

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50081842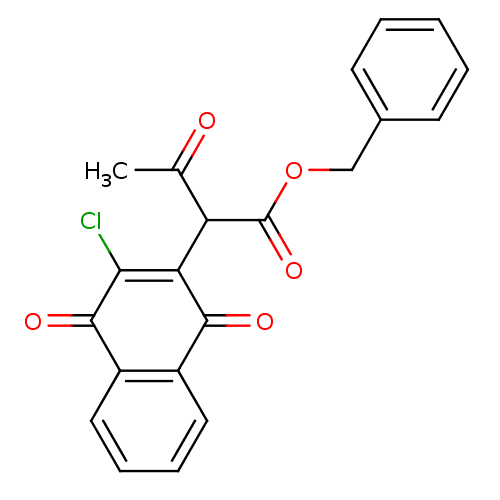 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by HPLC assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081846 (2-(1-Acetyl-2-oxo-propyl)-3-chloro-[1,4]naphthoqui...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by HPLC assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081842 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081846 (2-(1-Acetyl-2-oxo-propyl)-3-chloro-[1,4]naphthoqui...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Rev (Human immunodeficiency virus 1) | BDBM50503730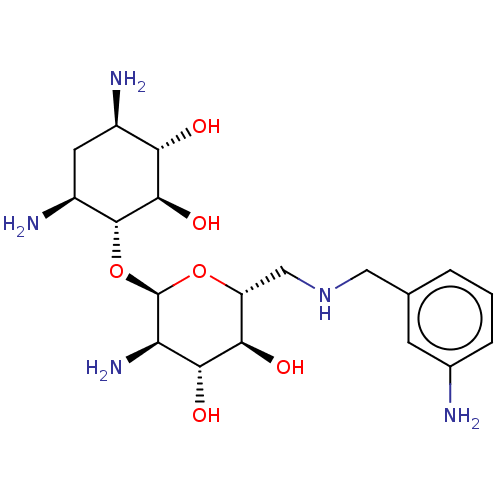 (CHEMBL4443278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 BindingDB Entry DOI: 10.7270/Q2Z60S9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081843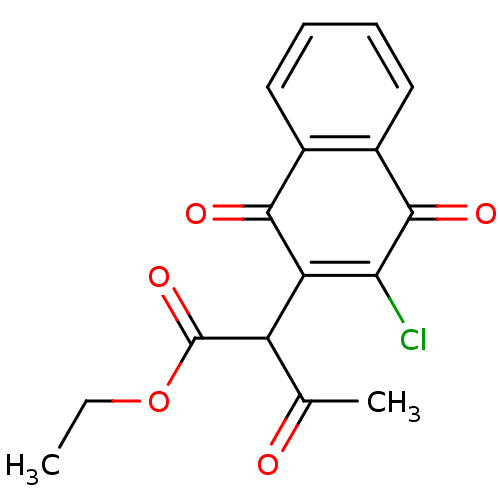 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777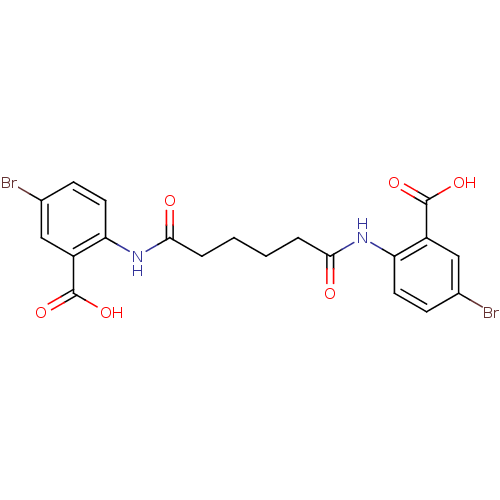 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071776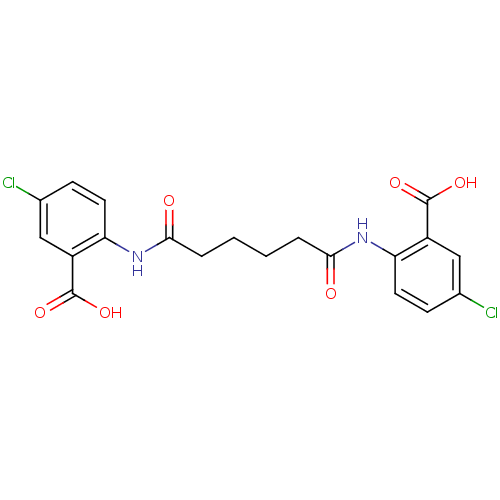 (2-({6-[(2-carboxy-4-chlorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081845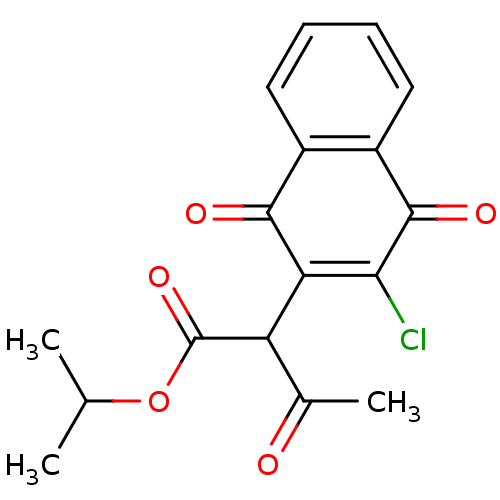 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071777 (5-bromo-2-({6-[(4-bromo-2-carboxyphenyl)amino]-6-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766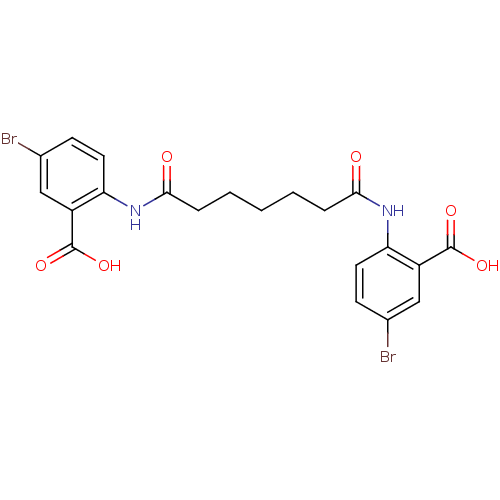 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM24774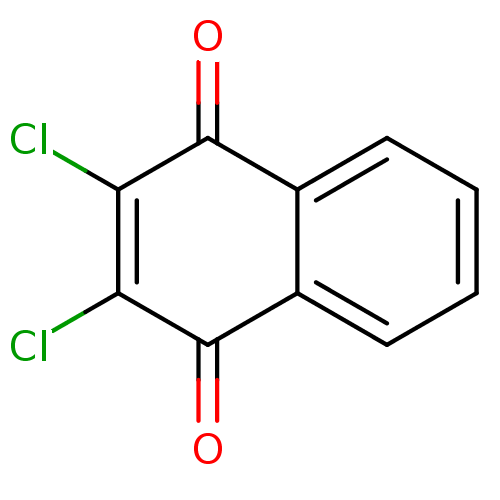 (2,3-dichloro-1,4-dihydronaphthalene-1,4-dione | 2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071768 (2-({6-[(2-carboxy-4-iodophenyl)amino]-6-oxohexanoy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071765 (2-[4-(2-carboxy-4-methoxyphenylcarbamoyl)butylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071766 (5-bromo-2-[5-(4-bromo-2-carboxyphenylcarbamoyl)pen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081843 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by HPLC assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM24774 (2,3-dichloro-1,4-dihydronaphthalene-1,4-dione | 2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by HPLC assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071771 (5-bromo-2-{[(5-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Rev (Human immunodeficiency virus 1) | BDBM50248131 (CHEMBL3754093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 BindingDB Entry DOI: 10.7270/Q2Z60S9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071767 (2-({6-[(2-carboxy-4-hydroxyphenyl)amino]-6-oxohexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071779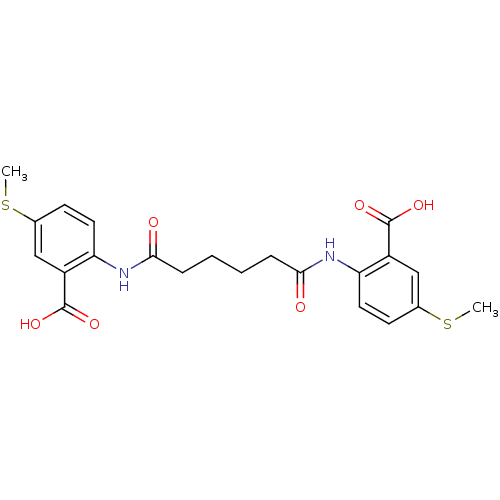 (2-[4-(2-carboxy-4-methylsulfanylphenylcarbamoyl)bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071762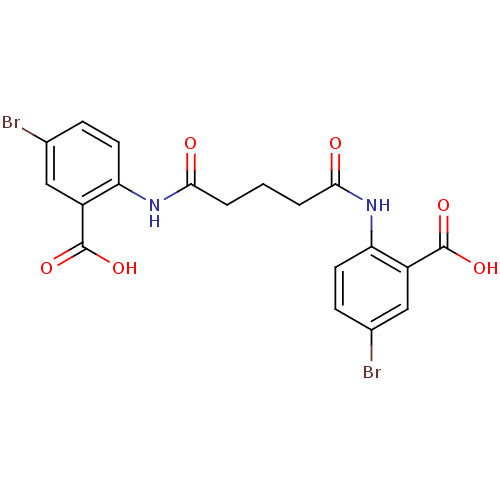 (5-bromo-2-({5-[(4-bromo-2-carboxyphenyl)amino]-5-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071761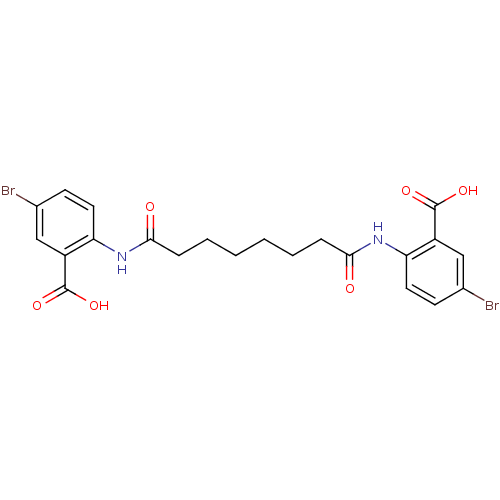 (5-bromo-2-[6-(4-bromo-2-carboxyphenylcarbamoyl)hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-2 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 microg/ml | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071778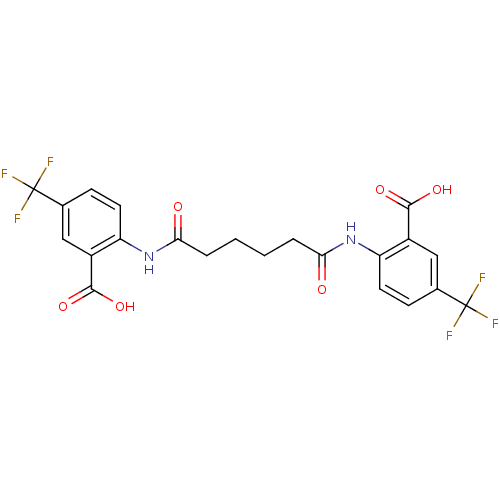 (2-[4-(2-carboxy-4-trifluoromethylphenylcarbamoyl)b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071768 (2-({6-[(2-carboxy-4-iodophenyl)amino]-6-oxohexanoy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071776 (2-({6-[(2-carboxy-4-chlorophenyl)amino]-6-oxohexan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071774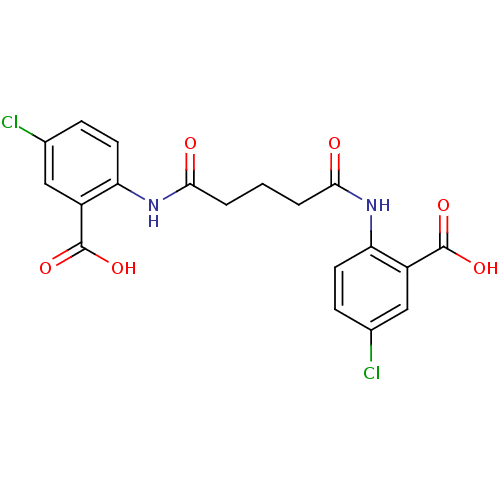 (2-({5-[(2-carboxy-4-chlorophenyl)amino]-5-oxopenta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071763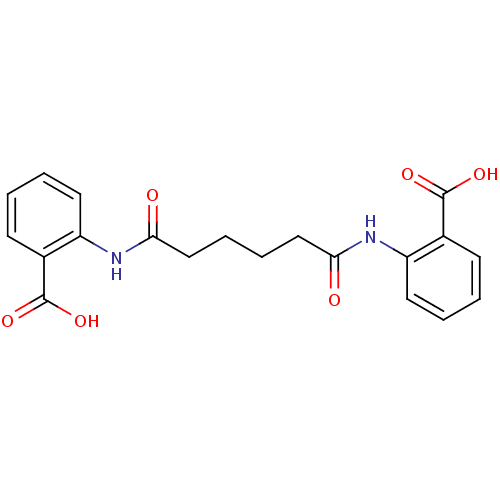 (2-({6-[(2-carboxyphenyl)amino]-6-oxohexanoyl}amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071773 (2-[5-(2-carboxy-4-chlorophenylcarbamoyl)pentylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071773 (2-[5-(2-carboxy-4-chlorophenylcarbamoyl)pentylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071763 (2-({6-[(2-carboxyphenyl)amino]-6-oxohexanoyl}amino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081845 (2-(3-Chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by HPLC assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071770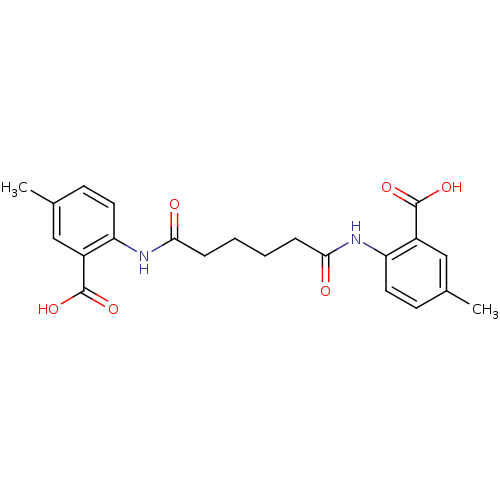 (2-[4-(2-carboxy-4-methylphenylcarbamoyl)butylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071774 (2-({5-[(2-carboxy-4-chlorophenyl)amino]-5-oxopenta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071762 (5-bromo-2-({5-[(4-bromo-2-carboxyphenyl)amino]-5-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139 (Homo sapiens (Human)) | BDBM50218757 (CHEMBL61867) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of protein kinase C | Bioorg Med Chem Lett 11: 1993-5 (2001) BindingDB Entry DOI: 10.7270/Q2B85B9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071765 (2-[4-(2-carboxy-4-methoxyphenylcarbamoyl)butylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071778 (2-[4-(2-carboxy-4-trifluoromethylphenylcarbamoyl)b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50081847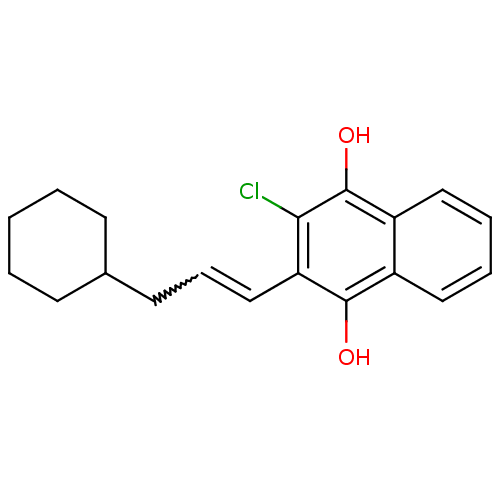 (2-Chloro-3-(3-cyclohexyl-propyl)-[1,4]naphthoquino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against HCMV protease by SPA assay | Bioorg Med Chem Lett 9: 2863-6 (1999) BindingDB Entry DOI: 10.7270/Q298867T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071780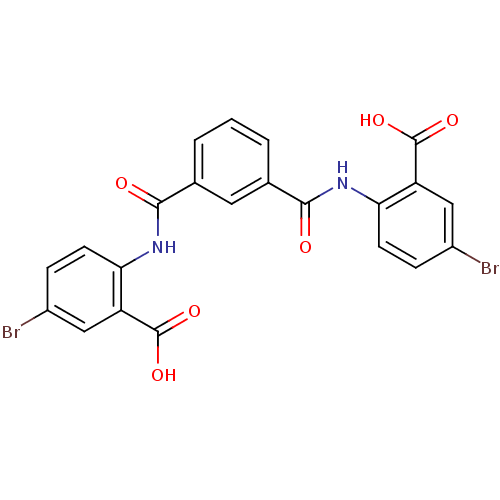 (5-bromo-2-[(3-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071770 (2-[4-(2-carboxy-4-methylphenylcarbamoyl)butylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071764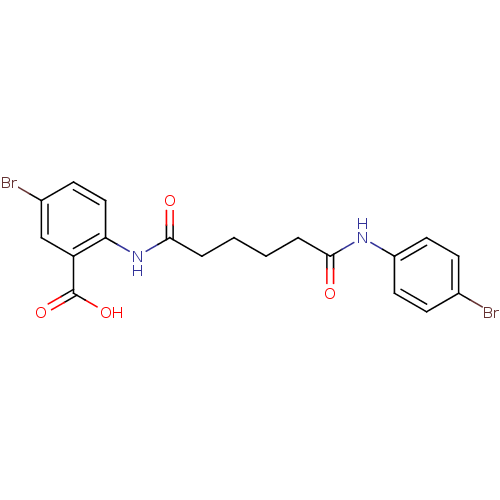 (5-Bromo-2-[5-(4-bromo-phenylcarbamoyl)-pentanoylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-2 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071760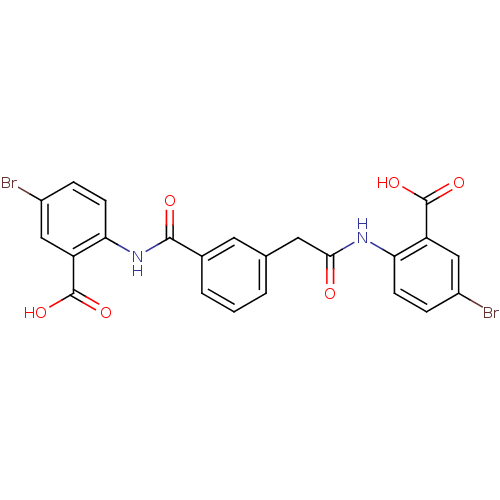 (5-bromo-2-{[(3-{[(4-bromo-2-carboxyphenyl)amino]ca...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Rev (Human immunodeficiency virus 1) | BDBM50503727 (CHEMBL4560686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 BindingDB Entry DOI: 10.7270/Q2Z60S9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071761 (5-bromo-2-[6-(4-bromo-2-carboxyphenylcarbamoyl)hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of