

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition Protein kinase C (PKC) | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117342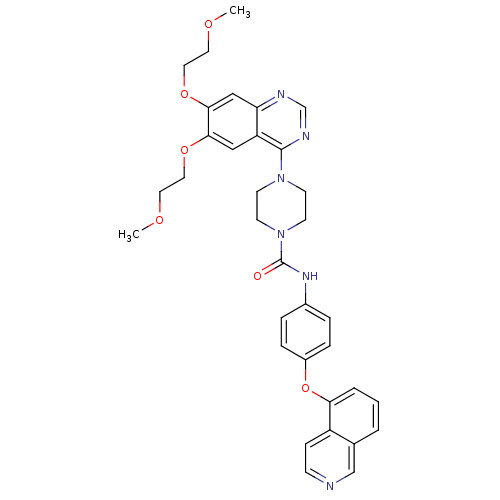 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117334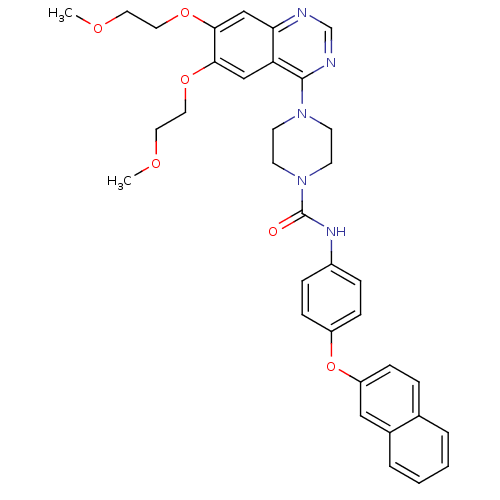 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM100350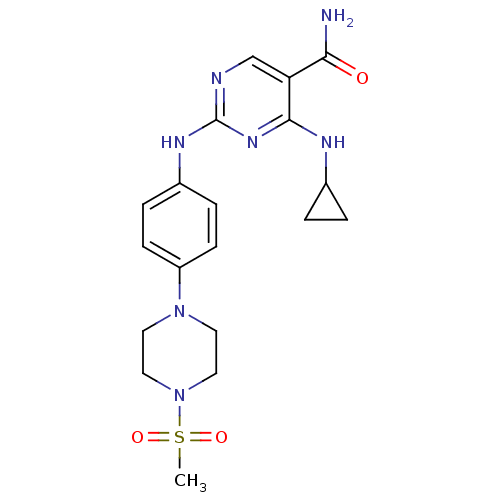 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
PORTOLA PHARMACEUTICALS, INC. US Patent | Assay Description Syk and JAK: The activity of a specified compound as an inhibitor of a JAK kinase may be assessed in vitro or in vivo. In some embodiments, the activ... | US Patent US10533001 (2020) BindingDB Entry DOI: 10.7270/Q2Z03BJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM100350 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The kinase reaction is performed in a black U-bottom 96-well microtitre plate. The final reaction volume is 50 μL and contains a final concentra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DR2ZQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM100350 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | 2.70 | n/a | n/a | n/a | n/a | n/a |
