 Found 1769 hits with Last Name = 'skerratt' and Initial = 'se'
Found 1769 hits with Last Name = 'skerratt' and Initial = 'se' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789
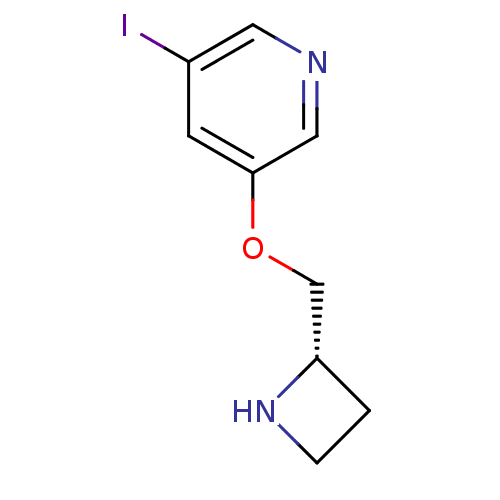
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain membrane |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR in rat cortex |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50067424
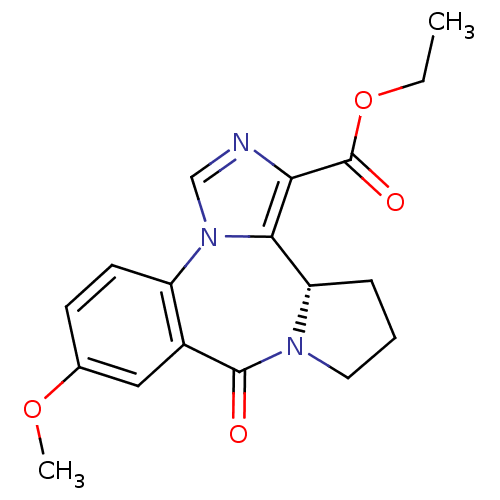
((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50142570
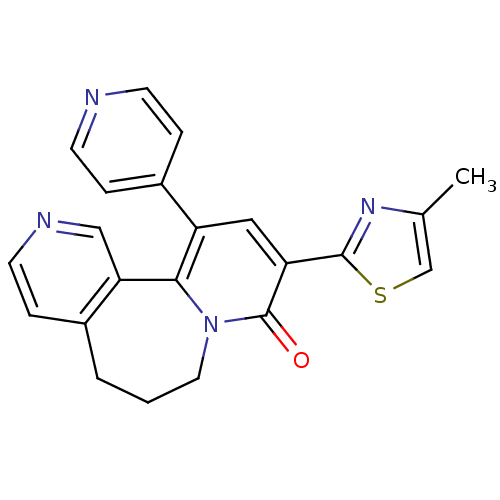
(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50179998
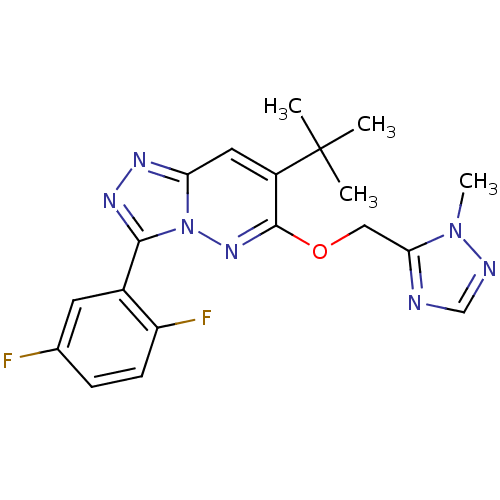
(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM186364
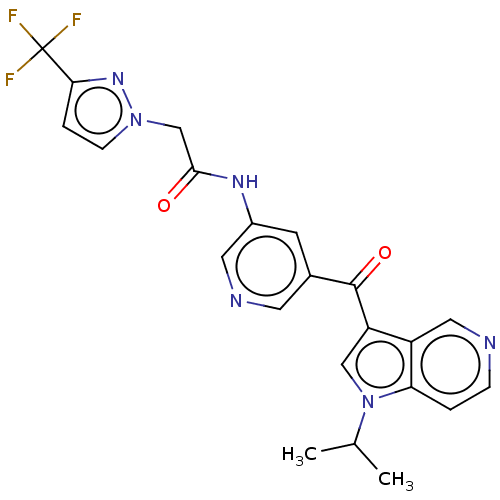
(US9163021, 21)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C(F)(F)F)c2)c2cnccc12 Show InChI InChI=1S/C22H19F3N6O2/c1-13(2)31-11-17(16-10-26-5-3-18(16)31)21(33)14-7-15(9-27-8-14)28-20(32)12-30-6-4-19(29-30)22(23,24)25/h3-11,13H,12H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM186369
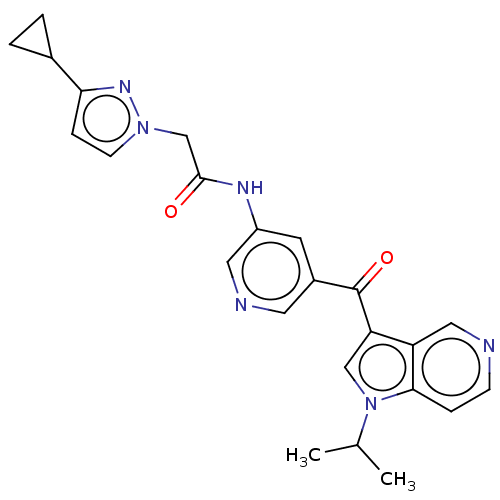
(US9163021, 26)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C3CC3)c2)c2cnccc12 Show InChI InChI=1S/C24H24N6O2/c1-15(2)30-13-20(19-12-25-7-5-22(19)30)24(32)17-9-18(11-26-10-17)27-23(31)14-29-8-6-21(28-29)16-3-4-16/h5-13,15-16H,3-4,14H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550245
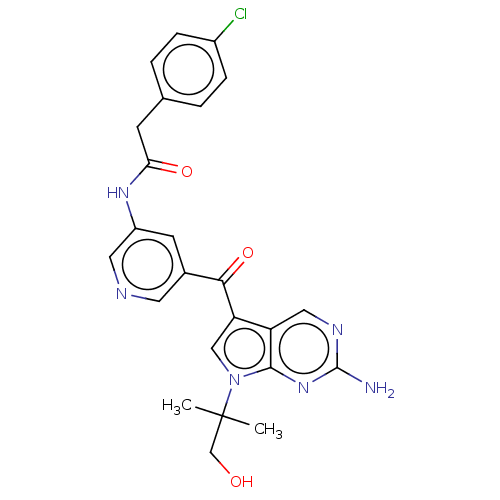
(CHEMBL4748276)Show SMILES CC(C)(CO)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2cnc(N)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| >4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM134606

