

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542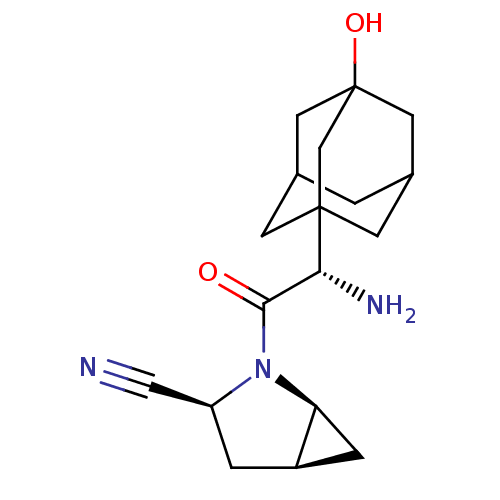 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225074 ((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 6476-80 (2007) Article DOI: 10.1016/j.bmcl.2007.09.090 BindingDB Entry DOI: 10.7270/Q2B56JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11541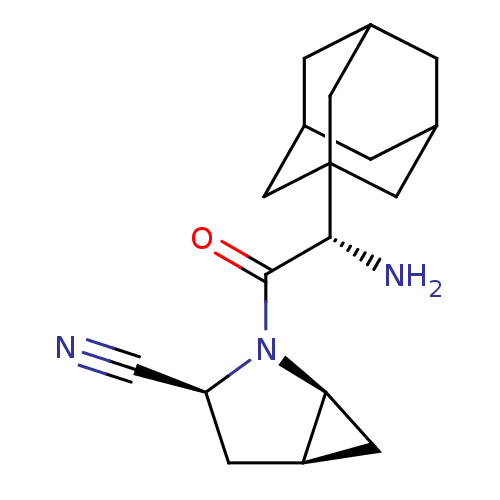 ((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11530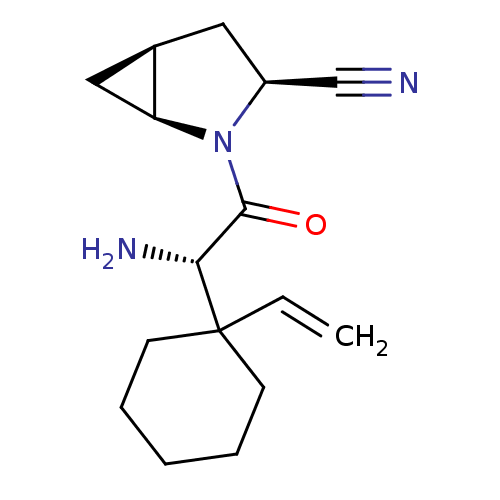 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11544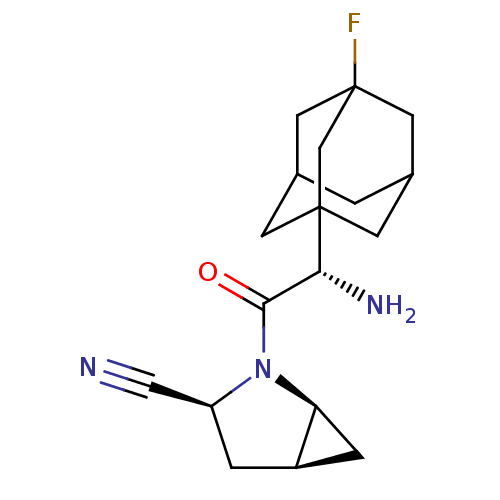 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50287059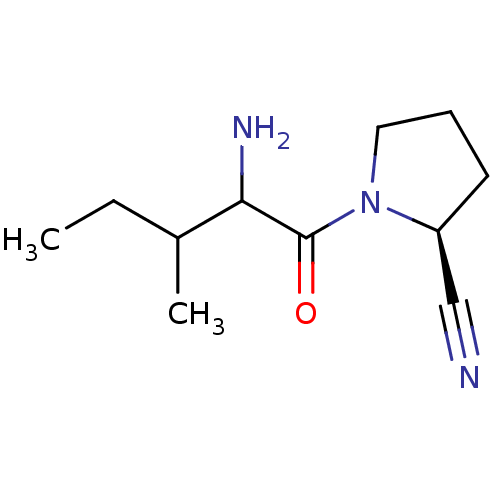 ((S)-1-(2-Amino-3-methyl-pentanoyl)-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430412 (CHEMBL2334700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50212876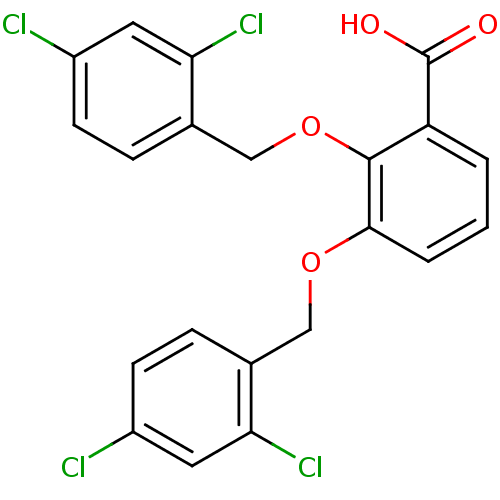 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from eFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212876 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11543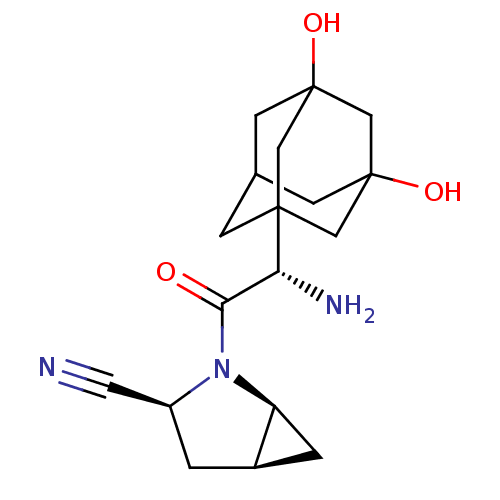 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50192462 ((S)-2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to ap2 | J Med Chem 49: 5013-7 (2006) Article DOI: 10.1021/jm060360i BindingDB Entry DOI: 10.7270/Q2TQ6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212885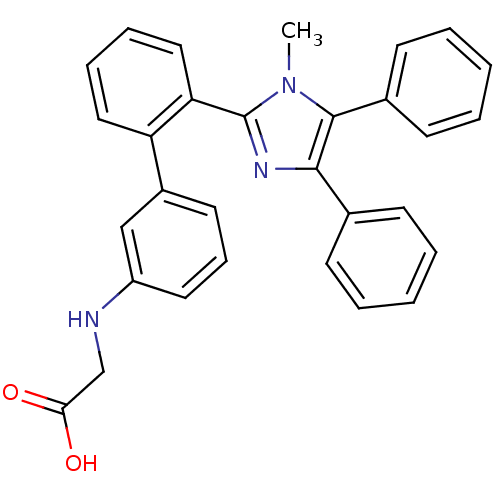 (CHEMBL245282 | [2'-(1-methyl-4,5-diphenyl-1H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225073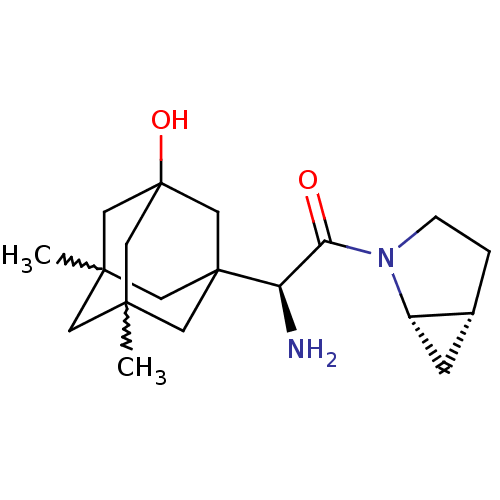 ((S)-2-amino-1-(1S,5R)-2-aza-bicyclo[3.1.0]hex-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 6476-80 (2007) Article DOI: 10.1016/j.bmcl.2007.09.090 BindingDB Entry DOI: 10.7270/Q2B56JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50192463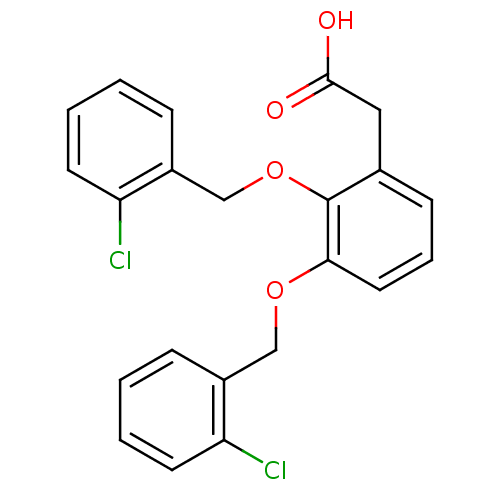 (2-(2,3-bis(2-chlorobenzyloxy)phenyl)acetic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human kFABP | J Med Chem 49: 5013-7 (2006) Article DOI: 10.1021/jm060360i BindingDB Entry DOI: 10.7270/Q2TQ6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212884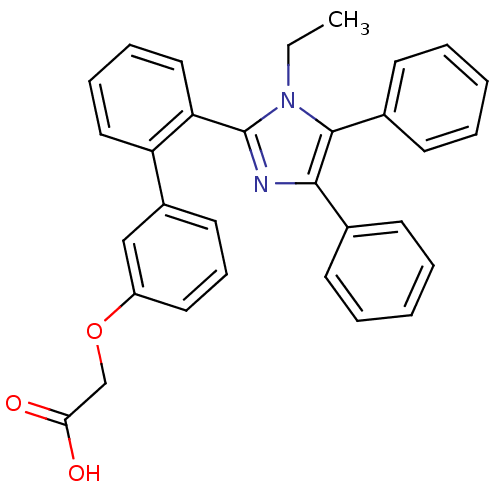 (CHEMBL245284 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid from aFABP | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212877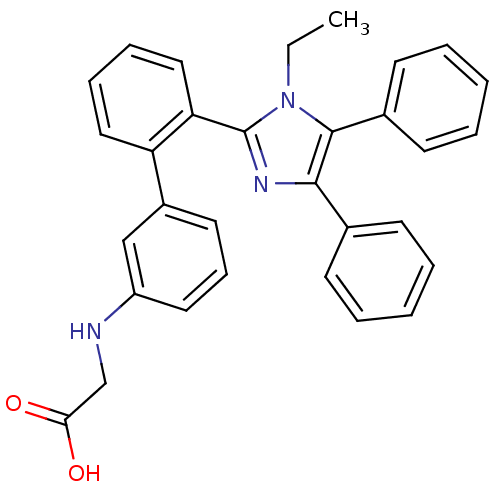 (CHEMBL245653 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212886 (CHEMBL247529 | [2'-(3-ethyl-4,5-diphenyl-furan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM11928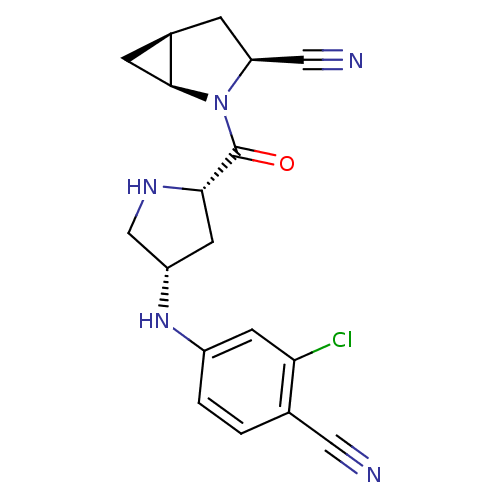 ((1S,3S,5S)-2-{[(2S,4S)-4-[(3-chloro-4-cyanophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | Bioorg Med Chem Lett 15: 3992-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.043 BindingDB Entry DOI: 10.7270/Q2CN7259 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11529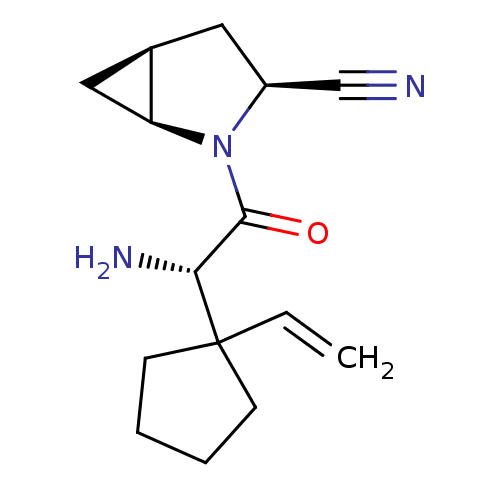 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of TYRO3 (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430413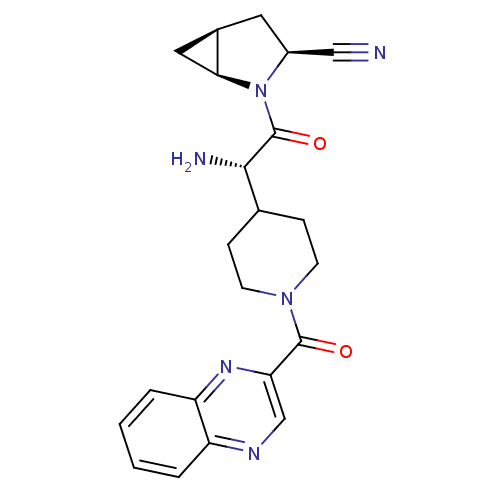 (CHEMBL2334699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430423 (CHEMBL2334689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145999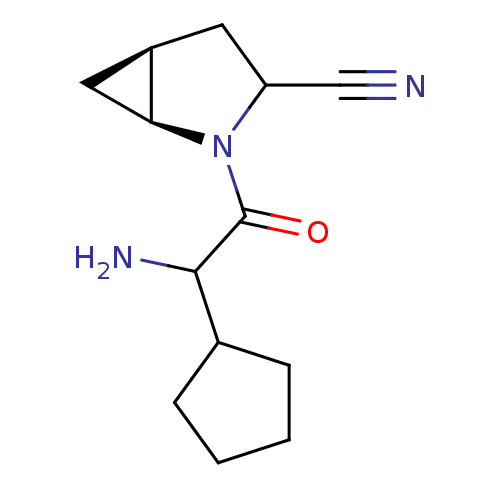 ((1S,5S)-2-(2-Amino-2-cyclopentyl-acetyl)-2-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, heart (Homo sapiens (Human)) | BDBM50212876 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from mFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430424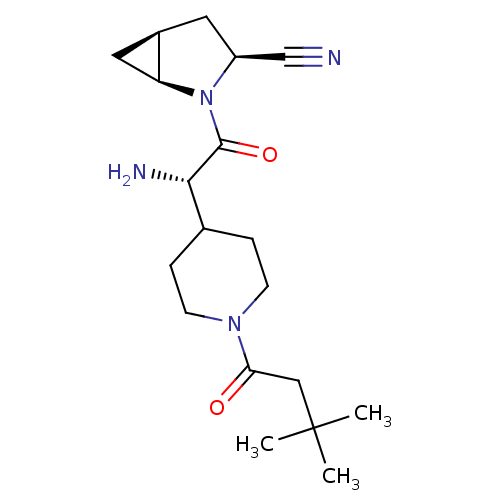 (CHEMBL2334688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430414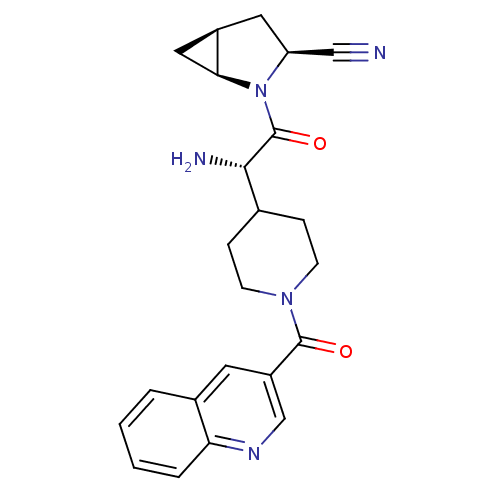 (CHEMBL2334698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11535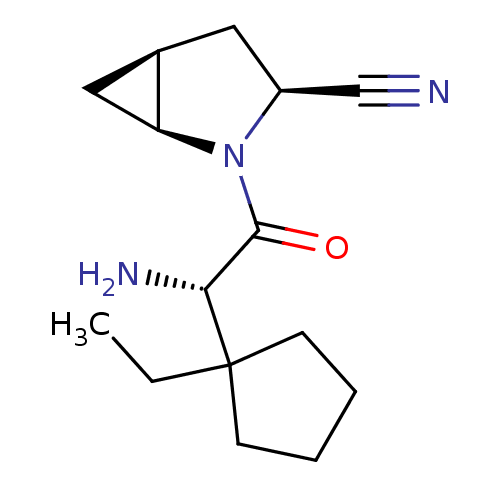 