Portola Pharmaceuticals, Inc. US Patent | Assay Description ATP-site dependent competition binding assay using Ambit KinomeScan Screening kit. | US Patent US8501944 (2013) BindingDB Entry DOI: 10.7270/Q20Z71W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM290819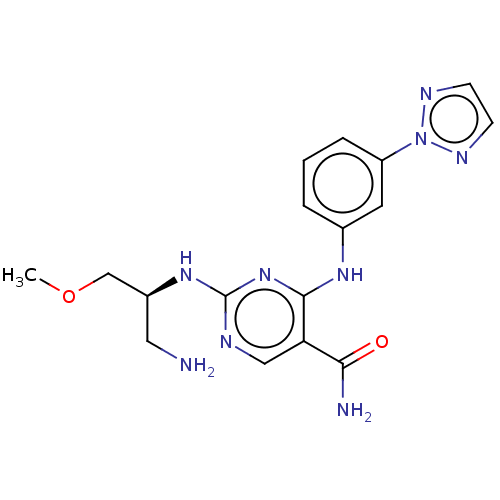 ((S)-4-(3-(2H-1,2,3-triazol-2-yl)phenylamino)-2-(1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Portola Pharmaceuticals, Inc. US Patent | Assay Description Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi... | US Patent US9579320 (2017) BindingDB Entry DOI: 10.7270/Q2K07692 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Extracellular signal-regulated kinase 2 (Erk2) | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 6 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mitogen activated protein kinase kinase 6 | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 4 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mitogen activated protein kinase kinase 4 | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117354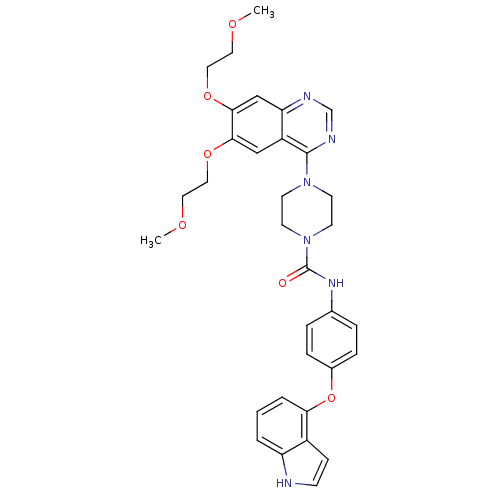 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400047 (BIIB-057 | CHEMBL2177736 | US9579320, Example 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Portola Pharmaceuticals, Inc. US Patent | Assay Description Employing the Milipore panel of purified kinases EXAMPLE 87 (IC50=1 nM) inhibited 98% of purified Syk kinase activity at 50 nM. IC50 values were dete... | US Patent US8952027 (2015) BindingDB