(US8846698, 69)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2cncnc12 Show InChI InChI=1S/C23H20ClN5O2/c1-14(2)29-12-20(19-11-26-13-27-23(19)29)22(31)16-8-18(10-25-9-16)28-21(30)7-15-3-5-17(24)6-4-15/h3-6,8-14H,7H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM134608
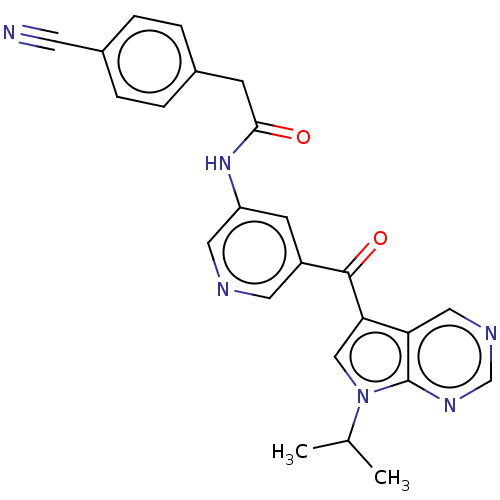
(US8846698, 71)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(cc3)C#N)c2)c2cncnc12 Show InChI InChI=1S/C24H20N6O2/c1-15(2)30-13-21(20-12-27-14-28-24(20)30)23(32)18-8-19(11-26-10-18)29-22(31)7-16-3-5-17(9-25)6-4-16/h3-6,8,10-15H,7H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550242
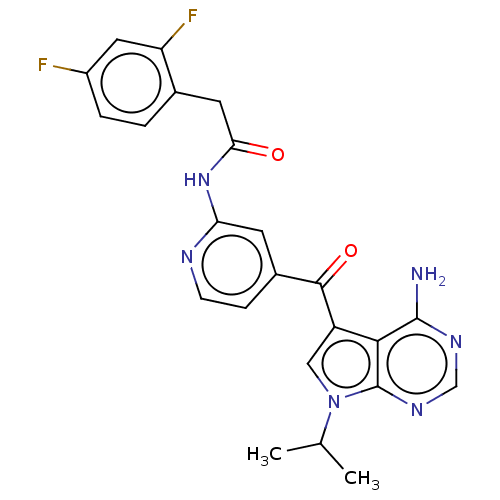
(CHEMBL4748010)Show SMILES CC(C)n1cc(C(=O)c2ccnc(NC(=O)Cc3ccc(F)cc3F)c2)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550244
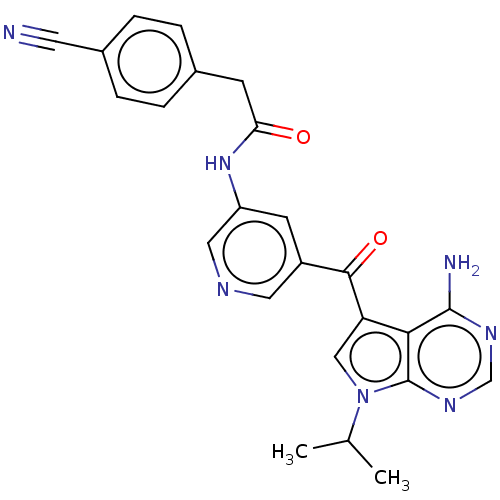
(CHEMBL4794903)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(cc3)C#N)c2)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550243
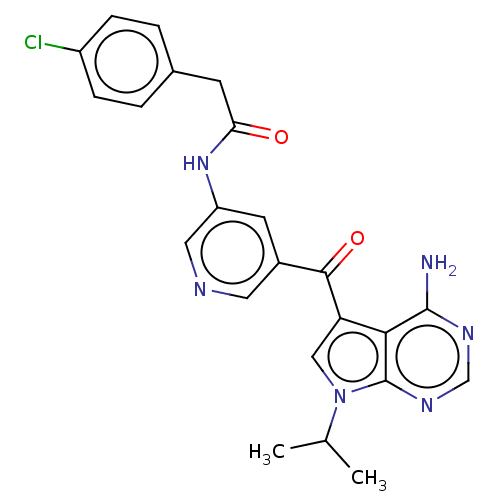
(CHEMBL4790022)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM186432
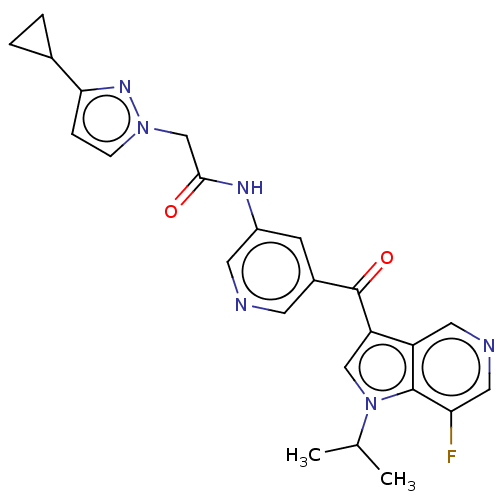
(US9163021, 90)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C3CC3)c2)c2cncc(F)c12 Show InChI InChI=1S/C24H23FN6O2/c1-14(2)31-12-19(18-10-27-11-20(25)23(18)31)24(33)16-7-17(9-26-8-16)28-22(32)13-30-6-5-21(29-30)15-3-4-15/h5-12,14-15H,3-4,13H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM134627

(US8846698, 90)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C3CC3)c2)c2cncnc12 Show InChI InChI=1S/C23H23N7O2/c1-14(2)30-11-19(18-10-25-13-26-23(18)30)22(32)16-7-17(9-24-8-16)27-21(31)12-29-6-5-20(28-29)15-3-4-15/h5-11,13-15H,3-4,12H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM134787
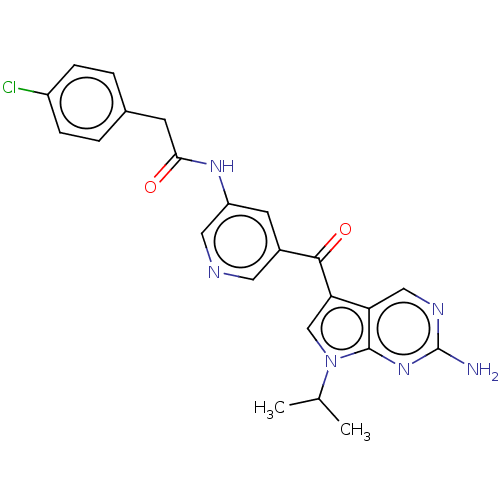
