,18,22-23,29H,10,12-13,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50185810
(3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fluoro-1H...)Show InChI InChI=1S/C22H31FN2/c23-19-8-9-22-20(14-19)21(15-24-22)18-10-12-25(13-11-18)16-17-6-4-2-1-3-5-7-17/h8-9,14-15,17-18,24H,1-7,10-13,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433371
(CHEMBL2375519)Show InChI InChI=1S/C15H13NO3/c17-15(18)12-8-13-14(16-12)11(9-19-13)7-6-10-4-2-1-3-5-10/h1-5,8-9,16H,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203788
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.90,36.37,12.13,106.109,wD:66.69,24.24,95.98,16.16,44.45,(-5.72,-19.26,;-5.69,-20.8,;-7.02,-21.58,;-4.35,-21.54,;-4.34,-23.1,;-5.68,-23.87,;-3.01,-23.87,;-3.01,-25.41,;-1.68,-23.11,;-1.2,-21.65,;.34,-21.65,;.82,-23.1,;-.43,-24.02,;-.43,-25.56,;-1.77,-26.32,;.9,-26.33,;.89,-27.87,;-.44,-28.64,;-.44,-30.18,;.89,-30.95,;-1.78,-30.94,;2.22,-28.64,;2.22,-30.18,;3.56,-27.87,;4.89,-28.64,;4.89,-30.18,;6.23,-30.95,;6.22,-32.49,;7.55,-33.26,;8.89,-32.49,;10.22,-33.26,;8.88,-30.94,;7.56,-30.18,;6.23,-27.87,;6.23,-26.33,;7.56,-28.64,;8.89,-27.88,;8.89,-26.34,;10.23,-25.57,;11.56,-26.34,;10.23,-24.03,;10.23,-28.65,;10.23,-30.19,;11.56,-27.88,;12.9,-28.65,;12.9,-30.19,;14.22,-30.96,;15.48,-29.97,;16.71,-30.96,;16.24,-32.43,;17,-33.74,;16.24,-35.07,;14.7,-35.07,;13.94,-33.74,;14.71,-32.42,;14.23,-27.88,;14.23,-26.34,;15.57,-28.65,;16.9,-27.89,;16.9,-26.35,;18.23,-25.58,;19.57,-26.35,;18.23,-24.04,;18.23,-28.66,;18.23,-30.2,;19.57,-27.89,;20.9,-28.66,;20.89,-30.2,;22.23,-30.97,;22.24,-27.89,;22.24,-26.35,;23.57,-28.66,;24.9,-27.9,;24.9,-26.36,;26.24,-25.59,;26.24,-24.05,;24.9,-23.28,;24.9,-21.74,;23.56,-20.97,;26.23,-20.97,;26.23,-28.67,;26.23,-30.21,;27.57,-27.9,;28.9,-28.66,;30.23,-27.89,;30.24,-26.35,;31.56,-28.66,;32.9,-27.9,;32.9,-26.36,;34.24,-25.59,;34.24,-24.05,;35.57,-26.36,;34.23,-28.67,;34.23,-30.21,;35.57,-27.9,;36.9,-28.67,;36.9,-30.21,;38.24,-30.98,;38.24,-32.52,;39.56,-33.29,;39.56,-34.83,;40.89,-35.61,;38.23,-35.6,;38.24,-27.9,;38.24,-26.36,;39.57,-28.67,;40.9,-27.91,;40.9,-26.37,;42.24,-25.6,;43.57,-26.38,;44.91,-25.61,;44.91,-24.07,;43.56,-23.3,;42.24,-24.07,;42.24,-28.68,;43.57,-27.91,;42.24,-30.22,)| Show InChI InChI=1S/C75H110N24O18/c1-38(2)27-45(76)73(117)99-26-12-19-57(99)72(116)97-55(34-60(79)104)69(113)93-51(30-41-20-22-43(101)23-21-41)66(110)95-53(32-58(77)102)68(112)94-52(31-42-35-87-46-16-9-8-15-44(42)46)67(111)96-54(33-59(78)103)70(114)98-56(37-100)71(115)90-47(17-10-24-85-74(81)82)63(107)88-36-61(105)89-50(28-39(3)4)65(109)91-48(18-11-25-86-75(83)84)64(108)92-49(62(80)106)29-40-13-6-5-7-14-40/h5-9,13-16,20-23,35,38-39,45,47-57,87,100-101H,10-12,17-19,24-34,36-37,76H2,1-4H3,(H2,77,102)(H2,78,103)(H2,79,104)(H2,80,106)(H,88,107)(H,89,105)(H,90,115)(H,91,109)(H,92,108)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,81,82,85)(H4,83,84,86)/t45-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203796