Entry DOI: 10.7270/Q2CF9NV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143844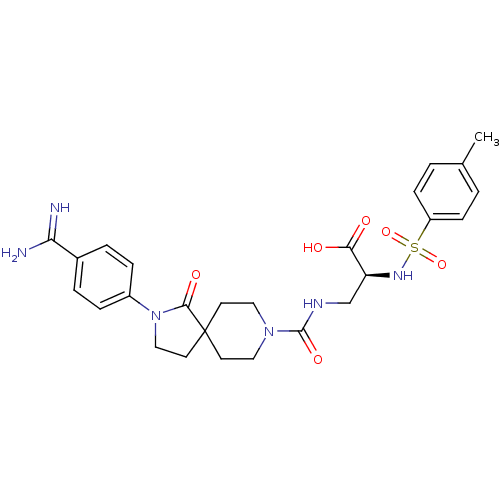 (3-{[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50092123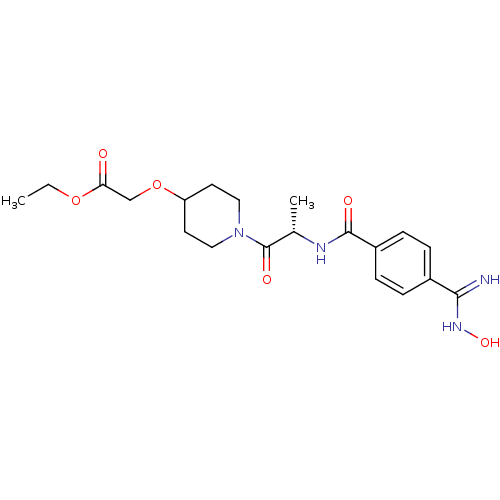 (((S)-1-{2-[4-(N-Hydroxycarbamimidoyl)-benzoylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117330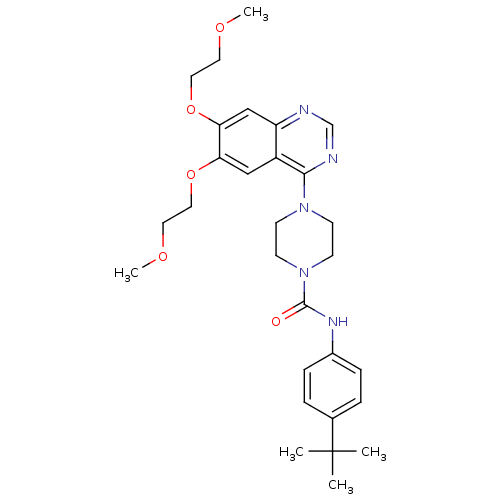 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117348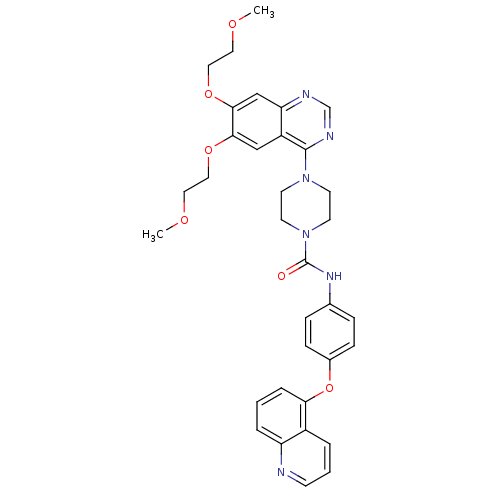 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061849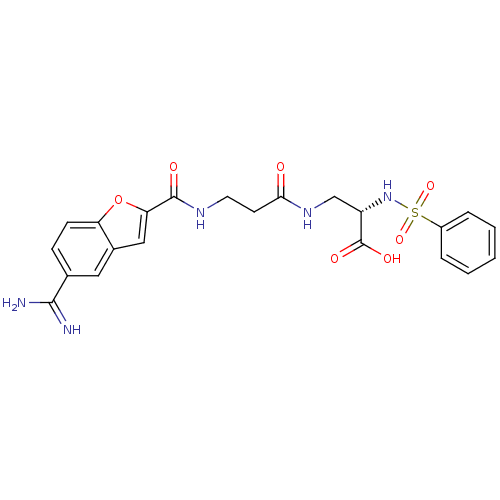 ((S)-2-Benzenesulfonylamino-3-{3-[(5-carbamimidoyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117337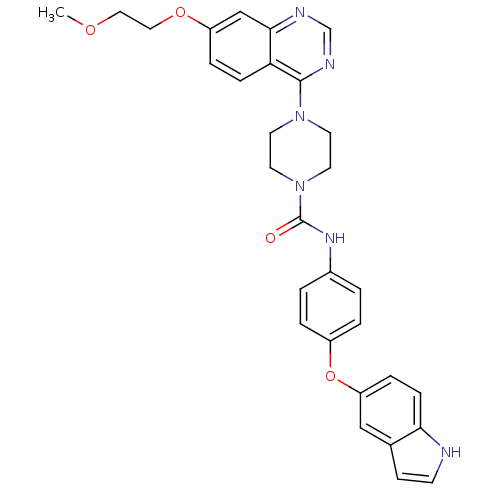 (4-[7-(2-Methoxy-ethoxy)-quinazolin-4-yl]-piperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061847 ((S)-2-(Butane-1-sulfonylamino)-3-{3-[(5-carbamimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143865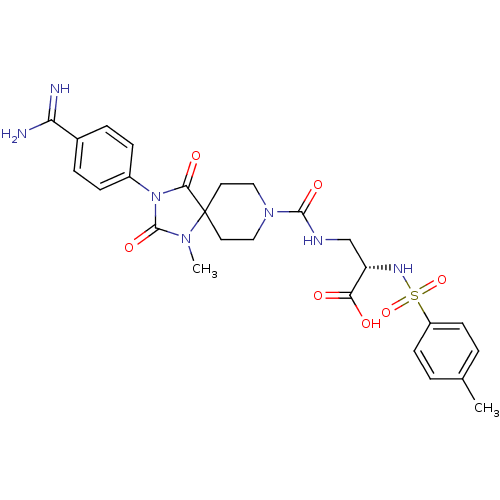 ((S)-3-{[3-(4-Carbamimidoyl-phenyl)-1-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061839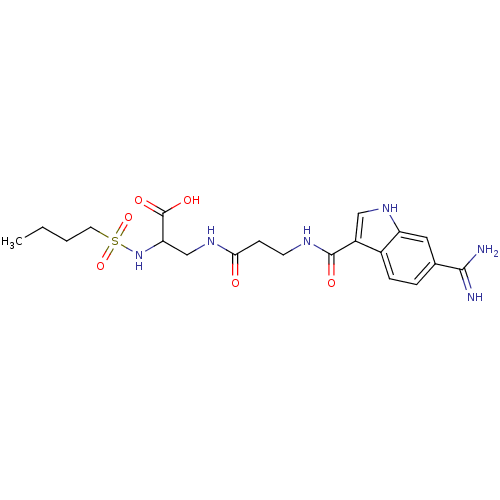 (2-(Butane-1-sulfonylamino)-3-{3-[(6-carbamimidoyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061840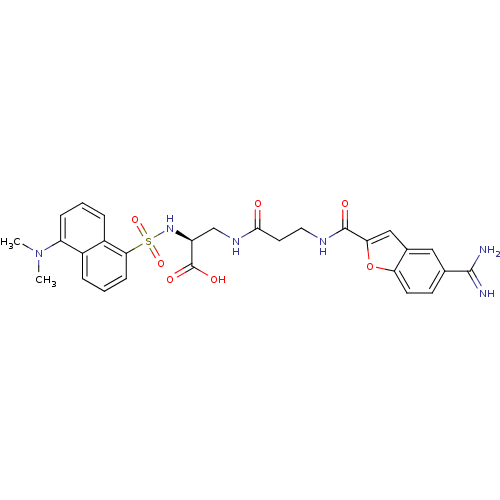 ((S)-3-{3-[(5-Carbamimidoyl-benzofuran-2-carbonyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061859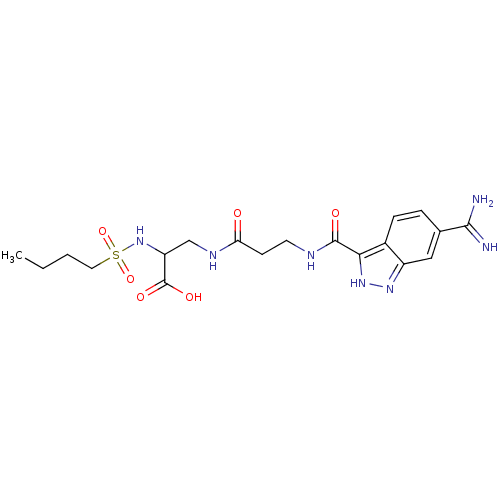 (2-(Butane-1-sulfonylamino)-3-{3-[(6-carbamimidoyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50061862 ((S)-3-{3-[(5-Carbamimidoyl-benzofuran-2-carbonyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of fibrinogen binding to purified immobilized glycoprotein (fibrinogen receptor). | J Med Chem 40: 4308-18 (1998) Article DOI: 10.1021/jm9704863 BindingDB Entry DOI: 10.7270/Q2HM57KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50111398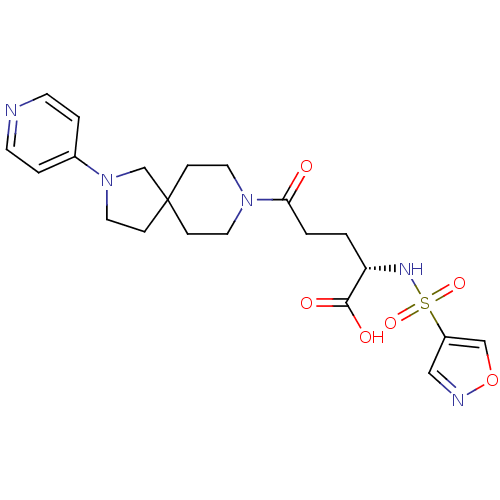 ((S)-2-(Isoxazole-4-sulfonylamino)-5-oxo-5-(2-pyrid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description In vitro activity to block the binding of fibrinogen to purified human fibrinogen receptor. | Bioorg Med Chem Lett 12: 1103-7 (2002) BindingDB Entry DOI: 10.7270/Q2H131BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50092109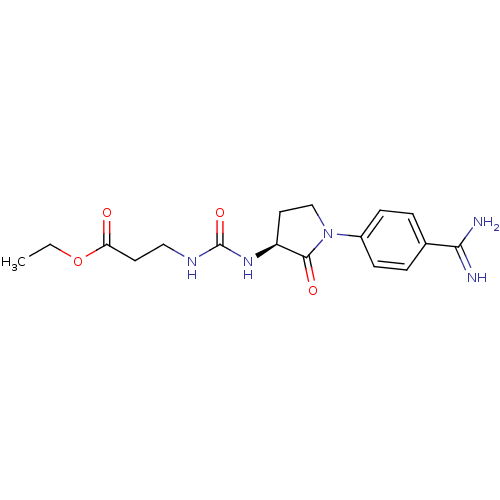 (3-{(S)-3-[1-(4-Carbamimidoyl-phenyl)-2-oxo-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117335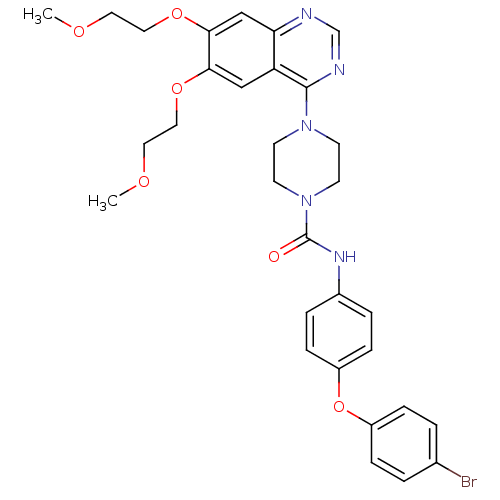 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM100350 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The kinase reaction is performed in a black U-bottom 96-well microtitre plate. The final reaction volume is 50 μL and contains a final