(US8846698, 250)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2cnc(N)nc12 Show InChI InChI=1S/C23H21ClN6O2/c1-13(2)30-12-19(18-11-27-23(25)29-22(18)30)21(32)15-8-17(10-26-9-15)28-20(31)7-14-3-5-16(24)6-4-14/h3-6,8-13H,7H2,1-2H3,(H,28,31)(H2,25,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM134790
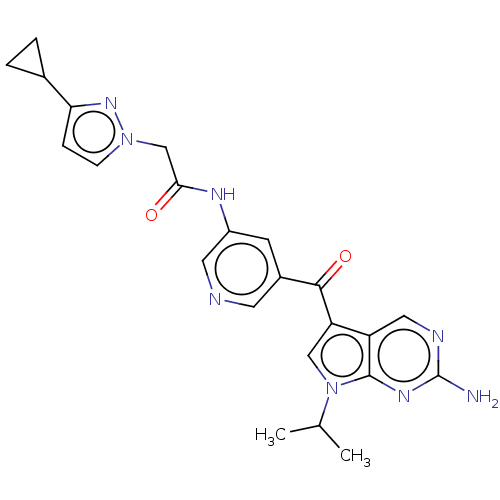
(US8846698, 253)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C3CC3)c2)c2cnc(N)nc12 Show InChI InChI=1S/C23H24N8O2/c1-13(2)31-11-18(17-10-26-23(24)28-22(17)31)21(33)15-7-16(9-25-8-15)27-20(32)12-30-6-5-19(29-30)14-3-4-14/h5-11,13-14H,3-4,12H2,1-2H3,(H,27,32)(H2,24,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550241
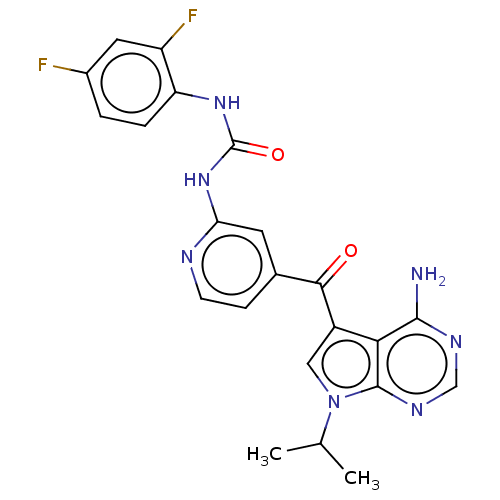
(CHEMBL4744936)Show SMILES CC(C)n1cc(C(=O)c2ccnc(NC(=O)Nc3ccc(F)cc3F)c2)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50550246
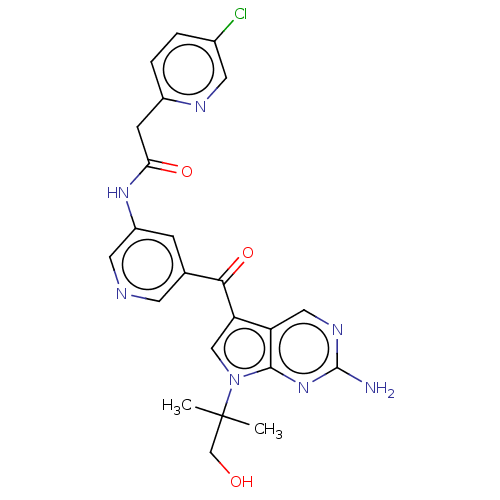
(PF06273340 | Pf-06273340)Show SMILES CC(C)(CO)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cn3)c2)c2cnc(N)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| >4.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dofetilide binding to human ERG |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50426571
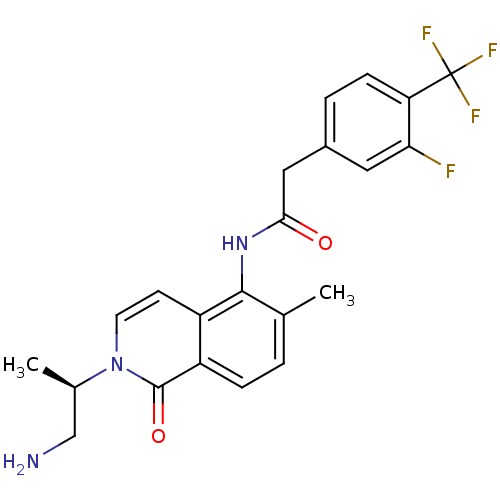
(CHEMBL2324343)Show SMILES C[C@H](CN)n1ccc2c(NC(=O)Cc3ccc(c(F)c3)C(F)(F)F)c(C)ccc2c1=O |r| Show InChI InChI=1S/C22H21F4N3O2/c1-12-3-5-16-15(7-8-29(21(16)31)13(2)11-27)20(12)28-19(30)10-14-4-6-17(18(23)9-14)22(24,25)26/h3-9,13H,10-11,27H2,1-2H3,(H,28,30)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of P2X7 receptor (unknown origin) assessed as inhibition of IL1beta production |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM30547
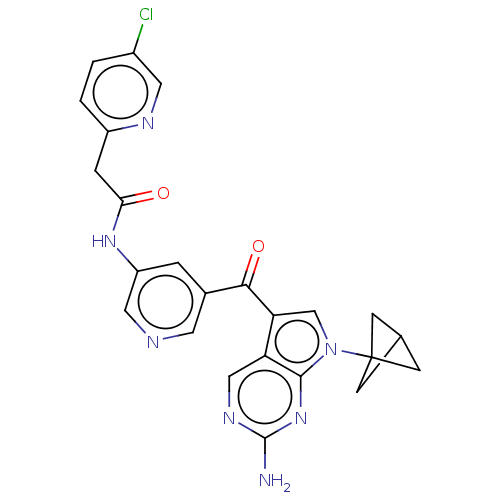
(US8846698, 583)Show SMILES Nc1ncc2c(cn(c2n1)C12CC(C1)C2)C(=O)c1cncc(NC(=O)Cc2ccc(Cl)cn2)c1 Show InChI InChI=1S/C24H20ClN7O2/c25-15-1-2-16(28-9-15)4-20(33)30-17-3-14(8-27-10-17)21(34)19-12-32(24-5-13(6-24)7-24)22-18(19)11-29-23(26)31-22/h1-3,8-13H,4-7H2,(H,30,33)(H2,26,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.681 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM30449

(US8846698, 580)Show SMILES Nc1ncc2c(cn(c2n1)C12CC(C1)C2)C(=O)c1cncc(NC(=O)Cn2ccc(n2)C(F)(F)F)c1 Show InChI InChI=1S/C23H19F3N8O2/c24-23(25,26)17-1-2-33(32-17)11-18(35)30-14-3-13(7-28-8-14)19(36)16-10-34(22-4-12(5-22)6-22)20-15(16)9-29-21(27)31-20/h1-3,7-10,12H,4-6,11H2,(H,30,35)(H2,27,29,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.825 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50457826

(CHEMBL4216500)Show SMILES Cn1cnc(c1)-c1cnc(N)c(O[C@H]2CN(CC2(F)F)C(=O)Cc2ccc(OC(F)(F)F)cc2)c1 |r| Show InChI InChI=1S/C22H20F5N5O3/c1-31-9-16(30-12-31)14-7-17(20(28)29-8-14)34-18-10-32(11-21(18,23)24)19(33)6-13-2-4-15(5-3-13)35-22(25,26)27/h2-5,7-9,12,18H,6,10-11H2,1H3,(H2,28,29)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at prolink-tagged TrkA in human U2OS cells assessed as inhibition of beta-NGF-induced receptor phosphorylation by measuring reduc... |
J Med Chem 61: 6779-6800 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00633
BindingDB Entry DOI: 10.7270/Q2K64MPW |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50457839

(CHEMBL4211921)Show SMILES Cn1cc(cn1)-c1ccc(O[C@@H]2CCN(C2)C(=O)Cc2ccc(OC(F)(F)F)cc2)c(n1)C(N)=O |r| Show InChI InChI=1S/C23H22F3N5O4/c1-30-12-15(11-28-30)18-6-7-19(21(29-18)22(27)33)34-17-8-9-31(13-17)20(32)10-14-2-4-16(5-3-14)35-23(24,25)26/h2-7,11-12,17H,8-10,13H2,1H3,(H2,27,33)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... |
J Med Chem 61: 6779-6800 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00633
BindingDB Entry DOI: 10.7270/Q2K64MPW |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50550243

(CHEMBL4790022)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2c(N)ncnc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TrkC expressed in human U2OS cells pre-incubated 30 mins before neurotrophin addition and measured after 2 hrs by luminescence ba... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM134787

(US8846698, 250)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cc3)c2)c2cnc(N)nc12 Show InChI InChI=1S/C23H21ClN6O2/c1-13(2)30-12-19(18-11-27-23(25)29-22(18)30)21(32)15-8-17(10-26-9-15)28-20(31)7-14-3-5-16(24)6-4-14/h3-6,8-13H,7H2,1-2H3,(H,28,31)(H2,25,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TrkC expressed in human U2OS cells pre-incubated 30 mins before neurotrophin addition and measured after 2 hrs by luminescence ba... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2CF9TQ8 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226864
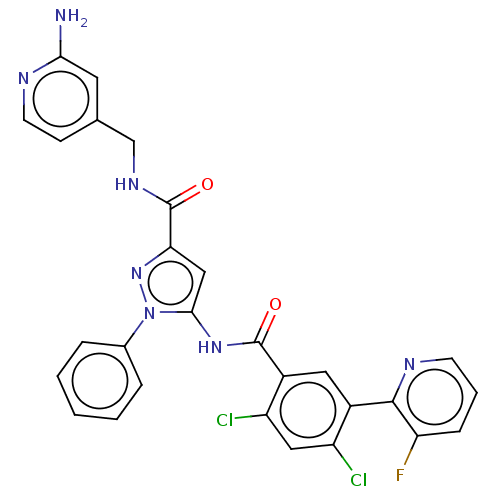
(US9328096, 557)Show SMILES Nc1cc(CNC(=O)c2cc(NC(=O)c3cc(c(Cl)cc3Cl)-c3ncccc3F)n(n2)-c2ccccc2)ccn1 Show InChI InChI=1S/C28H20Cl2FN7O2/c29-20-13-21(30)19(12-18(20)26-22(31)7-4-9-34-26)27(39)36-25-14-23(37-38(25)17-5-2-1-3-6-17)28(40)35-15-16-8-10-33-24(32)11-16/h1-14H,15H2,(H2,32,33)(H,35,40)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM227148
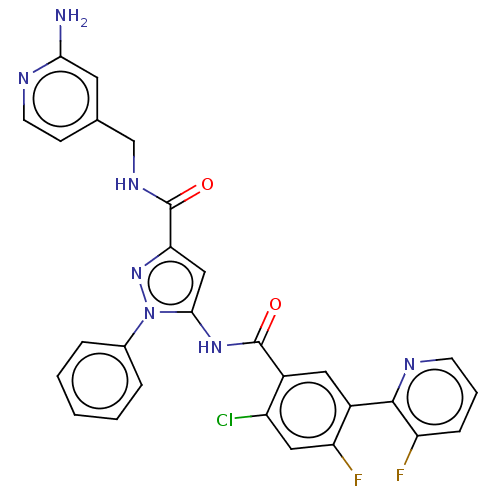