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50029969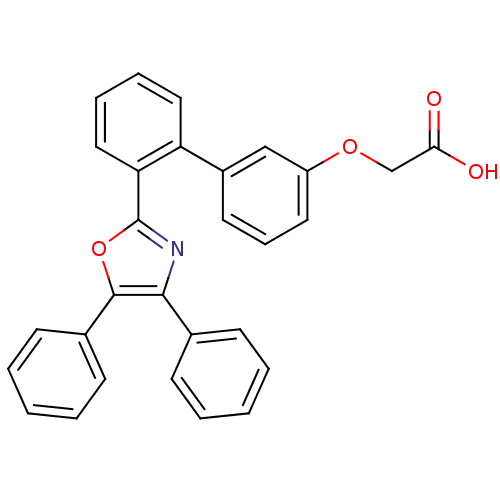 (CHEMBL126078 | [2'-(4,5-Diphenyl-oxazol-2-yl)-biph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25761 (Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for beta-receptor affinity determined in rat brain membrane fraction with [3H]- dihydroalprenolol | J Med Chem 30: 788-92 (1987) BindingDB Entry DOI: 10.7270/Q2FT8P8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146015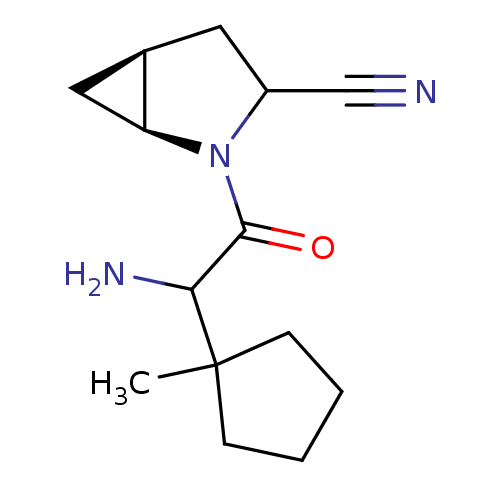 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclopentyl)-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146002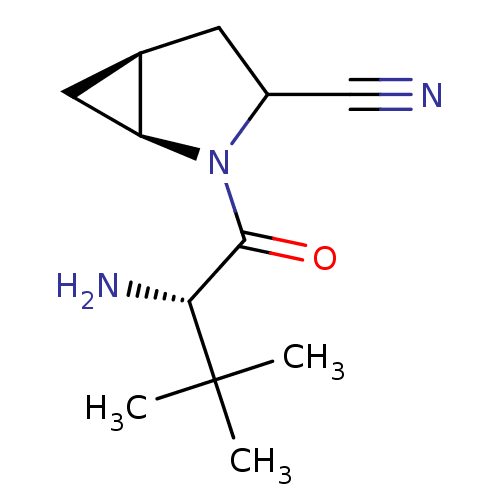 ((S)-2-((S)-2-Amino-3-methyl-3-(S)-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11533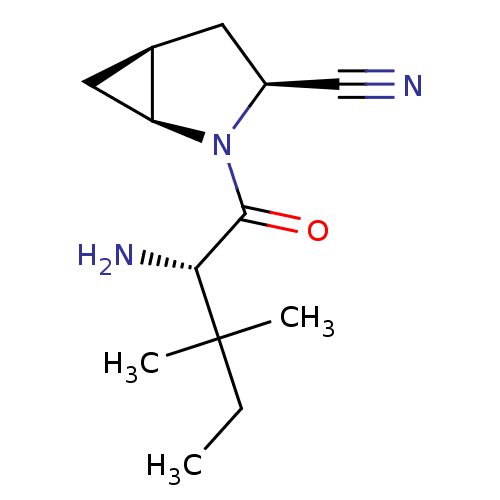 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212873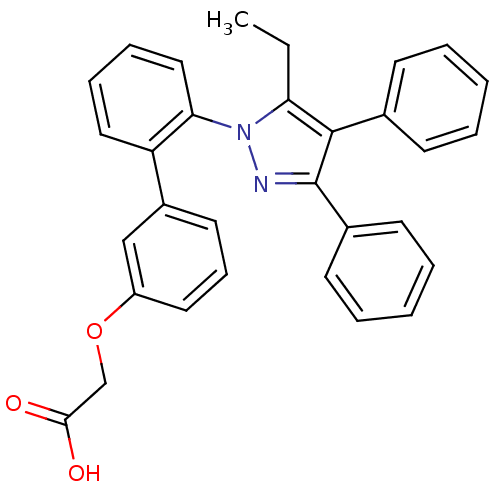 (((2'-(5-ETHYL-3,4-DIPHENYL-1H-PYRAZOL-1-YL)-3-BIPH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid from aFABP | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11538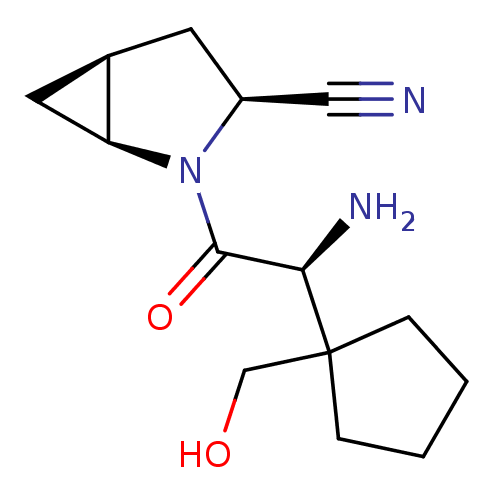 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146009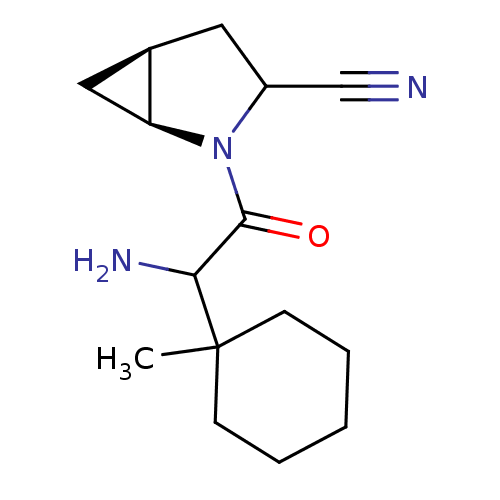 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclohexyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430415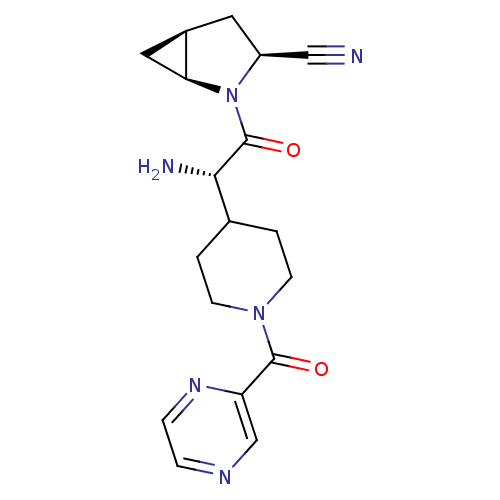 (CHEMBL2334697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50151003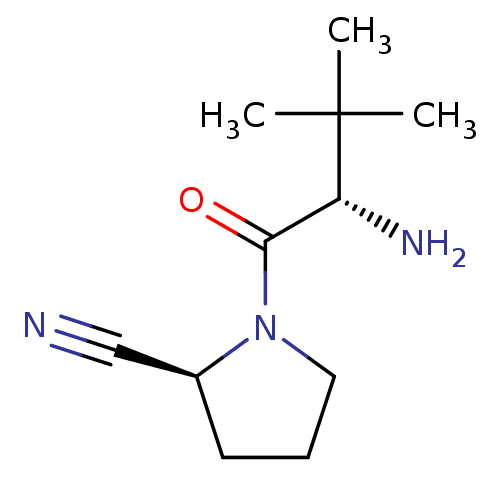 ((S)-1-((S)-2-Amino-3,3-dimethyl-butyryl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11539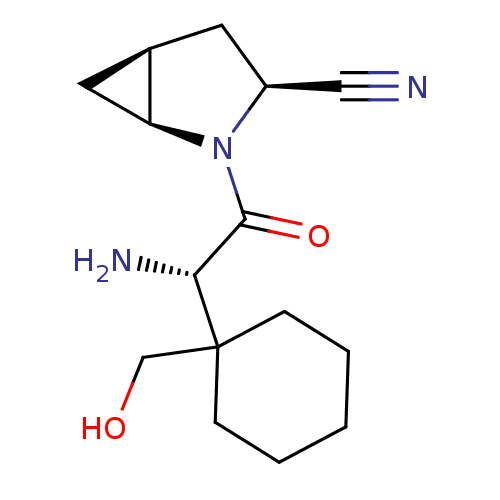 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212883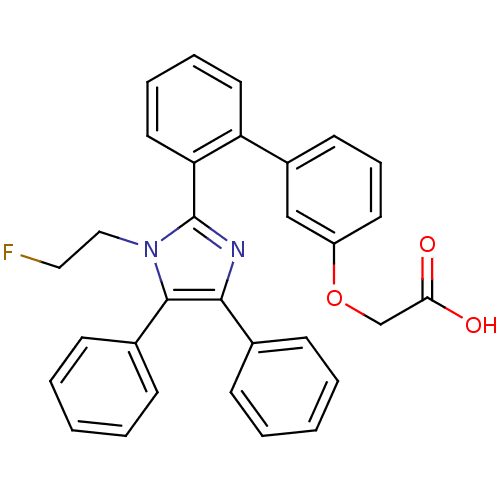 (CHEMBL397385 | {2'-[1-(2-fluoro-ethyl)-4,5-dipheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid from aFABP | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50192465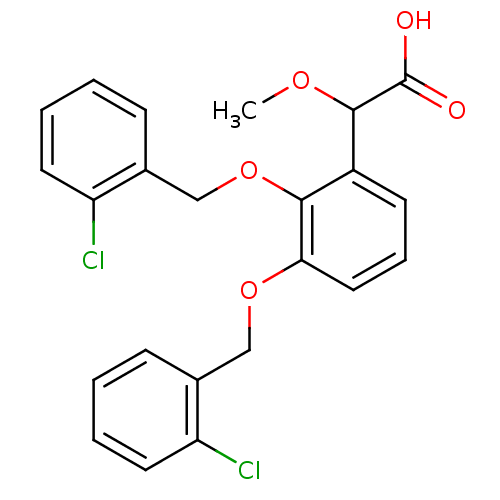 (2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-methoxyacet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human kFABP | J Med Chem 49: 5013-7 (2006) Article DOI: 10.1021/jm060360i BindingDB Entry DOI: 10.7270/Q2TQ6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212879 (CHEMBL248144 | [2'-(4,5-diphenyl-1H-imidazol-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430408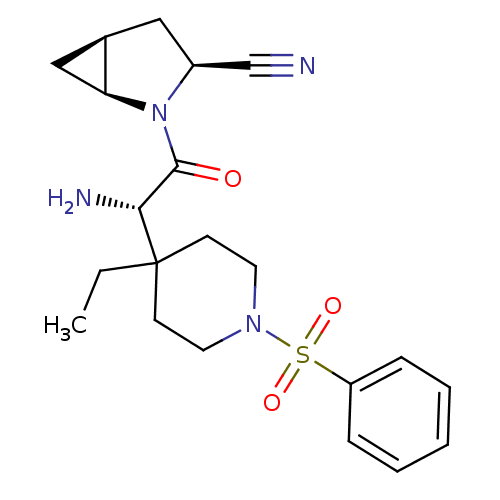 (CHEMBL2334680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225053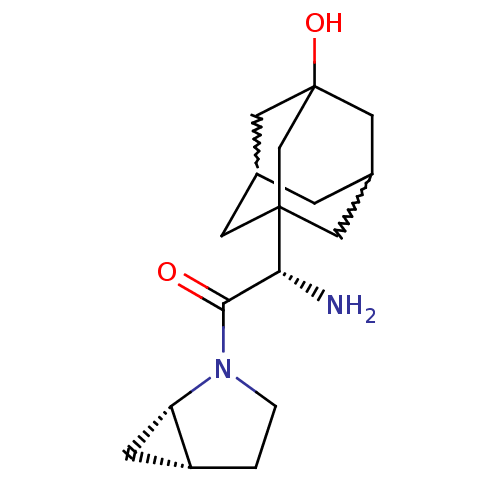 (2-(S)-amino-1-(1S,5R)-2-aza-bicyclo[3.1.0]hex-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 6476-80 (2007) Article DOI: 10.1016/j.bmcl.2007.09.090 BindingDB Entry DOI: 10.7270/Q2B56JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11532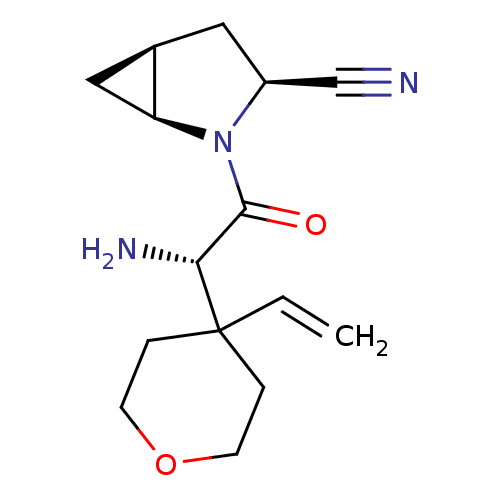 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11531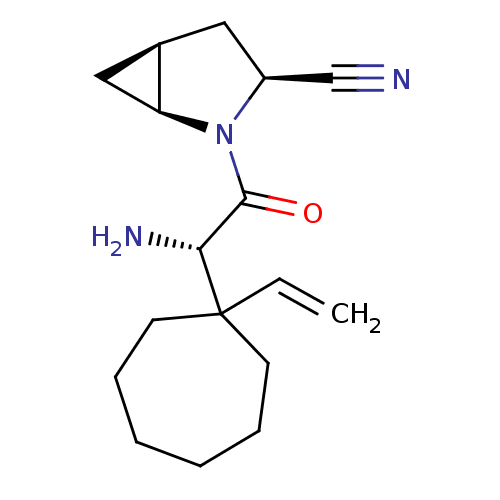 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50430419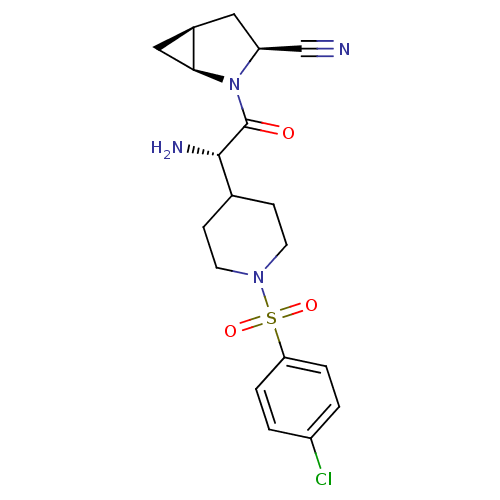 (CHEMBL2334693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 using gly-pro-para-nitroanilide as substrate | Bioorg Med Chem Lett 23: 1622-5 (2013) Article DOI: 10.1016/j.bmcl.2013.01.104 BindingDB Entry DOI: 10.7270/Q2K075N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146010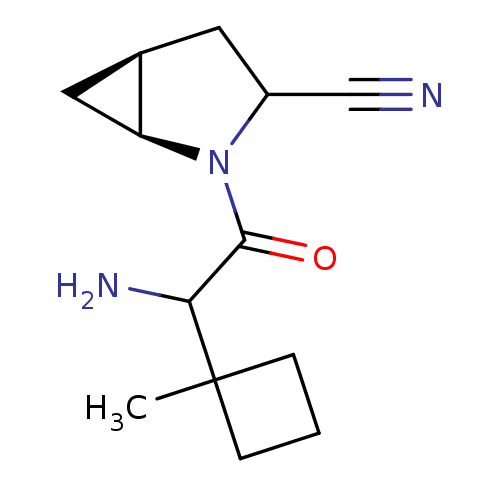 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclobutyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146007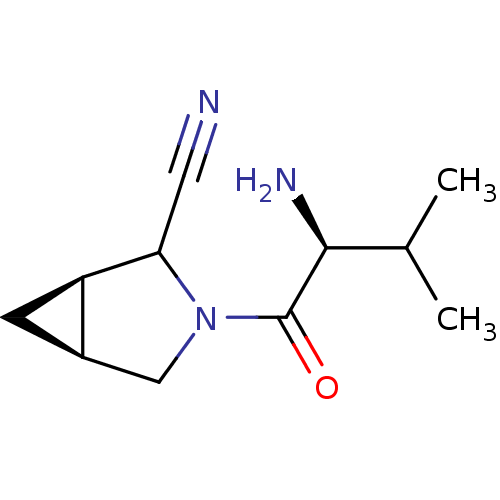 ((1R,5S)-3-((S)-2-Amino-3-methyl-butyryl)-3-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145997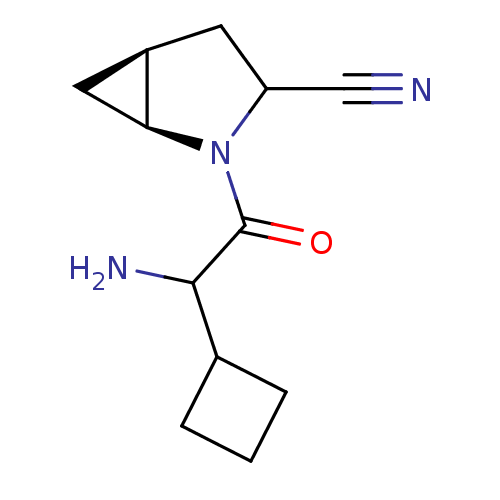 ((1S,5S)-2-(2-Amino-2-cyclobutyl-acetyl)-2-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 421 total ) | Next | Last >> |