concentra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DR2ZQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM100350 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PORTOLA PHARMACEUTICALS, INC. US Patent | Assay Description Syk and JAK: The activity of a specified compound as an inhibitor of a JAK kinase may be assessed in vitro or in vivo. In some embodiments, the activ... | US Patent US10533001 (2020) BindingDB Entry DOI: 10.7270/Q2Z03BJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM100350 (US10533001, Example 99 | US11414410, Example 99 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | 5.10 | n/a | n/a | n/a | n/a | n/a |
Portola Pharmaceuticals, Inc. US Patent | Assay Description ATP-site dependent competition binding assay using Ambit KinomeScan Screening kit. | US Patent US8501944 (2013) BindingDB Entry DOI: 10.7270/Q20Z71W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143842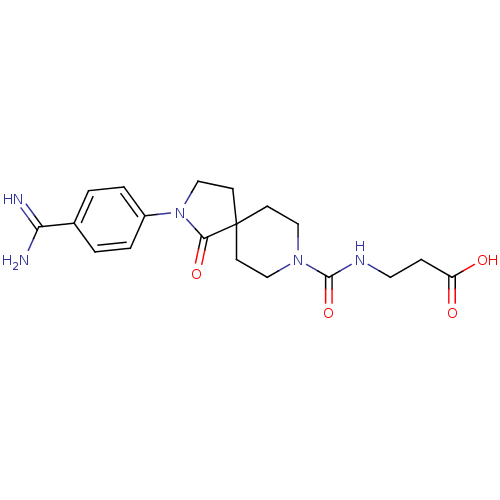 (3-{[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143872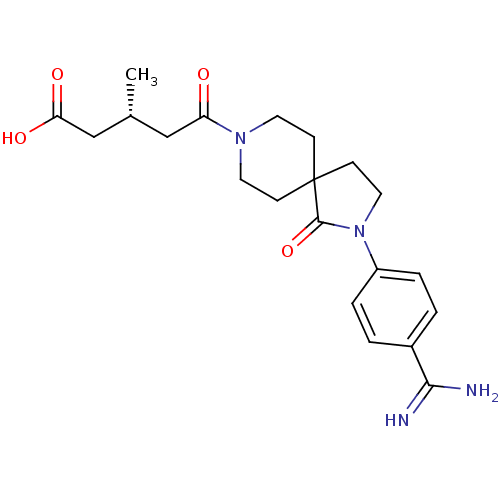 ((R)-5-[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117314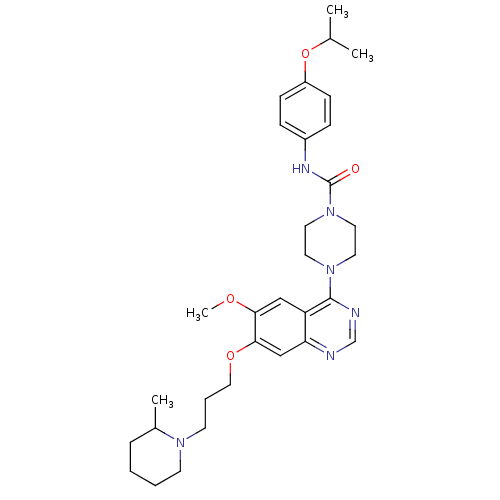 (4-{6-Methoxy-7-[3-(2-methyl-piperidin-1-yl)-propox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117312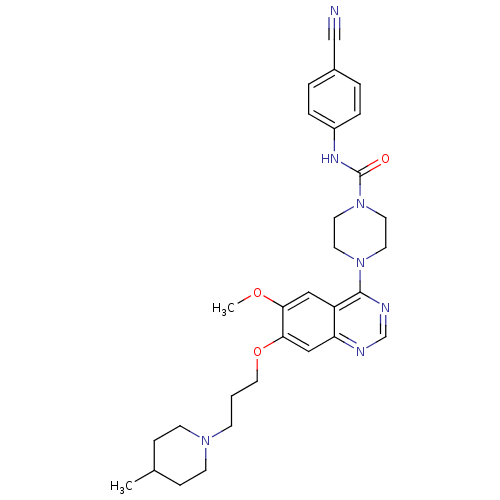 (4-{6-Methoxy-7-[3-(4-methyl-piperidin-1-yl)-propox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117326 (4-{6-Methoxy-7-[3-(2-methyl-piperidin-1-yl)-propox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117311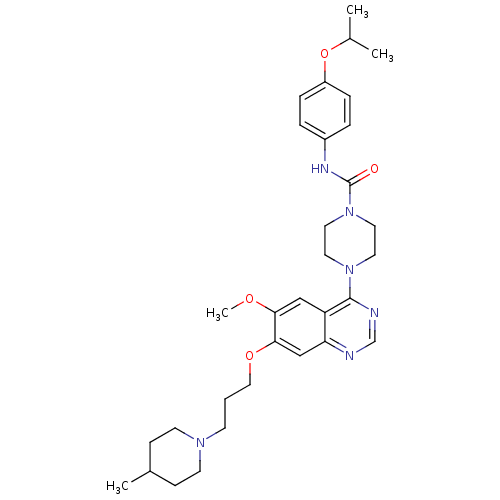 (4-{6-Methoxy-7-[3-(4-methyl-piperidin-1-yl)-propox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143849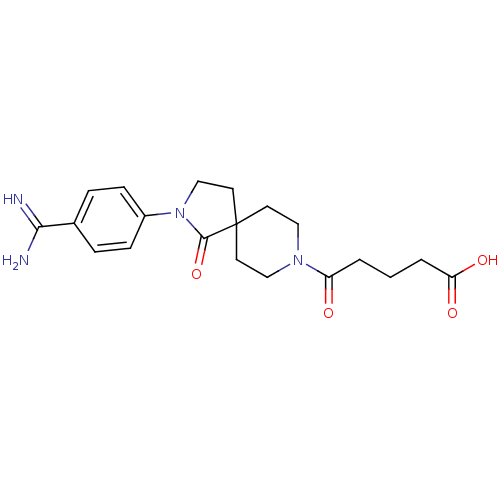 (5-[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spir...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50396073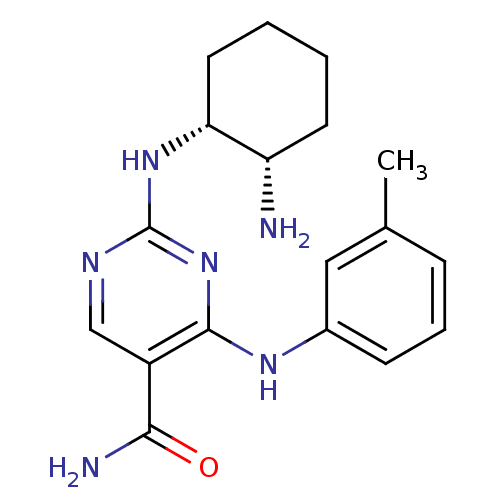 (CHEMBL1235110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Portola Pharmaceuticals, Inc. US Patent | Assay Description SYK tyrosine phosphorylation activity is measured using the LANCE Technology developed by Perkin Elmer Life and Analytical Sciences (Boston, Mass.). ... | US Patent US8952027 (2015) BindingDB Entry DOI: 10.7270/Q2CF9NV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50111419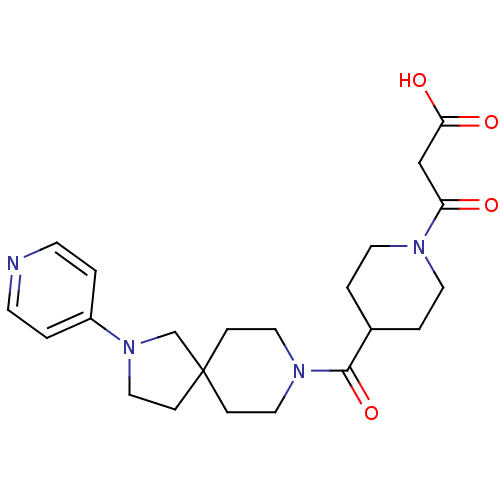 (3-Oxo-3-[4-(2-pyridin-4-yl-2,8-diaza-spiro[4.5]dec...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc. Curated by ChEMBL | Assay Description In vitro activity to block the binding of fibrinogen to purified human fibrinogen receptor. | Bioorg Med Chem Lett 12: 1103-7 (2002) BindingDB Entry DOI: 