(US9328096, 836)Show SMILES Nc1cc(CNC(=O)c2cc(NC(=O)c3cc(c(F)cc3Cl)-c3ncccc3F)n(n2)-c2ccccc2)ccn1 Show InChI InChI=1S/C28H20ClF2N7O2/c29-20-13-22(31)19(26-21(30)7-4-9-34-26)12-18(20)27(39)36-25-14-23(37-38(25)17-5-2-1-3-6-17)28(40)35-15-16-8-10-33-24(32)11-16/h1-14H,15H2,(H2,32,33)(H,35,40)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM134792
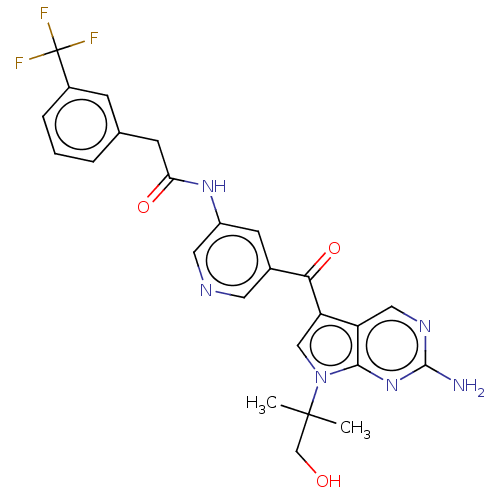
(US8846698, 256)Show SMILES CC(C)(CO)n1cc(C(=O)c2cncc(NC(=O)Cc3cccc(c3)C(F)(F)F)c2)c2cnc(N)nc12 Show InChI InChI=1S/C25H23F3N6O3/c1-24(2,13-35)34-12-19(18-11-31-23(29)33-22(18)34)21(37)15-8-17(10-30-9-15)32-20(36)7-14-4-3-5-16(6-14)25(26,27)28/h3-6,8-12,35H,7,13H2,1-2H3,(H,32,36)(H2,29,31,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50457833
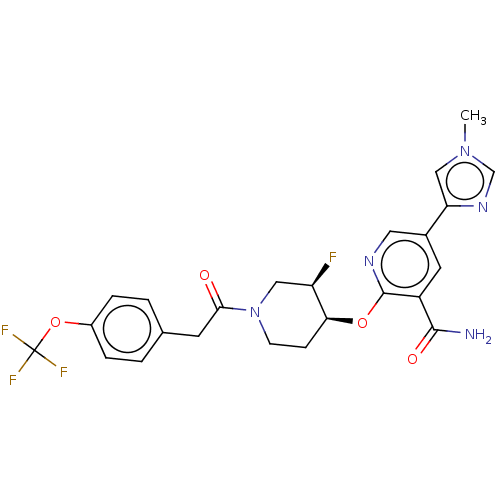
(CHEMBL4210892)Show SMILES Cn1cnc(c1)-c1cnc(O[C@H]2CCN(C[C@H]2F)C(=O)Cc2ccc(OC(F)(F)F)cc2)c(c1)C(N)=O |r| Show InChI InChI=1S/C24H23F4N5O4/c1-32-12-19(31-13-32)15-9-17(22(29)35)23(30-10-15)36-20-6-7-33(11-18(20)25)21(34)8-14-2-4-16(5-3-14)37-24(26,27)28/h2-5,9-10,12-13,18,20H,6-8,11H2,1H3,(H2,29,35)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... |
J Med Chem 61: 6779-6800 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00633
BindingDB Entry DOI: 10.7270/Q2K64MPW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226929
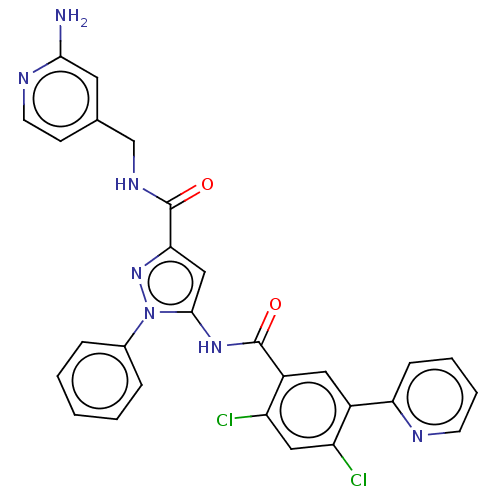
(US9328096, 617)Show SMILES Nc1cc(CNC(=O)c2cc(NC(=O)c3cc(c(Cl)cc3Cl)-c3ccccn3)n(n2)-c2ccccc2)ccn1 Show InChI InChI=1S/C28H21Cl2N7O2/c29-21-14-22(30)20(13-19(21)23-8-4-5-10-32-23)27(38)35-26-15-24(36-37(26)18-6-2-1-3-7-18)28(39)34-16-17-9-11-33-25(31)12-17/h1-15H,16H2,(H2,31,33)(H,34,39)(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226759