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.76,81.84,36.37,12.13,100.103,wD:66.69,24.24,89.92,16.16,44.45,(-9.93,-20.61,;-9.9,-22.15,;-11.22,-22.94,;-8.55,-22.89,;-8.55,-24.45,;-9.88,-25.22,;-7.21,-25.22,;-7.21,-26.76,;-5.88,-24.45,;-5.4,-22.99,;-3.86,-23,;-3.39,-24.46,;-4.64,-25.37,;-4.64,-26.91,;-5.98,-27.68,;-3.31,-27.68,;-3.31,-29.22,;-4.65,-29.99,;-4.65,-31.53,;-3.32,-32.3,;-5.98,-32.3,;-1.98,-29.99,;-1.98,-31.53,;-.64,-29.22,;.69,-29.99,;.69,-31.53,;2.02,-32.3,;2.01,-33.84,;3.34,-34.61,;4.68,-33.84,;6.01,-34.61,;4.68,-32.3,;3.35,-31.54,;2.02,-29.22,;2.02,-27.68,;3.36,-30,;4.69,-29.23,;4.69,-27.69,;6.03,-26.92,;7.36,-27.69,;6.03,-25.38,;6.02,-30,;6.02,-31.54,;7.36,-29.23,;8.69,-30,;8.69,-31.54,;10.02,-32.31,;11.27,-31.32,;12.51,-32.31,;12.03,-33.78,;12.79,-35.1,;12.03,-36.42,;10.49,-36.42,;9.73,-35.09,;10.5,-33.77,;10.03,-29.23,;10.03,-27.69,;11.36,-30,;12.69,-29.23,;12.69,-27.69,;14.03,-26.93,;15.36,-27.7,;14.03,-25.39,;14.03,-30.01,;14.02,-31.55,;15.36,-29.24,;16.69,-30.01,;16.69,-31.55,;15.36,-32.32,;18.03,-29.24,;18.03,-27.7,;19.36,-30.01,;20.69,-29.24,;20.7,-27.7,;22.03,-30.01,;22.03,-31.55,;23.36,-29.25,;24.7,-30.02,;26.03,-29.25,;26.03,-27.71,;27.36,-30.02,;28.7,-29.25,;28.7,-27.71,;30.03,-26.94,;30.03,-25.4,;31.37,-27.71,;30.03,-30.02,;30.03,-31.56,;31.36,-29.25,;32.7,-30.03,;32.7,-31.57,;34.03,-32.34,;34.03,-33.88,;35.36,-34.65,;35.36,-36.19,;36.69,-36.96,;34.02,-36.96,;34.03,-29.26,;34.03,-27.72,;35.36,-30.03,;36.7,-29.26,;36.7,-27.72,;38.04,-26.95,;39.36,-27.73,;40.7,-26.97,;40.7,-25.42,;39.36,-24.65,;38.03,-25.42,;38.03,-30.03,;39.37,-29.26,;38.03,-31.57,)| Show InChI InChI=1S/C72H103N21O18/c1-36(2)25-44(73)71(111)93-24-12-18-55(93)70(110)91-53(32-58(76)98)67(107)87-49(28-40-19-21-42(95)22-20-40)64(104)89-51(30-56(74)96)66(106)88-50(29-41-33-81-45-16-10-9-15-43(41)45)65(105)90-52(31-57(75)97)68(108)92-54(35-94)69(109)83-38(5)61(101)82-34-59(99)84-48(26-37(3)4)63(103)85-46(17-11-23-80-72(78)79)62(102)86-47(60(77)100)27-39-13-7-6-8-14-39/h6-10,13-16,19-22,33,36-38,44,46-55,81,94-95H,11-12,17-18,23-32,34-35,73H2,1-5H3,(H2,74,96)(H2,75,97)(H2,76,98)(H2,77,100)(H,82,101)(H,83,109)(H,84,99)(H,85,103)(H,86,102)(H,87,107)(H,88,106)(H,89,104)(H,90,105)(H,91,110)(H,92,108)(H4,78,79,80)/t38-,44-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50185792
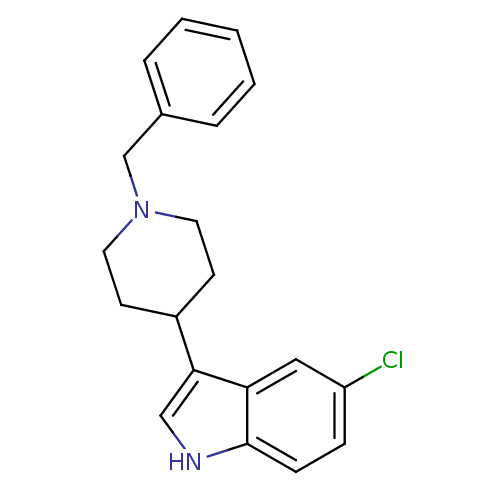
(3-(1-benzylpiperidin-4-yl)-5-chloro-1H-indole | CH...)Show InChI InChI=1S/C20H21ClN2/c21-17-6-7-20-18(12-17)19(13-22-20)16-8-10-23(11-9-16)14-15-4-2-1-3-5-15/h1-7,12-13,16,22H,8-11,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 3524-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.094
BindingDB Entry DOI: 10.7270/Q2VT1RQJ |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203785
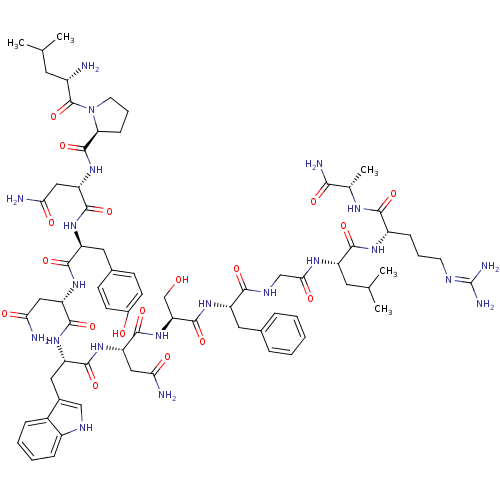
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.111,wD:66.69,24.24,95.99,16.16,44.45,(-9.14,-21.26,;-9.11,-22.8,;-10.43,-23.59,;-7.77,-23.54,;-7.76,-25.1,;-9.09,-25.87,;-6.43,-25.87,;-6.43,-27.41,;-5.1,-25.1,;-4.61,-23.64,;-3.08,-23.65,;-2.6,-25.11,;-3.85,-26.02,;-3.86,-27.56,;-5.19,-28.33,;-2.52,-28.33,;-2.52,-29.87,;-3.86,-30.64,;-3.86,-32.18,;-2.53,-32.95,;-5.2,-32.95,;-1.19,-30.64,;-1.19,-32.18,;.14,-29.87,;1.48,-30.64,;1.47,-32.18,;2.81,-32.95,;2.8,-34.49,;4.13,-35.26,;5.47,-34.49,;6.8,-35.26,;5.46,-32.95,;4.14,-32.19,;2.81,-29.87,;2.81,-28.33,;4.14,-30.65,;5.48,-29.88,;5.48,-28.34,;6.81,-27.57,;8.15,-28.34,;6.82,-26.03,;6.81,-30.65,;6.81,-32.19,;8.14,-29.88,;9.48,-30.65,;9.48,-32.19,;10.8,-32.96,;12.06,-31.97,;13.29,-32.96,;12.82,-34.43,;13.58,-35.75,;12.82,-37.07,;11.28,-37.07,;10.52,-35.74,;11.28,-34.42,;10.81,-29.88,;10.81,-28.34,;12.15,-30.65,;13.48,-29.89,;13.48,-28.35,;14.82,-27.58,;16.15,-28.35,;14.82,-26.04,;14.81,-30.66,;14.81,-32.2,;16.15,-29.89,;17.48,-30.66,;17.48,-32.2,;18.81,-32.97,;18.81,-29.89,;18.82,-28.35,;20.15,-30.66,;21.48,-29.89,;21.48,-28.35,;22.82,-27.59,;24.15,-28.36,;25.48,-27.59,;25.49,-26.05,;24.14,-25.28,;22.81,-26.05,;22.81,-30.67,;22.81,-32.21,;24.15,-29.9,;25.48,-30.67,;26.82,-29.9,;26.82,-28.36,;28.15,-30.67,;29.48,-29.9,;29.49,-28.36,;30.82,-27.59,;30.82,-26.05,;32.15,-28.36,;30.82,-30.67,;30.82,-32.21,;32.15,-29.91,;33.48,-30.68,;33.48,-32.22,;34.82,-32.99,;34.81,-34.53,;36.15,-35.3,;36.15,-36.84,;37.48,-37.61,;34.81,-37.61,;34.82,-29.91,;34.82,-28.37,;36.15,-30.68,;37.49,-29.91,;37.49,-28.37,;38.82,-30.68,;40.15,-29.91,;38.82,-32.22,)| Show InChI InChI=1S/C72H103N21O18/c1-36(2)25-44(73)71(111)93-24-12-18-55(93)70(110)91-53(32-58(76)98)67(107)87-49(28-40-19-21-42(95)22-20-40)64(104)89-51(30-56(74)96)66(106)88-50(29-41-33-81-45-16-10-9-15-43(41)45)65(105)90-52(31-57(75)97)68(108)92-54(35-94)69(109)86-48(27-39-13-7-6-8-14-39)61(101)82-34-59(99)84-47(26-37(3)4)63(103)85-46(17-11-23-80-72(78)79)62(102)83-38(5)60(77)100/h6-10,13-16,19-22,33,36-38,44,46-55,81,94-95H,11-12,17-18,23-32,34-35,73H2,1-5H3,(H2,74,96)(H2,75,97)(H2,76,98)(H2,77,100)(H,82,101)(H,83,102)(H,84,99)(H,85,103)(H,86,109)(H,87,107)(H,88,106)(H,89,104)(H,90,105)(H,91,110)(H,92,108)(H4,78,79,80)/t38-,44-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




















