10.7270/Q2H131BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143866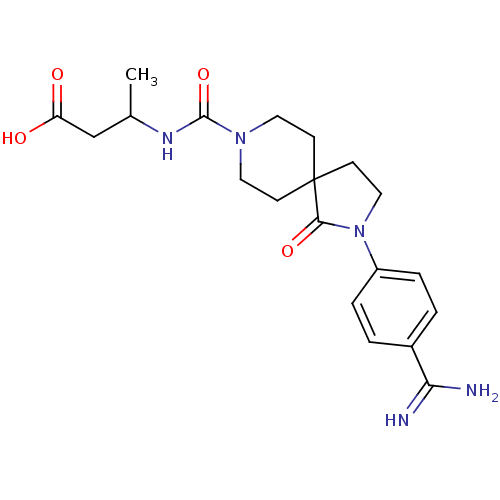 (3-{[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143842 (3-{[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50117312 (4-{6-Methoxy-7-[3-(4-methyl-piperidin-1-yl)-propox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of chimeric PDGF receptor with c-kit cytoplasmic domain phosphorylation in CHO cells | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143840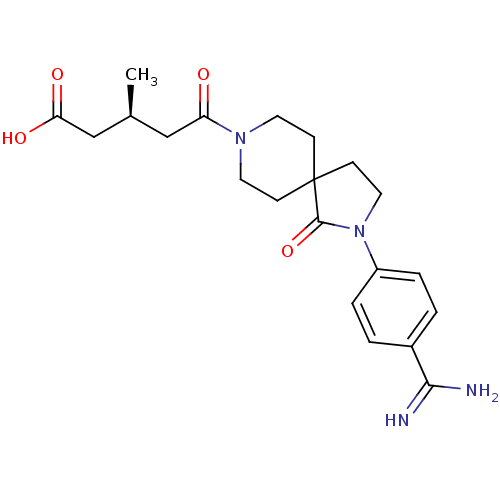 ((S)-5-[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143874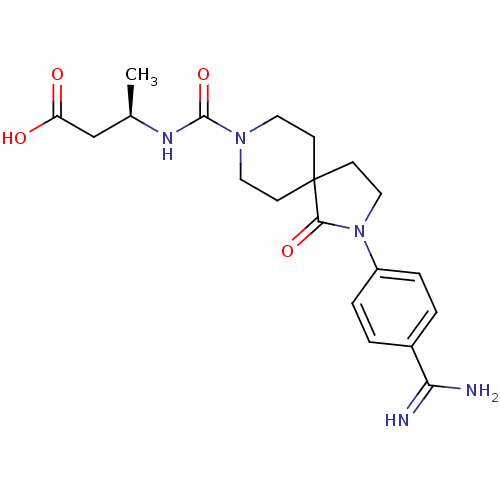 ((R)-3-{[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143850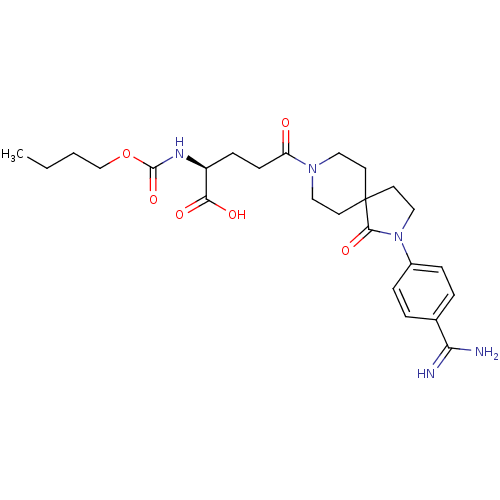 (2-Butoxycarbonylamino-5-[2-(4-carbamimidoyl-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50143864 (5-[2-(4-Carbamimidoyl-phenyl)-1-oxo-2,8-diaza-spir...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Tested for inhibition of the binding of fibrinogen to purified human GPIIb-IIIa in a 96-well format (ELISA assay) | J Med Chem 47: 2037-61 (2004) Article DOI: 10.1021/jm030354b BindingDB Entry DOI: 10.7270/Q2FQ9W1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM290809 (2-((1R,2S)-2-aminocyclohexylamino)-4-(6-methoxynap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Portola Pharmaceuticals, Inc. US Patent | Assay