(US9328096, 452)Show SMILES Nc1cc(CNC(=O)c2cc(NC(=O)c3cc(c(Cl)cc3Cl)-c3ccc(F)cn3)n(n2)-c2ccccc2)ccn1 Show InChI InChI=1S/C28H20Cl2FN7O2/c29-21-12-22(30)20(11-19(21)23-7-6-17(31)15-34-23)27(39)36-26-13-24(37-38(26)18-4-2-1-3-5-18)28(40)35-14-16-8-9-33-25(32)10-16/h1-13,15H,14H2,(H2,32,33)(H,35,40)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226847
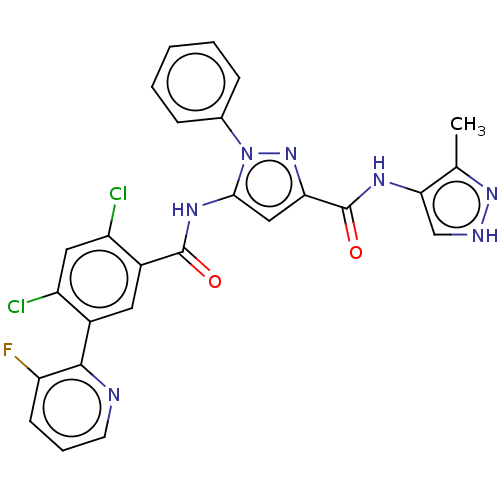
(US9328096, 540)Show SMILES Cc1n[nH]cc1NC(=O)c1cc(NC(=O)c2cc(c(Cl)cc2Cl)-c2ncccc2F)n(n1)-c1ccccc1 Show InChI InChI=1S/C26H18Cl2FN7O2/c1-14-22(13-31-34-14)32-26(38)21-12-23(36(35-21)15-6-3-2-4-7-15)33-25(37)17-10-16(18(27)11-19(17)28)24-20(29)8-5-9-30-24/h2-13H,1H3,(H,31,34)(H,32,38)(H,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM135100

(US8846698, 526)Show SMILES CC(C)n1cc(C(=O)c2cncc(NC(=O)Nc3cc(nn3-c3cnn(C)c3)C3CC3)c2)c2cncnc12 Show InChI InChI=1S/C26H26N10O2/c1-15(2)35-13-21(20-11-28-14-29-25(20)35)24(37)17-6-18(9-27-8-17)31-26(38)32-23-7-22(16-4-5-16)33-36(23)19-10-30-34(3)12-19/h6-16H,4-5H2,1-3H3,(H2,31,32,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM135053

(US8846698, 538)Show SMILES COCC(C)(C)n1cc(C(=O)c2cncc(NC(=O)Cn3ccc(n3)C3CC3)c2)c2cncnc12 Show InChI InChI=1S/C25H27N7O3/c1-25(2,14-35-3)32-12-20(19-11-27-15-28-24(19)32)23(34)17-8-18(10-26-9-17)29-22(33)13-31-7-6-21(30-31)16-4-5-16/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM135077
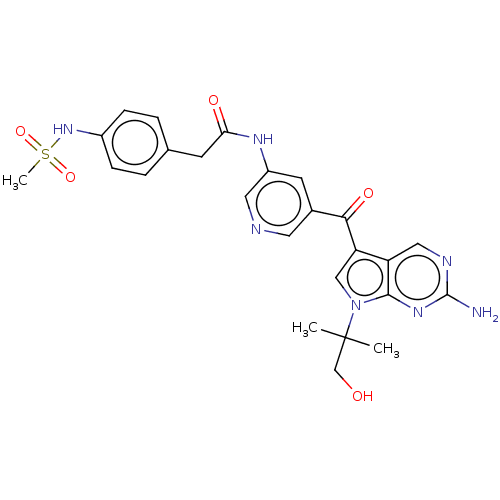
(US8846698, 564)Show SMILES CC(C)(CO)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(NS(C)(=O)=O)cc3)c2)c2cnc(N)nc12 Show InChI InChI=1S/C25H27N7O5S/c1-25(2,14-33)32-13-20(19-12-28-24(26)30-23(19)32)22(35)16-9-18(11-27-10-16)29-21(34)8-15-4-6-17(7-5-15)31-38(3,36)37/h4-7,9-13,31,33H,8,14H2,1-3H3,(H,29,34)(H2,26,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM135051
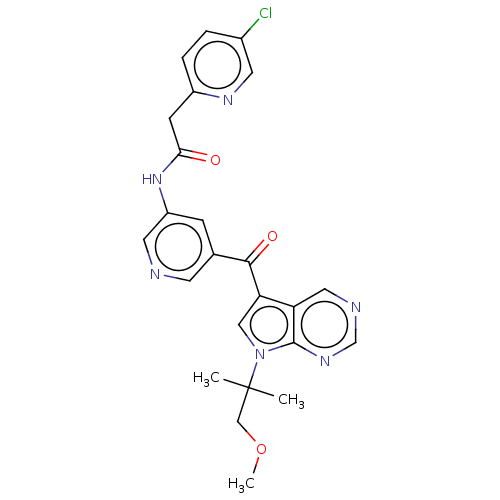
