

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186746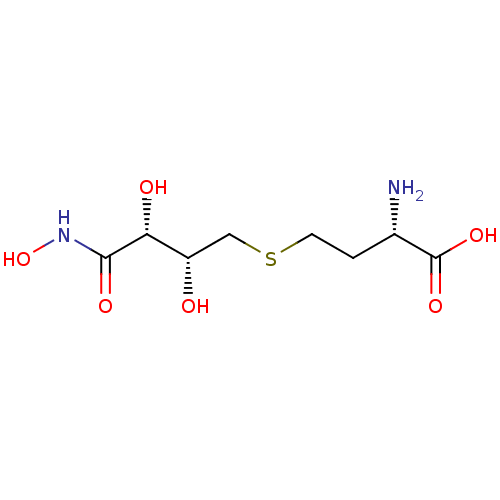 ((2S)-2-AMINO-4-[(2R,3R)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186746 ((2S)-2-AMINO-4-[(2R,3R)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of ferrous substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186747 ((2S)-2-AMINO-4-[(2R,3S)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of ferrous substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186747 ((2S)-2-AMINO-4-[(2R,3S)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Escherichia coli (strain K12)) | BDBM50186747 ((2S)-2-AMINO-4-[(2R,3S)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Escherichia coli LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Vibrio harveyi (strain ATCC BAA-1116 / BB120)) | BDBM50186747 ((2S)-2-AMINO-4-[(2R,3S)-2,3-DIHYDROXY-3-N-HYDROXYC...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Vibrio harveyi LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186746 ((2S)-2-AMINO-4-[(2R,3R)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of zinc substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Escherichia coli (strain K12)) | BDBM50186746 ((2S)-2-AMINO-4-[(2R,3R)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Escherichia coli LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Vibrio harveyi (strain ATCC BAA-1116 / BB120)) | BDBM50186746 ((2S)-2-AMINO-4-[(2R,3R)-2,3-DIHYDROXY-3-N-HYDROXYC...