

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Competitive inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate Linewea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Noncompetitive inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogeni... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444908 (CHEMBL3099624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Competitive inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate Linewea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444908 (CHEMBL3099624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Noncompetitive inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogeni... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444888 (CHEMBL3099621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Competitive inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate Linewea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444888 (CHEMBL3099621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Noncompetitive inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogeni... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069985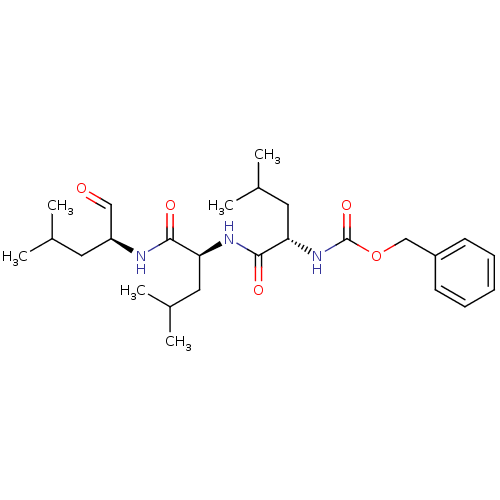 ((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 26S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50069985 ((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50432548 (LACTACYSTIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50324535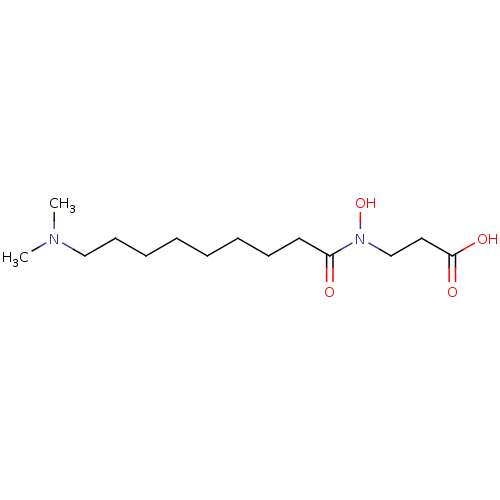 (3-{[9-(Dimethylamino)nonanoyl](hydroxy)amino}propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of KDM2A (unknown origin) | J Med Chem 56: 7222-31 (2013) Article DOI: 10.1021/jm400624b BindingDB Entry DOI: 10.7270/Q2SB475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50458013 (CHEMBL4215864) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome using Boc-LRR-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444899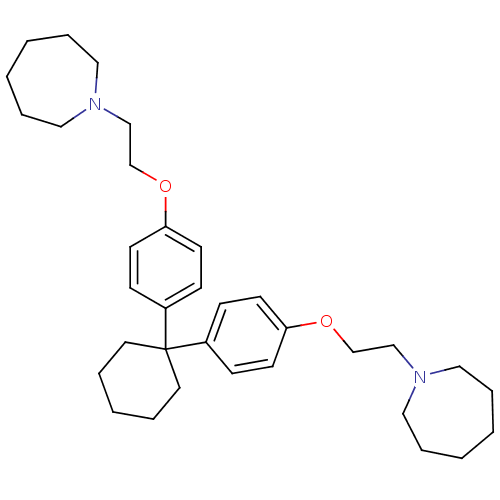 (CHEMBL3099640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50458009 (CHEMBL4205518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444904 (CHEMBL3099622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50121213 (CHEMBL3622371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University Curated by ChEMBL | Assay Description Inhibition of human LSD1 using H3K4 peptide as substrate by peroxidase-coupled assay | Bioorg Med Chem Lett 26: 1193-5 (2016) Article DOI: 10.1016/j.bmcl.2016.01.036 