Description Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi... | US Patent US9579320 (2017) BindingDB Entry DOI: 10.7270/Q2K07692 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM290831 (2-((1R,2S)-2-aminocyclohexylamino)-4-(1-ethyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Portola Pharmaceuticals, Inc. US Patent | Assay Description Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi... | US Patent US9579320 (2017) BindingDB Entry DOI: 10.7270/Q2K07692 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM291025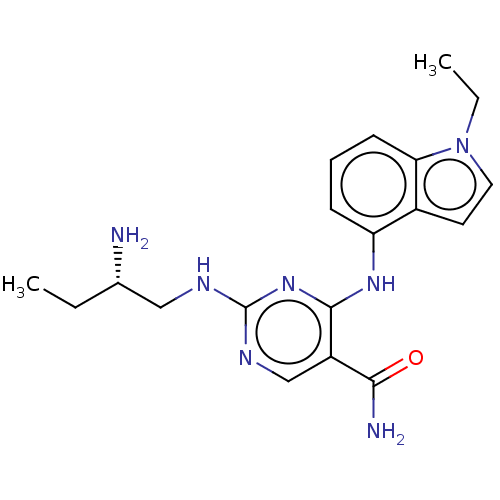 ((S)-2-(2-aminobutylamino)-4-(1-ethyl-1H-indol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Portola Pharmaceuticals, Inc. US Patent | Assay Description Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi... | US Patent US9579320 (2017) BindingDB Entry DOI: 10.7270/Q2K07692 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM365380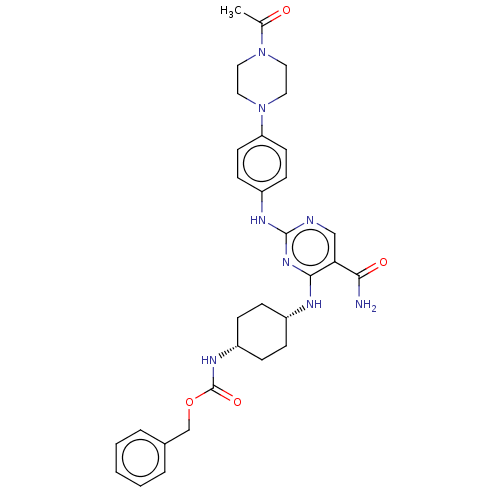 (US10533001, Example 41 | US11414410, Example 41 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The kinase reaction is performed in a black U-bottom 96-well microtitre plate. The final reaction volume is 50 μL and contains a final concentra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DR2ZQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1512 total ) | Next | Last >> |