(US8846698, 536)Show SMILES COCC(C)(C)n1cc(C(=O)c2cncc(NC(=O)Cc3ccc(Cl)cn3)c2)c2cncnc12 Show InChI InChI=1S/C24H23ClN6O3/c1-24(2,13-34-3)31-12-20(19-11-27-14-29-23(19)31)22(33)15-6-18(10-26-8-15)30-21(32)7-17-5-4-16(25)9-28-17/h4-6,8-12,14H,7,13H2,1-3H3,(H,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Limited
US Patent
| Assay Description
Isolated TRK Enzyme assays use the HTRF KinEASE-TK kit (Cisbio Cat# 62TK0PEJ) with recombinant His-tagged cytoplasmic domains of each TRK receptor so... |
US Patent US8846698 (2014)
BindingDB Entry DOI: 10.7270/Q2F47MTW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226527
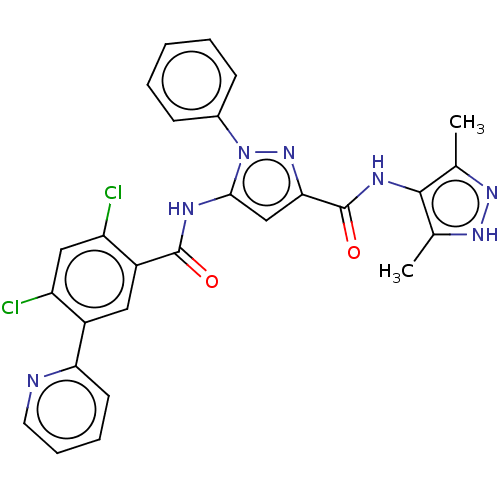
(US9328096, 220)Show SMILES Cc1n[nH]c(C)c1NC(=O)c1cc(NC(=O)c2cc(c(Cl)cc2Cl)-c2ccccn2)n(n1)-c1ccccc1 Show InChI InChI=1S/C27H21Cl2N7O2/c1-15-25(16(2)34-33-15)32-27(38)23-14-24(36(35-23)17-8-4-3-5-9-17)31-26(37)19-12-18(20(28)13-21(19)29)22-10-6-7-11-30-22/h3-14H,1-2H3,(H,31,37)(H,32,38)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [441-796]
(Homo sapiens (Human)) | BDBM226611
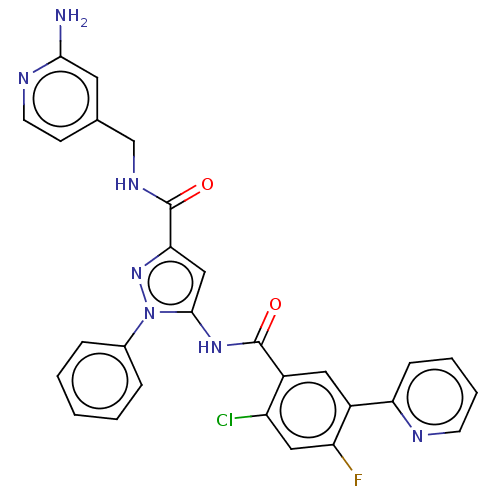
(US9328096, 304)Show SMILES Nc1cc(CNC(=O)c2cc(NC(=O)c3cc(c(F)cc3Cl)-c3ccccn3)n(n2)-c2ccccc2)ccn1 Show InChI InChI=1S/C28H21ClFN7O2/c29-21-14-22(30)20(23-8-4-5-10-32-23)13-19(21)27(38)35-26-15-24(36-37(26)18-6-2-1-3-7-18)28(39)34-16-17-9-11-33-25(31)12-17/h1-15H,16H2,(H2,31,33)(H,34,39)(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
TRKA co-expressed with p75: The assays are based upon DiscoveRx's proprietary Enzyme Fragment Complementation (EFC) technology. In the case of the TR... |
US Patent US9328096 (2016)
BindingDB Entry DOI: 10.7270/Q2FN152J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data