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Vibrio harveyi LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186747 ((2S)-2-AMINO-4-[(2R,3S)-2,3-DIHYDROXY-3-N-HYDROXYC...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of zinc substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50142500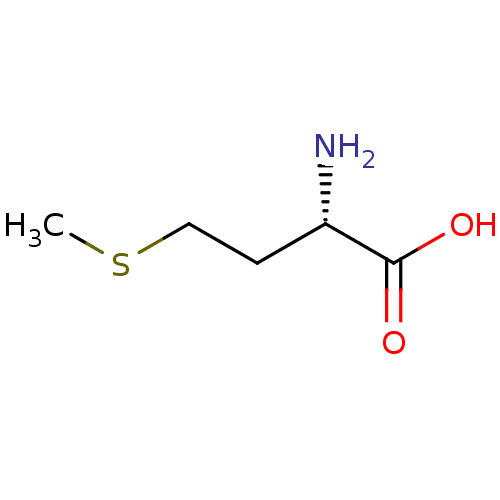 ((2S)-2-amino-4-(methylsulfanyl)butanoic acid | (S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186742 ((2S)-2-amino-6-(N-formyl-N-hydroxylamino)hexanoic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186743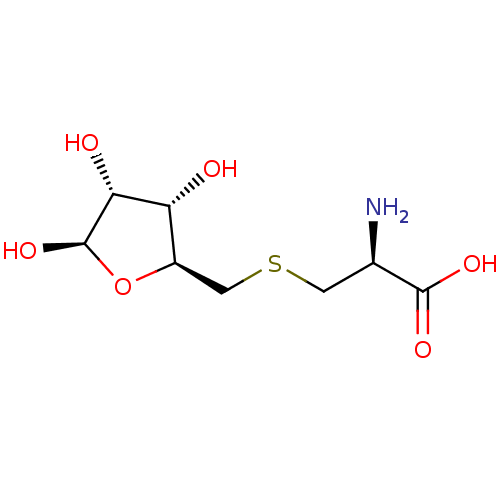 (CHEMBL383729 | S-ribosylcysteine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186748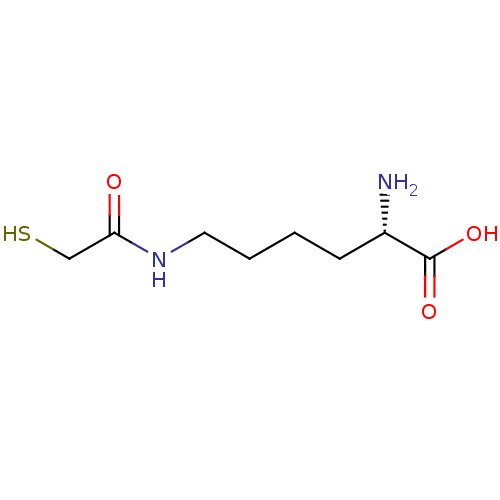 ((S)-2-amino-6-(2-mercaptoacetamido)hexanoic acid |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50148771 ((2R,3R)-N,2,3,4-TETRAHYDROXYBUTANAMIDE | (2R,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of ferrous substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186744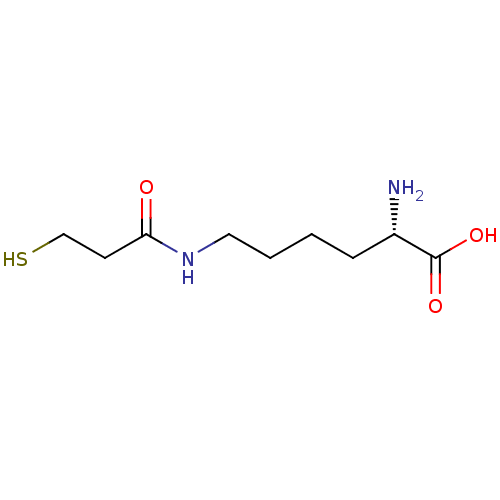 ((S)-2-amino-6-(3-mercaptopropanamido)hexanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50148771 ((2R,3R)-N,2,3,4-TETRAHYDROXYBUTANAMIDE | (2R,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50186745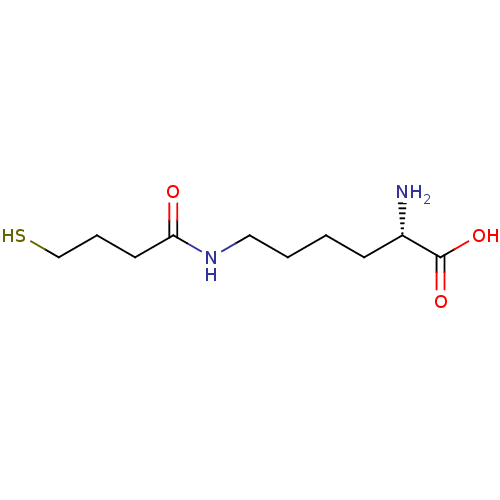 ((S)-2-amino-6-(4-mercaptobutanamido)hexanoic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 4.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Vibrio harveyi (strain ATCC BAA-1116 / BB120)) | BDBM50148771 ((2R,3R)-N,2,3,4-TETRAHYDROXYBUTANAMIDE | (2R,4R)-2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Vibrio harveyi LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Escherichia coli (strain K12)) | BDBM50148771 ((2R,3R)-N,2,3,4-TETRAHYDROXYBUTANAMIDE | (2R,4R)-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 7.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of cobalt substituted Escherichia coli LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-ribosylhomocysteine lyase (Bacillus subtilis) | BDBM50148771 ((2R,3R)-N,2,3,4-TETRAHYDROXYBUTANAMIDE | (2R,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of zinc substituted Bacillus subtilis LuxS | J Med Chem 49: 3003-11 (2006) Article DOI: 10.1021/jm060047g BindingDB Entry DOI: 10.7270/Q25X28JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Campus Curated by ChEMBL | Assay Description Inhibition of PDE4D (unknown origin) | Eur J Med Chem 174: 198-215 (2019) Article DOI: 10.1016/j.ejmech.2019.04.020 BindingDB Entry DOI: 10.7270/Q2G44TQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267826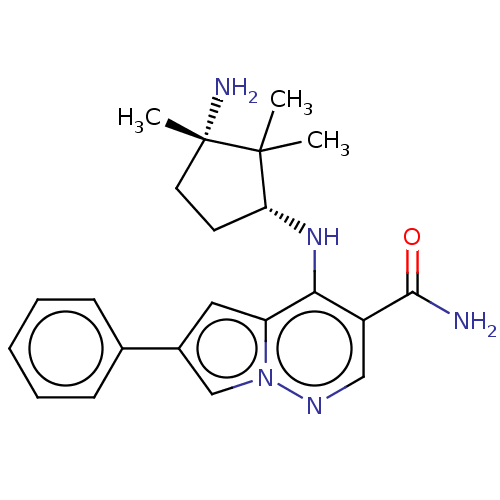 (CHEMBL4096145) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267827 (CHEMBL4076794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267796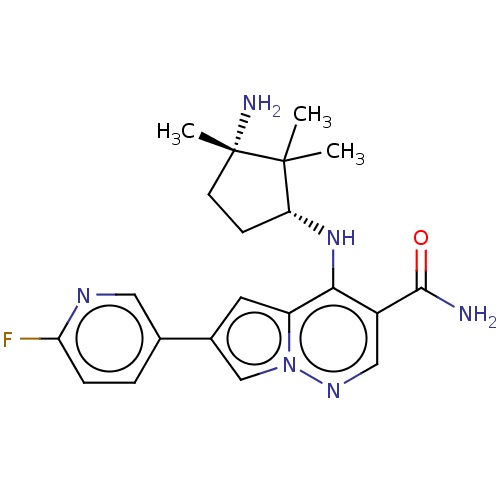 (CHEMBL4098840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267804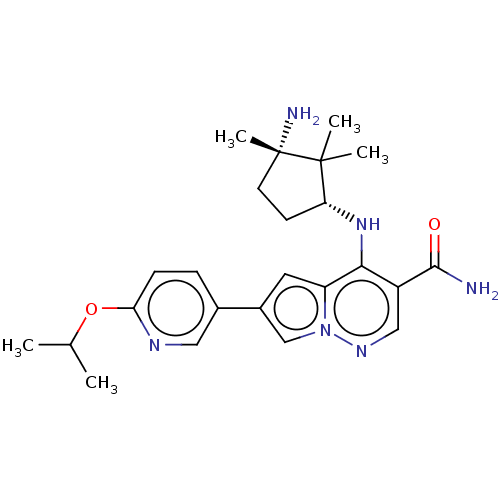 (CHEMBL4070136) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267806 (CHEMBL4071255) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141473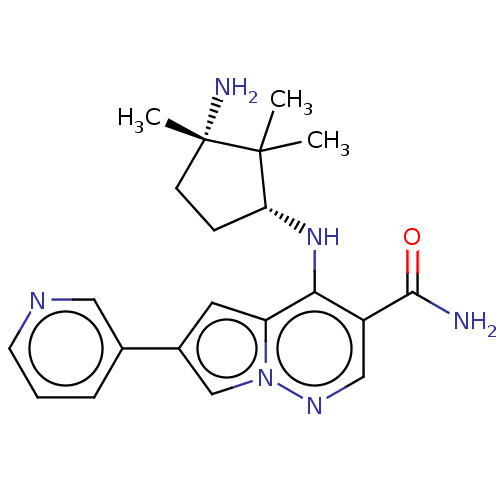 (US8921368, 192) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267852 (CHEMBL4073562) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267828 (CHEMBL4078773) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267852 (CHEMBL4073562) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM141473 (US8921368, 192) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267807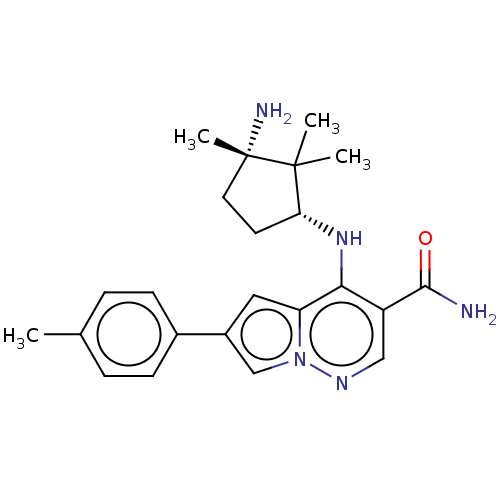 (CHEMBL4060536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267810 (CHEMBL4068856) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267807 (CHEMBL4060536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267836 (CHEMBL4105015) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267840 (CHEMBL4062062) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267837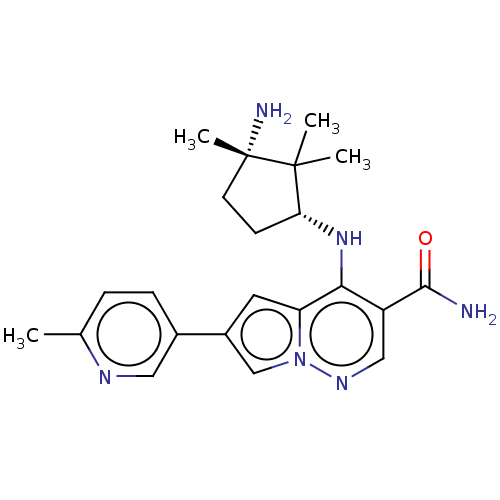 (CHEMBL4060444) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267797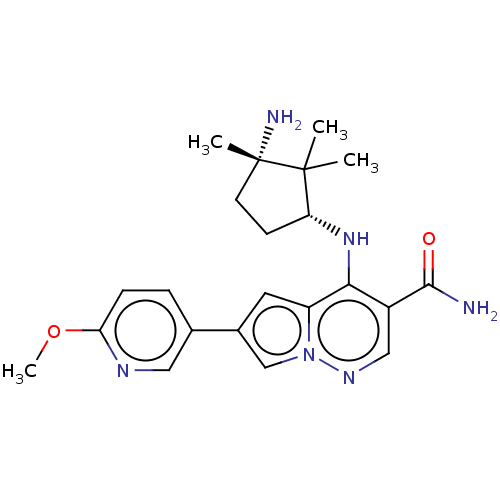 (CHEMBL4080797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267808 (CHEMBL4087655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267840 (CHEMBL4062062) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267808 (CHEMBL4087655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267848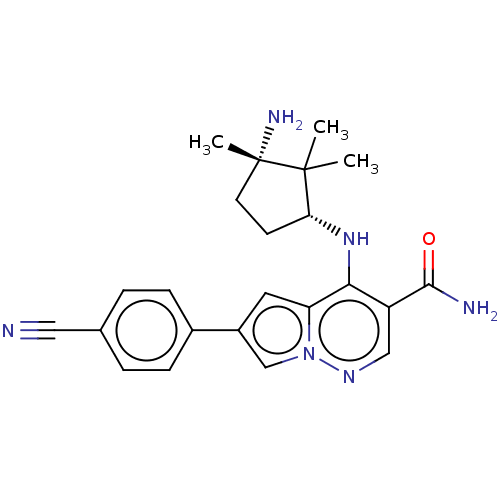 (CHEMBL4091582) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267826 (CHEMBL4096145) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267827 (CHEMBL4076794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28028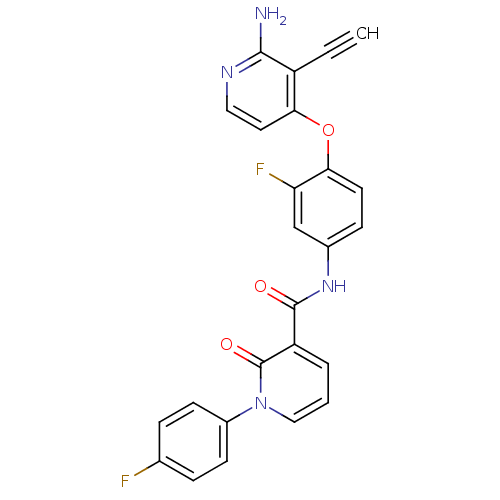 (2-aminopyridine analogue, 7 | N-{4-[(2-amino-3-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... | J Med Chem 52: 1251-4 (2009) Article DOI: 10.1021/jm801586s BindingDB Entry DOI: 10.7270/Q20863MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28030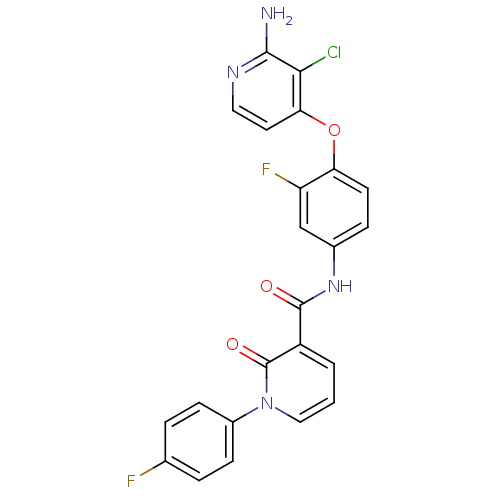 (2-aminopyridine analogue, 9 | N-{4-[(2-amino-3-chl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... | J Med Chem 52: 1251-4 (2009) Article DOI: 10.1021/jm801586s BindingDB Entry DOI: 10.7270/Q20863MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267839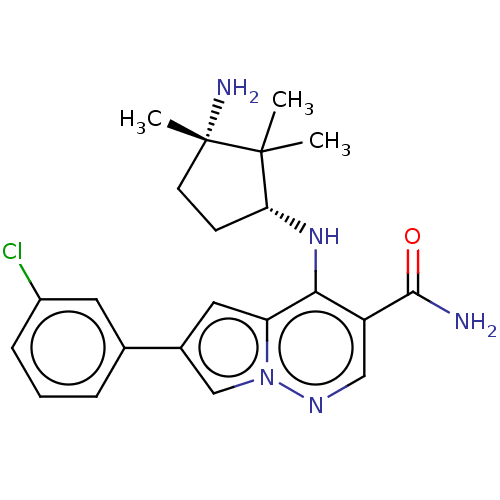 (CHEMBL4076823) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267848 (CHEMBL4091582) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331029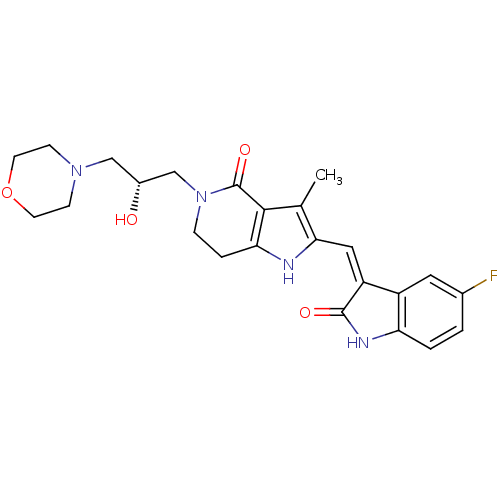 ((R,Z)-2-(5-Fluoro-2-oxo-1,2-dihydro-indol-3-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 after 30 mins by ELISA | J Med Chem 53: 8140-9 (2010) Article DOI: 10.1021/jm101036c BindingDB Entry DOI: 10.7270/Q2BV7GWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 771 total ) | Next | Last >> |