HIV-1 reverse transcriptase using rC.dG and [3H]-dGTP as substrates at 100 ug/mL | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071780 (5-bromo-2-[(3-{[(4-bromo-2-carboxyphenyl)amino]car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50071779 (2-[4-(2-carboxy-4-methylsulfanylphenylcarbamoyl)bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Center Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 reverse transcriptase | Bioorg Med Chem Lett 8: 2623-8 (1999) BindingDB Entry DOI: 10.7270/Q2RF5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Rev (Human immunodeficiency virus 1) | BDBM50503726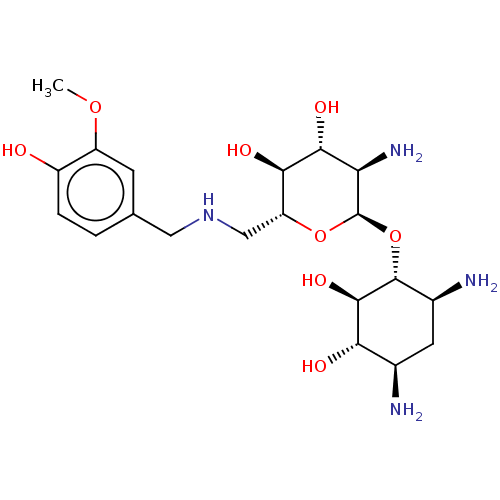 (CHEMBL4483318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 BindingDB Entry DOI: 10.7270/Q2Z60S9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Rev (Human immunodeficiency virus 1) | BDBM50503720 (CHEMBL4583631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of [3H]Rev response element binding to HIV1 biotinylated-Rev protein expressed in Escherichia coli after 15 mins by scintillation proximit... | Bioorg Med Chem Lett 29: 339-341 (2019) Article DOI: 10.1016/j.bmcl.2018.11.004 BindingDB Entry DOI: 10.7270/Q2Z60S9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50120982 ((S)-4-Acetylamino-4-[2-carboxy-1-((S)-1-{(S)-1-[(S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3/4A protease on 18 hr preincubation in chromogenic assay | Bioorg Med Chem Lett 12: 3359-62 (2002) BindingDB Entry DOI: 10.7270/Q2S181W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |