 Found 74 hits with Last Name = 'skinner-adams' and Initial = 'ts'
Found 74 hits with Last Name = 'skinner-adams' and Initial = 'ts' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293820

(2-(2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)e...)Show InChI InChI=1S/C9H16N5O5P/c10-9-12-7-6(8(15)13-9)11-5-14(7)1-2-19-3-4-20(16,17)18/h11H,1-5H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293821

(2-(2-(6-oxo-1,6,7,8-tetrahydropurin-9-yl)ethoxy)et...)Show InChI InChI=1S/C9H15N4O5P/c14-9-7-8(10-5-11-9)13(6-12-7)1-2-18-3-4-19(15,16)17/h5,12H,1-4,6H2,(H,10,11,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293826
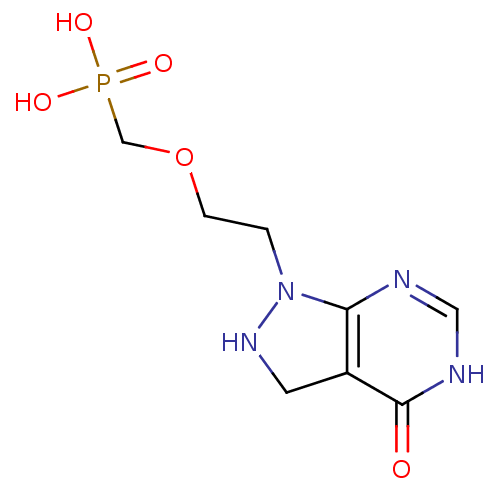
((2-(4-oxo-2,3,4,5-tetrahydropyrazolo[3,4-d]pyrimid...)Show InChI InChI=1S/C8H13N4O5P/c13-8-6-3-11-12(7(6)9-4-10-8)1-2-17-5-18(14,15)16/h4,11H,1-3,5H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293819
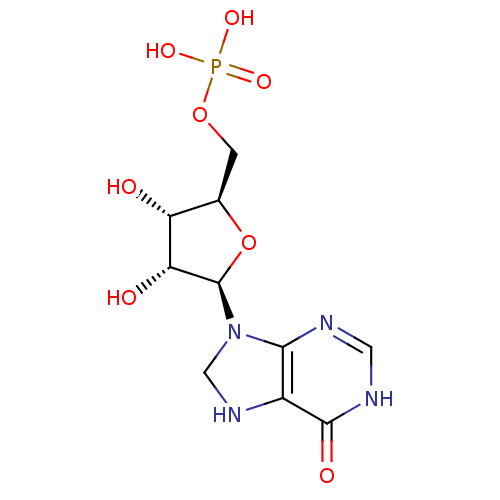
(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-oxo-1,6,7,8-tetr...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)N1CNc2c1nc[nH]c2=O |r| Show InChI InChI=1S/C10H15N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2,4,6-7,10,13,15-16H,1,3H2,(H,11,12,17)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293818
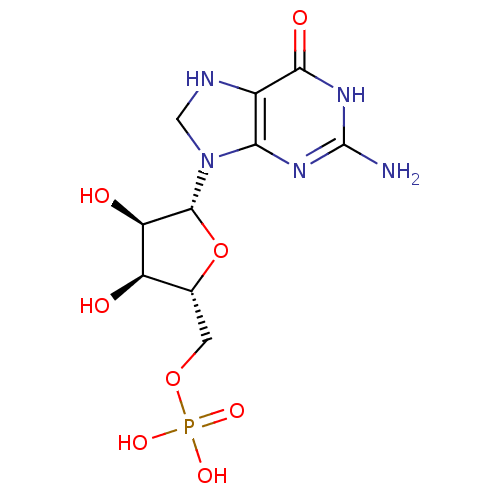
(((2R,3S,4R,5R)-5-(2-amino-6-oxo-1,6,7,8-tetrahydro...)Show SMILES Nc1nc2N(CNc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O8P/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(23-9)1-22-24(19,20)21/h3,5-6,9,12,16-17H,1-2H2,(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293830
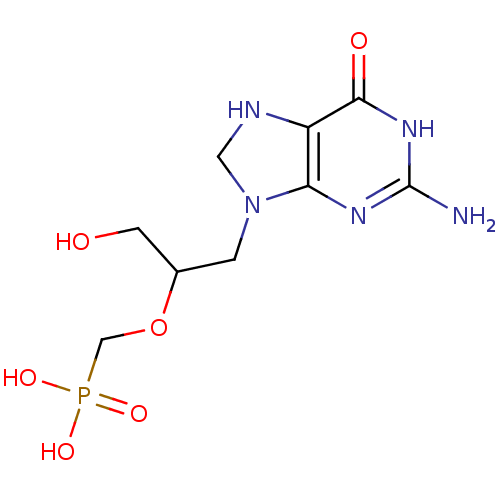
(CHEMBL561493 | RS-(3-(2-amino-6-oxo-1,6,7,8-tetrah...)Show InChI InChI=1S/C9H16N5O6P/c10-9-12-7-6(8(16)13-9)11-3-14(7)1-5(2-15)20-4-21(17,18)19/h5,11,15H,1-4H2,(H2,17,18,19)(H3,10,12,13,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293835
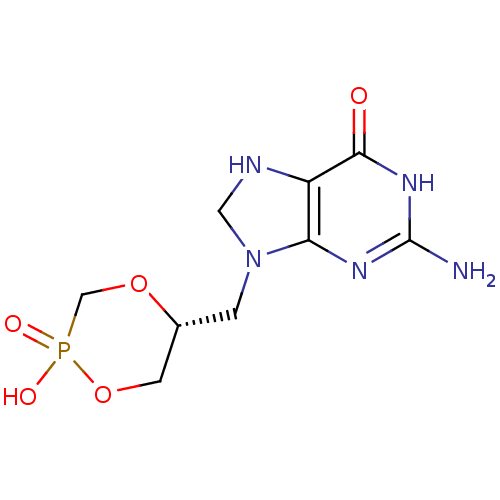
((R)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293831

((S)-(1-(5-amino-7-oxo-1,2,6,7-tetrahydro-[1,2,3]tr...)Show SMILES C[C@@H](CN1NNc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C8H15N6O5P/c1-4(19-3-20(16,17)18)2-14-6-5(12-13-14)7(15)11-8(9)10-6/h4,12-13H,2-3H2,1H3,(H2,16,17,18)(H3,9,10,11,15)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293835

((R)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293836
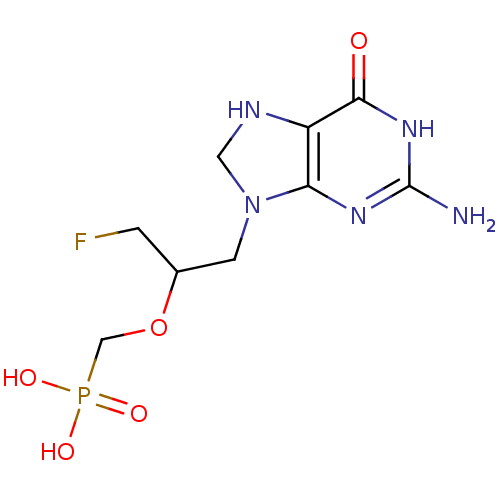
(CHEMBL541006 | RS-(1-(2-amino-6-oxo-7,8-dihydro-1H...)Show InChI InChI=1S/C9H15FN5O5P/c10-1-5(20-4-21(17,18)19)2-15-3-12-6-7(15)13-9(11)14-8(6)16/h5,12H,1-4H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50225188
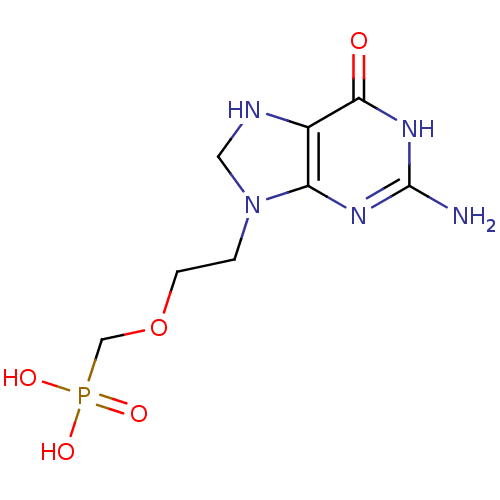
((2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)eth...)Show InChI InChI=1S/C8H14N5O5P/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-18-4-19(15,16)17/h10H,1-4H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293833
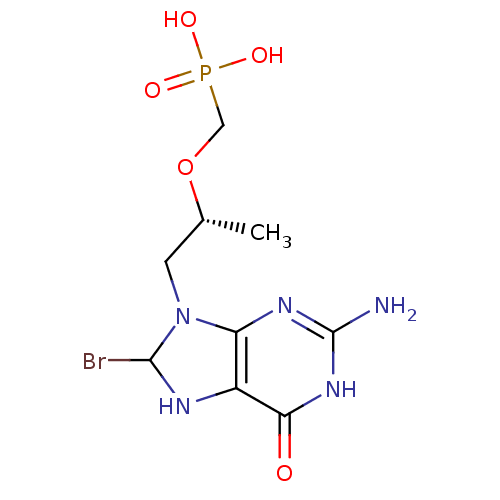
(((R)-1-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropu...)Show SMILES C[C@H](CN1C(Br)Nc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C9H15BrN5O5P/c1-4(20-3-21(17,18)19)2-15-6-5(12-8(15)10)7(16)14-9(11)13-6/h4,8,12H,2-3H2,1H3,(H2,17,18,19)(H3,11,13,14,16)/t4-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50225188

((2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)eth...)Show InChI InChI=1S/C8H14N5O5P/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-18-4-19(15,16)17/h10H,1-4H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293824

((2-(2-amino-8-hydroxy-6-oxo-1,6,7,8-tetrahydropuri...)Show InChI InChI=1S/C8H14N5O6P/c9-7-11-5-4(6(14)12-7)10-8(15)13(5)1-2-19-3-20(16,17)18/h8,10,15H,1-3H2,(H2,16,17,18)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293834
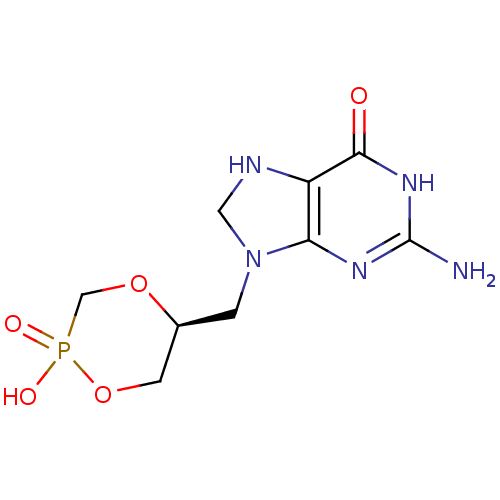
((S)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293834

((S)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293828
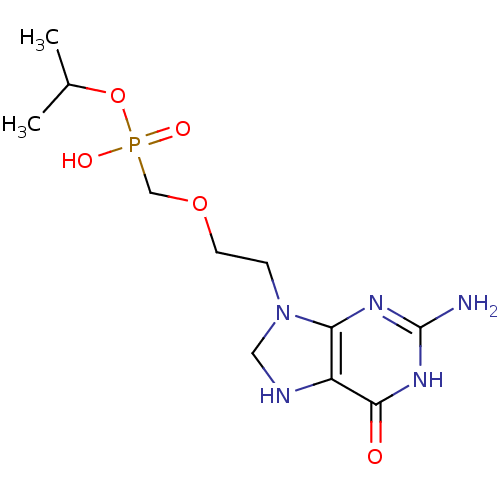
(CHEMBL560759 | isopropyl(2-(2-amino-6-oxo-1,6,7,8-...)Show InChI InChI=1S/C11H20N5O5P/c1-7(2)21-22(18,19)6-20-4-3-16-5-13-8-9(16)14-11(12)15-10(8)17/h7,13H,3-6H2,1-2H3,(H,18,19)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293823

((2-(5-amino-7-oxo-1,2,6,7-tetrahydro-[1,2,3]triazo...)Show InChI InChI=1S/C7H13N6O5P/c8-7-9-5-4(6(14)10-7)11-12-13(5)1-2-18-3-19(15,16)17/h11-12H,1-3H2,(H2,15,16,17)(H3,8,9,10,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293837
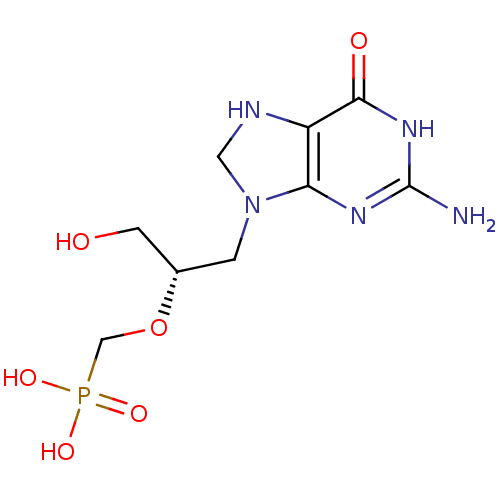
((S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]guanin...)Show SMILES Nc1nc2N(C[C@@H](CO)OCP(O)(O)=O)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H16N5O6P/c10-9-12-7-6(8(16)13-9)11-3-14(7)1-5(2-15)20-4-21(17,18)19/h5,11,15H,1-4H2,(H2,17,18,19)(H3,10,12,13,16)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293838

((2-(6-oxo-1,6,7,8-tetrahydropurin-9-yl)ethoxy)meth...)Show InChI InChI=1S/C8H13N4O5P/c13-8-6-7(9-3-10-8)12(4-11-6)1-2-17-5-18(14,15)16/h3,11H,1-2,4-5H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293832
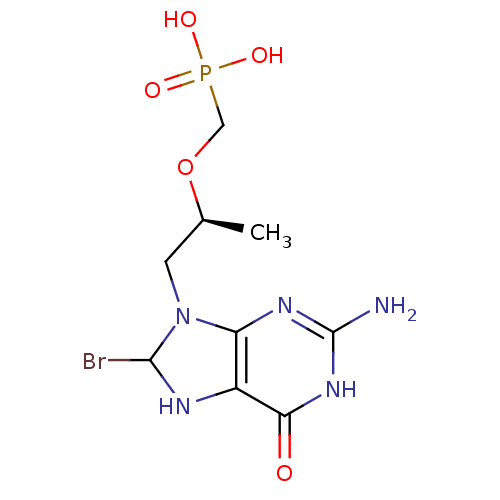
(((S)-1-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropu...)Show SMILES C[C@@H](CN1C(Br)Nc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C9H15BrN5O5P/c1-4(20-3-21(17,18)19)2-15-6-5(12-8(15)10)7(16)14-9(11)13-6/h4,8,12H,2-3H2,1H3,(H2,17,18,19)(H3,11,13,14,16)/t4-,8?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293825
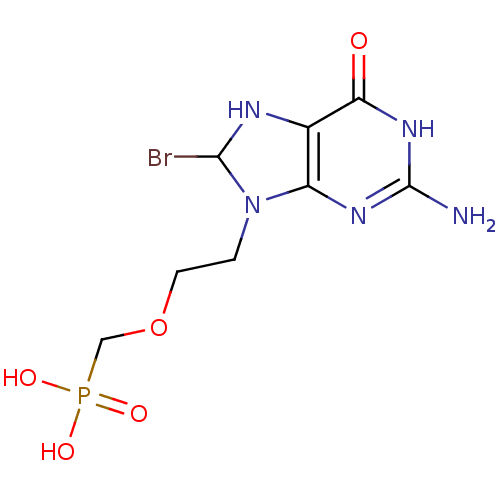
((2-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropurin-...)Show InChI InChI=1S/C8H13BrN5O5P/c9-7-11-4-5(12-8(10)13-6(4)15)14(7)1-2-19-3-20(16,17)18/h7,11H,1-3H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293829
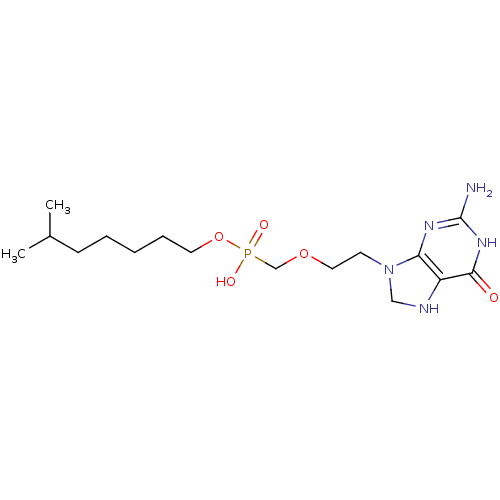
(6-methylheptyl(2-(2-amino-6-oxo-1,6,7,8-tetrahydro...)Show SMILES CC(C)CCCCCOP(O)(=O)COCCN1CNc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C16H30N5O5P/c1-12(2)6-4-3-5-8-26-27(23,24)11-25-9-7-21-10-18-13-14(21)19-16(17)20-15(13)22/h12,18H,3-11H2,1-2H3,(H,23,24)(H3,17,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293827
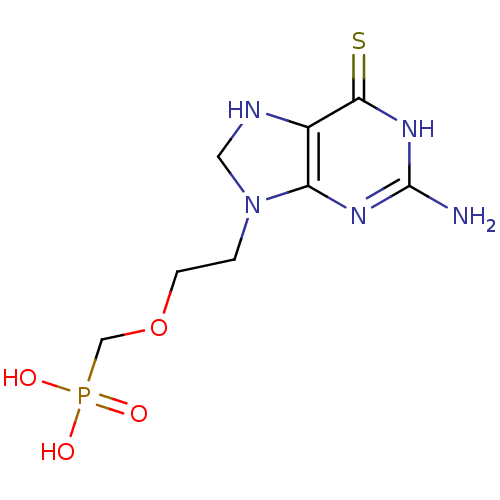
((2-(2-amino-6-thioxo-1,6,7,8-tetrahydropurin-9-yl)...)Show InChI InChI=1S/C8H14N5O4PS/c9-8-11-6-5(7(19)12-8)10-3-13(6)1-2-17-4-18(14,15)16/h10H,1-4H2,(H2,14,15,16)(H3,9,11,12,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506775

(CHEMBL4593437)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O3S/c26-20(25-28)9-5-2-6-14-29-22-23-19(15-21(27)24-22)18-12-10-17(11-13-18)16-7-3-1-4-8-16/h1,3-4,7-8,10-13,15,28H,2,5-6,9,14H2,(H,25,26)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506777

(CHEMBL4476057)Show SMILES ONC(=O)CCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N3O3S/c23-17(22-25)7-3-4-10-26-19-20-16(12-18(24)21-19)15-9-8-13-5-1-2-6-14(13)11-15/h1-2,5-6,8-9,11-12,25H,3-4,7,10H2,(H,22,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506780

(CHEMBL4550522)Show SMILES ONC(=O)\C=C\c1ccc(NC(=O)Cc2cccc3ccccc23)cn1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-26)11-10-16-8-9-17(13-21-16)22-20(25)12-15-6-3-5-14-4-1-2-7-18(14)15/h1-11,13,26H,12H2,(H,22,25)(H,23,24)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506778

(CHEMBL4469596)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H21N3O3S/c24-18(23-26)8-2-1-5-11-27-20-21-17(13-19(25)22-20)16-10-9-14-6-3-4-7-15(14)12-16/h3-4,6-7,9-10,12-13,26H,1-2,5,8,11H2,(H,23,24)(H,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506779

(CHEMBL4521590)Show InChI InChI=1S/C18H15N3O3/c22-17(20-14-8-9-16(19-11-14)18(23)21-24)10-13-6-3-5-12-4-1-2-7-15(12)13/h1-9,11,24H,10H2,(H,20,22)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50506781

(CHEMBL4435005)Show InChI InChI=1S/C14H13N3O3/c18-13(8-10-4-2-1-3-5-10)16-11-6-7-12(15-9-11)14(19)17-20/h1-7,9,20H,8H2,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506775

(CHEMBL4593437)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O3S/c26-20(25-28)9-5-2-6-14-29-22-23-19(15-21(27)24-22)18-12-10-17(11-13-18)16-7-3-1-4-8-16/h1,3-4,7-8,10-13,15,28H,2,5-6,9,14H2,(H,25,26)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506780

(CHEMBL4550522)Show SMILES ONC(=O)\C=C\c1ccc(NC(=O)Cc2cccc3ccccc23)cn1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-26)11-10-16-8-9-17(13-21-16)22-20(25)12-15-6-3-5-14-4-1-2-7-18(14)15/h1-11,13,26H,12H2,(H,22,25)(H,23,24)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506781

(CHEMBL4435005)Show InChI InChI=1S/C14H13N3O3/c18-13(8-10-4-2-1-3-5-10)16-11-6-7-12(15-9-11)14(19)17-20/h1-7,9,20H,8H2,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506779

(CHEMBL4521590)Show InChI InChI=1S/C18H15N3O3/c22-17(20-14-8-9-16(19-11-14)18(23)21-24)10-13-6-3-5-12-4-1-2-7-15(12)13/h1-9,11,24H,10H2,(H,20,22)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Carbamate kinase
(Giardia intestinalis) | BDBM50058655

(1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...)Show InChI InChI=1S/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Giardia lamblia carbamate Kinase preincubated for 15 mins followed by ADP and carbamate phosphate addition and measured after 20 mins b... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00910
BindingDB Entry DOI: 10.7270/Q2NK3JR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506778

(CHEMBL4469596)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H21N3O3S/c24-18(23-26)8-2-1-5-11-27-20-21-17(13-19(25)22-20)16-10-9-14-6-3-4-7-15(14)12-16/h3-4,6-7,9-10,12-13,26H,1-2,5,8,11H2,(H,23,24)(H,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50506777

(CHEMBL4476057)Show SMILES ONC(=O)CCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N3O3S/c23-17(22-25)7-3-4-10-26-19-20-16(12-18(24)21-19)15-9-8-13-5-1-2-6-14(13)11-15/h1-2,5-6,8-9,11-12,25H,3-4,7,10H2,(H,22,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506780

(CHEMBL4550522)Show SMILES ONC(=O)\C=C\c1ccc(NC(=O)Cc2cccc3ccccc23)cn1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-26)11-10-16-8-9-17(13-21-16)22-20(25)12-15-6-3-5-14-4-1-2-7-18(14)15/h1-11,13,26H,12H2,(H,22,25)(H,23,24)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506781

(CHEMBL4435005)Show InChI InChI=1S/C14H13N3O3/c18-13(8-10-4-2-1-3-5-10)16-11-6-7-12(15-9-11)14(19)17-20/h1-7,9,20H,8H2,(H,16,18)(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506778

(CHEMBL4469596)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C20H21N3O3S/c24-18(23-26)8-2-1-5-11-27-20-21-17(13-19(25)22-20)16-10-9-14-6-3-4-7-15(14)12-16/h3-4,6-7,9-10,12-13,26H,1-2,5,8,11H2,(H,23,24)(H,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506779

(CHEMBL4521590)Show InChI InChI=1S/C18H15N3O3/c22-17(20-14-8-9-16(19-11-14)18(23)21-24)10-13-6-3-5-12-4-1-2-7-15(12)13/h1-9,11,24H,10H2,(H,20,22)(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506777

(CHEMBL4476057)Show SMILES ONC(=O)CCCCSc1nc(cc(=O)[nH]1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N3O3S/c23-17(22-25)7-3-4-10-26-19-20-16(12-18(24)21-19)15-9-8-13-5-1-2-6-14(13)11-15/h1-2,5-6,8-9,11-12,25H,3-4,7,10H2,(H,22,23)(H,20,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50506775

(CHEMBL4593437)Show SMILES ONC(=O)CCCCCSc1nc(cc(=O)[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O3S/c26-20(25-28)9-5-2-6-14-29-22-23-19(15-21(27)24-22)18-12-10-17(11-13-18)16-7-3-1-4-8-16/h1,3-4,7-8,10-13,15,28H,2,5-6,9,14H2,(H,25,26)(H,23,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 3A
(Homo sapiens (Human)) | BDBM50506782
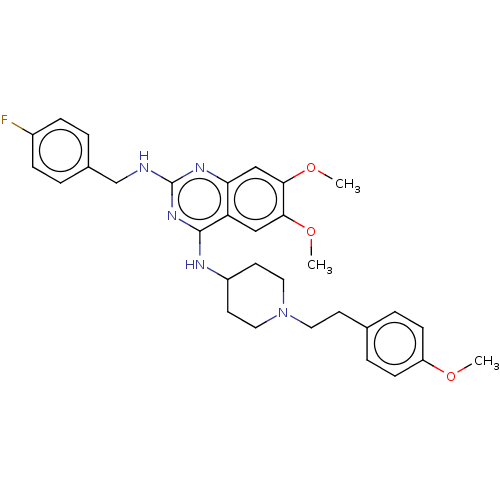
(CHEMBL4461581)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc(NCc3ccc(F)cc3)nc3cc(OC)c(OC)cc23)cc1 Show InChI InChI=1S/C31H36FN5O3/c1-38-25-10-6-21(7-11-25)12-15-37-16-13-24(14-17-37)34-30-26-18-28(39-2)29(40-3)19-27(26)35-31(36-30)33-20-22-4-8-23(32)9-5-22/h4-11,18-19,24H,12-17,20H2,1-3H3,(H2,33,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DNMT3a C-terminal catalytic domain (623 to 908 residues) using 5'-biotinylated/3'-FAM-oligonucleotide as substrate me... |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 3A
(Homo sapiens (Human)) | BDBM50506785

(CHEMBL4537137)Show SMILES COc1cc2c(NC3CCN(Cc4ccccc4)CC3)nc(nc2cc1O)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C31H36N6O2/c1-39-29-20-26-27(21-28(29)38)33-31(37-18-16-36(17-19-37)25-10-6-3-7-11-25)34-30(26)32-24-12-14-35(15-13-24)22-23-8-4-2-5-9-23/h2-11,20-21,24,38H,12-19,22H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DNMT3a C-terminal catalytic domain (623 to 908 residues) using 5'-biotinylated/3'-FAM-oligonucleotide as substrate me... |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50506784

(CHEMBL4527662)Show SMILES COc1cc2c(NC3CCN(Cc4ccccc4)CC3)nc(nc2cc1OCc1ccccc1)N1CCN(CC1)c1ccccc1 Show InChI InChI=1S/C38H42N6O2/c1-45-35-25-33-34(26-36(35)46-28-30-13-7-3-8-14-30)40-38(44-23-21-43(22-24-44)32-15-9-4-10-16-32)41-37(33)39-31-17-19-42(20-18-31)27-29-11-5-2-6-12-29/h2-16,25-26,31H,17-24,27-28H2,1H3,(H,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-fused G9a (786 to 1210 residues) expressed in Escherichia coli using biotinylated H3 (1 to 21 residues... |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data