BindingDB Entry DOI: 10.7270/Q2833TWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444889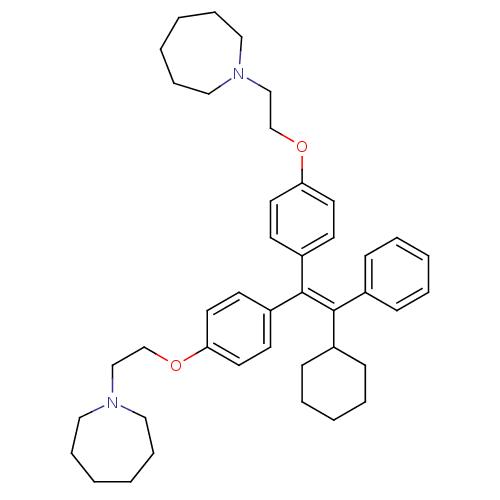 (CHEMBL3099620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50458008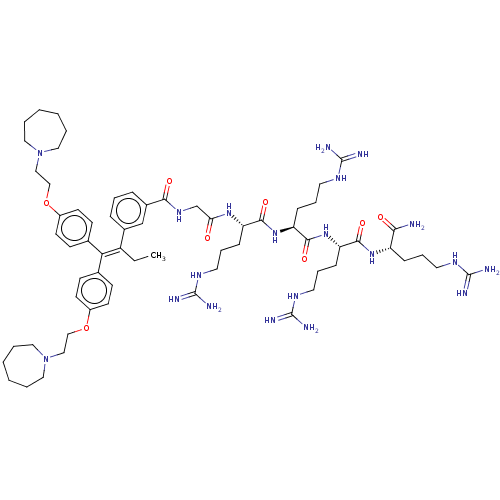 (CHEMBL4204198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome using Boc-LRR-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444907 (CHEMBL3099623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50458009 (CHEMBL4205518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444908 (CHEMBL3099624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444888 (CHEMBL3099621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50140044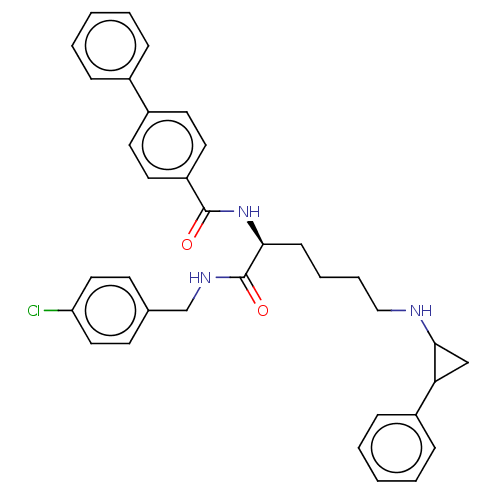 (CHEMBL3764353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University Curated by ChEMBL | Assay Description Inhibition of human LSD1 using H3K4 peptide as substrate by peroxidase-coupled assay | Bioorg Med Chem Lett 26: 1193-5 (2016) Article DOI: 10.1016/j.bmcl.2016.01.036 BindingDB Entry DOI: 10.7270/Q2833TWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50140043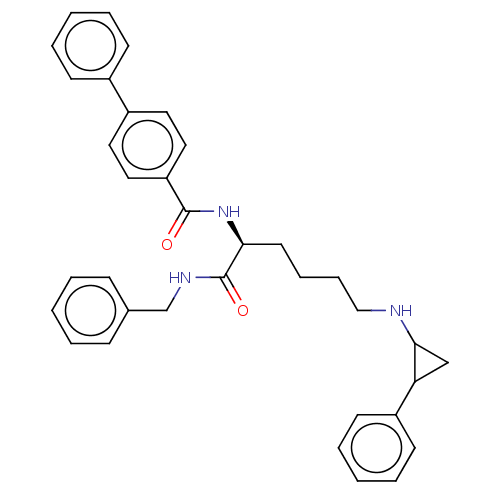 (CHEMBL3765529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University Curated by ChEMBL | Assay Description Inhibition of human LSD1 using H3K4 peptide as substrate by peroxidase-coupled assay | Bioorg Med Chem Lett 26: 1193-5 (2016) Article DOI: 10.1016/j.bmcl.2016.01.036 BindingDB Entry DOI: 10.7270/Q2833TWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444908 (CHEMBL3099624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50140045 (CHEMBL3764836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University Curated by ChEMBL | Assay Description Inhibition of human LSD1 using H3K4 peptide as substrate by peroxidase-coupled assay | Bioorg Med Chem Lett 26: 1193-5 (2016) Article DOI: 10.1016/j.bmcl.2016.01.036 BindingDB Entry DOI: 10.7270/Q2833TWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444906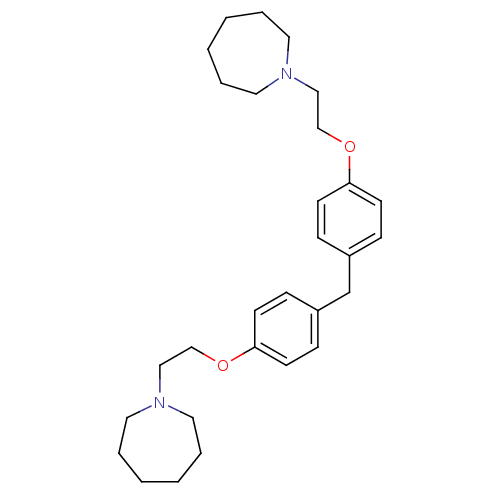 (CHEMBL3099636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444906 (CHEMBL3099636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444908 (CHEMBL3099624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444909 (CHEMBL3099618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444901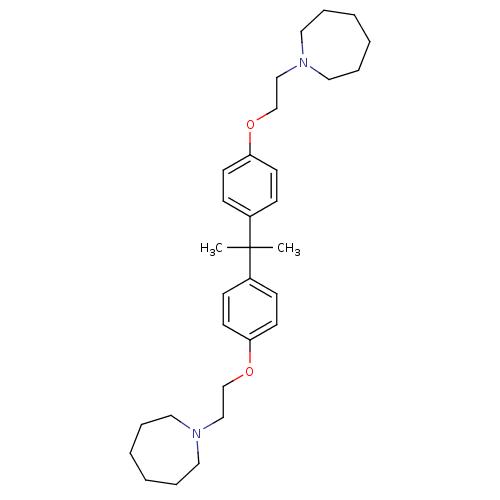 (CHEMBL3099638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444901 (CHEMBL3099638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444885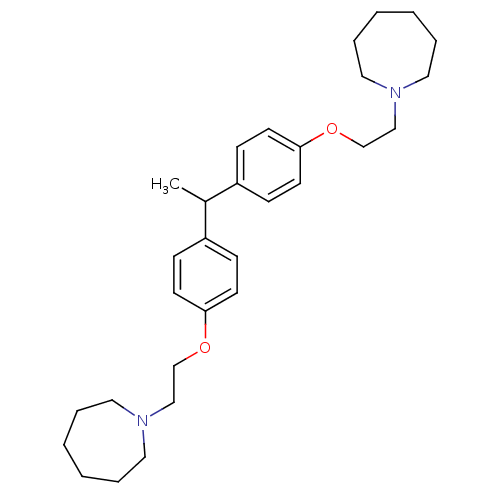 (CHEMBL3099637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4A (Homo sapiens (Human)) | BDBM50324535 (3-{[9-(Dimethylamino)nonanoyl](hydroxy)amino}propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of KDM4A (unknown origin) using H3K9me3 peptide and 2-oxoglutarate as substrate after 1 hr by FDH-coupled assay | J Med Chem 56: 7222-31 (2013) Article DOI: 10.1021/jm400624b BindingDB Entry DOI: 10.7270/Q2SB475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444885 (CHEMBL3099637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444903 (CHEMBL3099631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444907 (CHEMBL3099623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50444905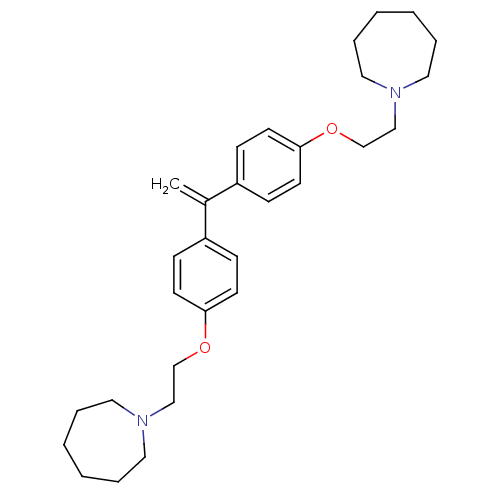 (CHEMBL3099635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit assessed as hydrolysis of Boc-LRR-AMC fluorogenic substrate measured for 1... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50458007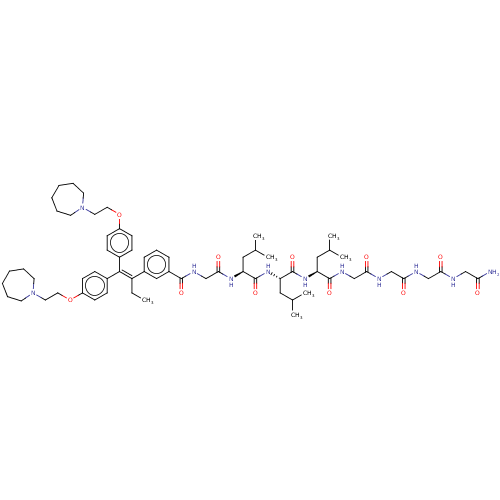 (CHEMBL4209883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50458014 (CHEMBL4206039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444898 (CHEMBL3099625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50458004 (CHEMBL4210397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50458014 (CHEMBL4206039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagahama Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH activity of human 20S proteasome using Z-LLE-MCA as substrate measured for 1 hr by fluorescence assay | Eur J Med Chem 146: 636-650 (2018) Article DOI: 10.1016/j.ejmech.2018.01.045 BindingDB Entry DOI: 10.7270/Q2DF6TV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50444907 (CHEMBL3099623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit assessed as hydrolysis of succinyl-LLVY-AMC fluorogenic substrate mea... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50444905 (CHEMBL3099635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bio-Science and Technology Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of human 20S proteasome beta 1 subunit assessed as hydrolysis of Z-LLE-AMC fluorogenic substrate measured for 1 hr b... | Eur J Med Chem 71: 290-305 (2014) Article DOI: 10.1016/j.ejmech.2013.11.009 BindingDB Entry DOI: 10.7270/Q2222W81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |