 Found 549 hits with Last Name = 'snow' and Initial = 'l'
Found 549 hits with Last Name = 'snow' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor (unknown origin) |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2C receptor (unknown origin) |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529935

(COCHLIOQUINONE A)Show SMILES CC[C@H](C)[C@@H](OC(C)=O)[C@@H](C)C1=CC(=O)C2=C(O[C@]3(C)CC[C@H]4O[C@H](CC[C@]4(C)[C@H]3[C@@H]2O)C(C)(C)O)C1=O |r,t:11,15| Show InChI InChI=1S/C30H44O8/c1-9-15(2)25(36-17(4)31)16(3)18-14-19(32)22-24(34)27-29(7)12-10-20(28(5,6)35)37-21(29)11-13-30(27,8)38-26(22)23(18)33/h14-16,20-21,24-25,27,34-35H,9-13H2,1-8H3/t15-,16-,20+,21+,24+,25+,27+,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529935

(COCHLIOQUINONE A)Show SMILES CC[C@H](C)[C@@H](OC(C)=O)[C@@H](C)C1=CC(=O)C2=C(O[C@]3(C)CC[C@H]4O[C@H](CC[C@]4(C)[C@H]3[C@@H]2O)C(C)(C)O)C1=O |r,t:11,15| Show InChI InChI=1S/C30H44O8/c1-9-15(2)25(36-17(4)31)16(3)18-14-19(32)22-24(34)27-29(7)12-10-20(28(5,6)35)37-21(29)11-13-30(27,8)38-26(22)23(18)33/h14-16,20-21,24-25,27,34-35H,9-13H2,1-8H3/t15-,16-,20+,21+,24+,25+,27+,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529932

(STEMPHONE)Show SMILES C\C=C(/C)[C@@H](OC(C)=O)[C@@H](C)C1=CC(=O)C2=C(O[C@]3(C)CC[C@H]4O[C@H](CC[C@]4(C)[C@H]3[C@@H]2O)C(C)(C)O)C1=O |r,t:11,15| Show InChI InChI=1S/C30H42O8/c1-9-15(2)25(36-17(4)31)16(3)18-14-19(32)22-24(34)27-29(7)12-10-20(28(5,6)35)37-21(29)11-13-30(27,8)38-26(22)23(18)33/h9,14,16,20-21,24-25,27,34-35H,10-13H2,1-8H3/b15-9+/t16-,20+,21+,24+,25+,27+,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529932

(STEMPHONE)Show SMILES C\C=C(/C)[C@@H](OC(C)=O)[C@@H](C)C1=CC(=O)C2=C(O[C@]3(C)CC[C@H]4O[C@H](CC[C@]4(C)[C@H]3[C@@H]2O)C(C)(C)O)C1=O |r,t:11,15| Show InChI InChI=1S/C30H42O8/c1-9-15(2)25(36-17(4)31)16(3)18-14-19(32)22-24(34)27-29(7)12-10-20(28(5,6)35)37-21(29)11-13-30(27,8)38-26(22)23(18)33/h9,14,16,20-21,24-25,27,34-35H,10-13H2,1-8H3/b15-9+/t16-,20+,21+,24+,25+,27+,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529934

(ETHYLENE GLYCOL DIOCTANOATE)Show InChI InChI=1S/C18H34O4/c1-3-5-7-9-11-13-17(19)21-15-16-22-18(20)14-12-10-8-6-4-2/h3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529934

(ETHYLENE GLYCOL DIOCTANOATE)Show InChI InChI=1S/C18H34O4/c1-3-5-7-9-11-13-17(19)21-15-16-22-18(20)14-12-10-8-6-4-2/h3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529937
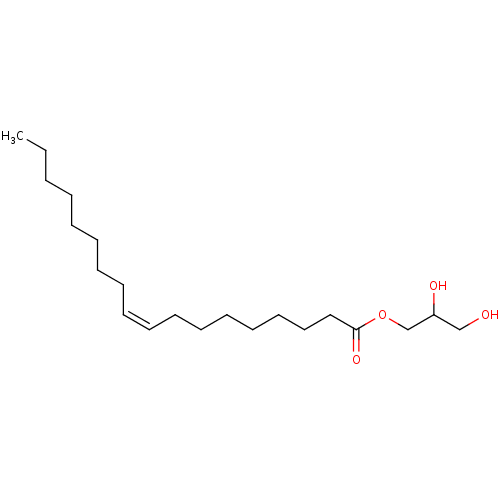
(CHEBI:75342 | Glyceryl monooleate)Show InChI InChI=1S/C21H40O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)25-19-20(23)18-22/h9-10,20,22-23H,2-8,11-19H2,1H3/b10-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase alpha
(Homo sapiens) | BDBM50529937

(CHEBI:75342 | Glyceryl monooleate)Show InChI InChI=1S/C21H40O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)25-19-20(23)18-22/h9-10,20,22-23H,2-8,11-19H2,1H3/b10-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of DGKalpha in human erythrocytes using OAG as substrate preincubated for 1 min followed by OAG addition and measured after 8 mins in pres... |
Eur J Med Chem 164: 378-390 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.061
BindingDB Entry DOI: 10.7270/Q2N01B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
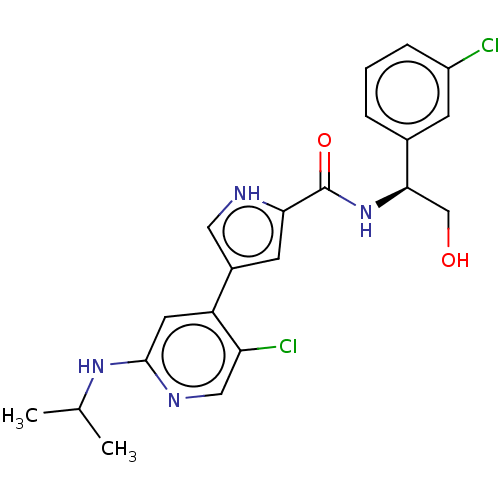
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465

(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151740
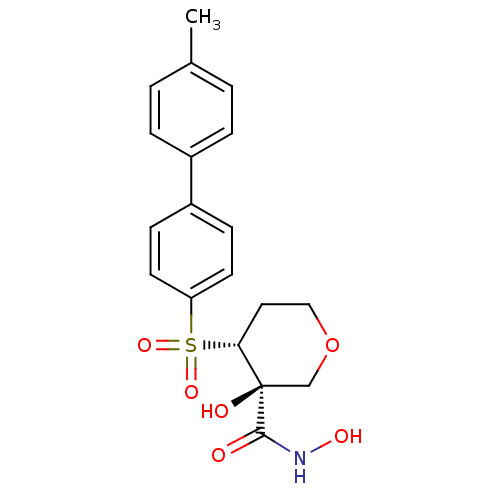
((3S,4R)-3-Hydroxy-4-(4''-methyl-biphenyl-4-sulfony...)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)[C@@H]1CCOC[C@]1(O)C(=O)NO Show InChI InChI=1S/C19H21NO6S/c1-13-2-4-14(5-3-13)15-6-8-16(9-7-15)27(24,25)17-10-11-26-12-19(17,22)18(21)20-23/h2-9,17,22-23H,10-12H2,1H3,(H,20,21)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151751

(3-Hydroxy-4-(3''-methyl-biphenyl-4-sulfonyl)-tetra...)Show SMILES Cc1cccc(c1)-c1ccc(cc1)S(=O)(=O)[C@@H]1CCOC[C@]1(O)C(=O)NO Show InChI InChI=1S/C19H21NO6S/c1-13-3-2-4-15(11-13)14-5-7-16(8-6-14)27(24,25)17-9-10-26-12-19(17,22)18(21)20-23/h2-8,11,17,22-23H,9-10,12H2,1H3,(H,20,21)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168742
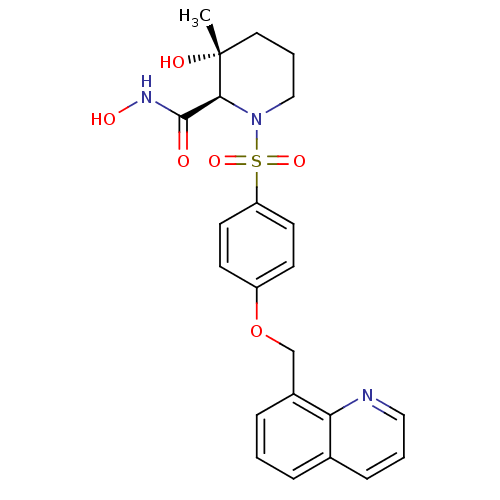
((2R,3R)-3-Hydroxy-3-methyl-1-[4-(quinolin-8-ylmeth...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2cccc3cccnc23)cc1 Show InChI InChI=1S/C23H25N3O6S/c1-23(28)12-4-14-26(21(23)22(27)25-29)33(30,31)19-10-8-18(9-11-19)32-15-17-6-2-5-16-7-3-13-24-20(16)17/h2-3,5-11,13,21,28-29H,4,12,14-15H2,1H3,(H,25,27)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50167611

((2R,5R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES CC1(C)C[C@@H](O)CN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C21H24ClFN2O6S/c1-21(2)10-15(26)11-25(19(21)20(27)24-28)32(29,30)17-7-5-16(6-8-17)31-12-13-3-4-14(23)9-18(13)22/h3-9,15,19,26,28H,10-12H2,1-2H3,(H,24,27)/t15-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Groton Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 15: 2808-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.105
BindingDB Entry DOI: 10.7270/Q2BC3Z24 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151734
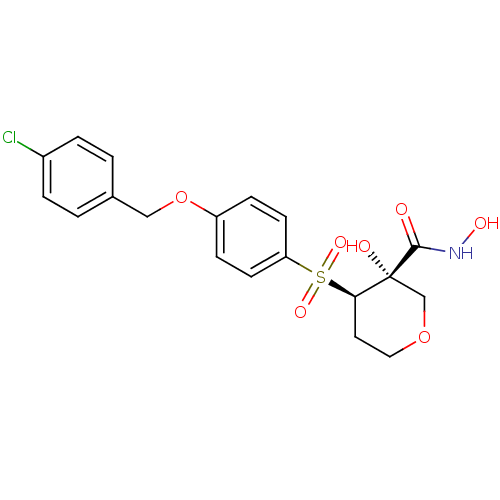
((3S,4R)-4-[4-(4-Chloro-benzyloxy)-benzenesulfonyl]...)Show SMILES ONC(=O)[C@@]1(O)COCC[C@H]1S(=O)(=O)c1ccc(OCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C19H20ClNO7S/c20-14-3-1-13(2-4-14)11-28-15-5-7-16(8-6-15)29(25,26)17-9-10-27-12-19(17,23)18(22)21-24/h1-8,17,23-24H,9-12H2,(H,21,22)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50151732

(4-[4-(2,4-Dichloro-benzyloxy)-benzenesulfonyl]-3-h...)Show SMILES ONC(=O)[C@@]1(O)COCC[C@H]1S(=O)(=O)c1ccc(OCc2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C19H19Cl2NO7S/c20-13-2-1-12(16(21)9-13)10-29-14-3-5-15(6-4-14)30(26,27)17-7-8-28-11-19(17,24)18(23)22-25/h1-6,9,17,24-25H,7-8,10-11H2,(H,22,23)/t17-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease 12 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151732

(4-[4-(2,4-Dichloro-benzyloxy)-benzenesulfonyl]-3-h...)Show SMILES ONC(=O)[C@@]1(O)COCC[C@H]1S(=O)(=O)c1ccc(OCc2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C19H19Cl2NO7S/c20-13-2-1-12(16(21)9-13)10-29-14-3-5-15(6-4-14)30(26,27)17-7-8-28-11-19(17,24)18(23)22-25/h1-6,9,17,24-25H,7-8,10-11H2,(H,22,23)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24922
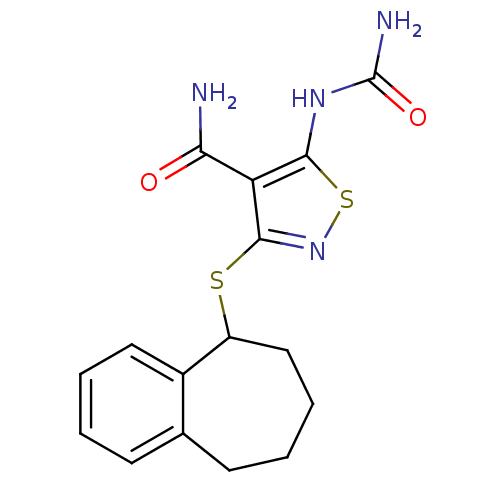
(5-(carbamoylamino)-3-{6,7,8,9-tetrahydro-5H-benzo[...)Show InChI InChI=1S/C16H18N4O2S2/c17-13(21)12-14(19-16(18)22)24-20-15(12)23-11-8-4-2-6-9-5-1-3-7-10(9)11/h1,3,5,7,11H,2,4,6,8H2,(H2,17,21)(H3,18,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118975
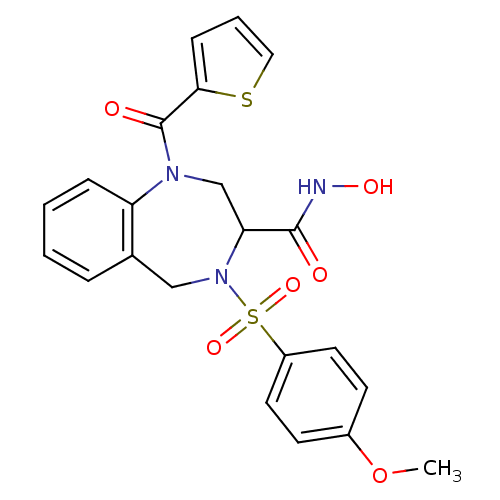
(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of matrix metalloprotease-13. |
Bioorg Med Chem Lett 13: 3243-6 (2003)
BindingDB Entry DOI: 10.7270/Q2Q23ZMM |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24923
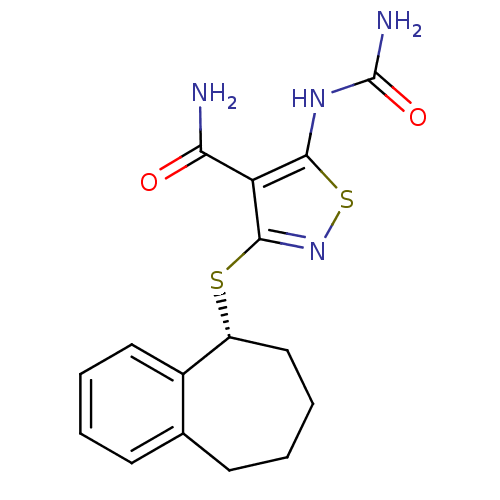
(5-(carbamoylamino)-3-[(5R)-6,7,8,9-tetrahydro-5H-b...)Show SMILES NC(=O)Nc1snc(S[C@@H]2CCCCc3ccccc23)c1C(N)=O |r| Show InChI InChI=1S/C16H18N4O2S2/c17-13(21)12-14(19-16(18)22)24-20-15(12)23-11-8-4-2-6-9-5-1-3-7-10(9)11/h1,3,5,7,11H,2,4,6,8H2,(H2,17,21)(H3,18,19,22)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-8 |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151736

(3-Hydroxy-4-(4-p-tolyloxy-benzenesulfonyl)-tetrahy...)Show SMILES Cc1ccc(Oc2ccc(cc2)S(=O)(=O)[C@@H]2CCOC[C@]2(O)C(=O)NO)cc1 Show InChI InChI=1S/C19H21NO7S/c1-13-2-4-14(5-3-13)27-15-6-8-16(9-7-15)28(24,25)17-10-11-26-12-19(17,22)18(21)20-23/h2-9,17,22-23H,10-12H2,1H3,(H,20,21)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151741

((3S,4R)-3-Hydroxy-4-(4-m-tolyloxy-benzenesulfonyl)...)Show SMILES Cc1cccc(Oc2ccc(cc2)S(=O)(=O)[C@@H]2CCOC[C@]2(O)C(=O)NO)c1 Show InChI InChI=1S/C19H21NO7S/c1-13-3-2-4-15(11-13)27-14-5-7-16(8-6-14)28(24,25)17-9-10-26-12-19(17,22)18(21)20-23/h2-8,11,17,22-23H,9-10,12H2,1H3,(H,20,21)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50167619
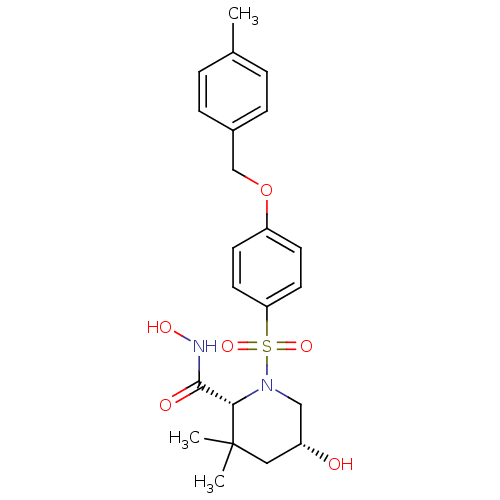
((2R,5R)-5-Hydroxy-3,3-dimethyl-1-[4-(4-methyl-benz...)Show SMILES Cc1ccc(COc2ccc(cc2)S(=O)(=O)N2C[C@H](O)CC(C)(C)[C@@H]2C(=O)NO)cc1 Show InChI InChI=1S/C22H28N2O6S/c1-15-4-6-16(7-5-15)14-30-18-8-10-19(11-9-18)31(28,29)24-13-17(25)12-22(2,3)20(24)21(26)23-27/h4-11,17,20,25,27H,12-14H2,1-3H3,(H,23,26)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Groton Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 15: 2808-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.105
BindingDB Entry DOI: 10.7270/Q2BC3Z24 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151738
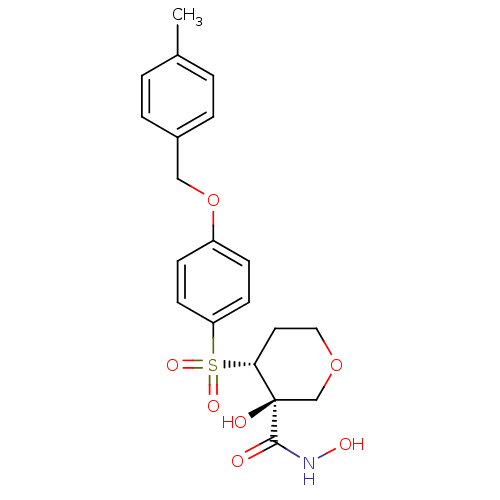
(3-Hydroxy-4-[4-(4-methyl-benzyloxy)-benzenesulfony...)Show SMILES Cc1ccc(COc2ccc(cc2)S(=O)(=O)[C@@H]2CCOC[C@]2(O)C(=O)NO)cc1 Show InChI InChI=1S/C20H23NO7S/c1-14-2-4-15(5-3-14)12-28-16-6-8-17(9-7-16)29(25,26)18-10-11-27-13-20(18,23)19(22)21-24/h2-9,18,23-24H,10-13H2,1H3,(H,21,22)/t18-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50167618

((2R,5R)-1-[4-(4-Bromo-benzyloxy)-benzenesulfonyl]-...)Show SMILES CC1(C)C[C@@H](O)CN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C21H25BrN2O6S/c1-21(2)11-16(25)12-24(19(21)20(26)23-27)31(28,29)18-9-7-17(8-10-18)30-13-14-3-5-15(22)6-4-14/h3-10,16,19,25,27H,11-13H2,1-2H3,(H,23,26)/t16-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Groton Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 15: 2808-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.105
BindingDB Entry DOI: 10.7270/Q2BC3Z24 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168743
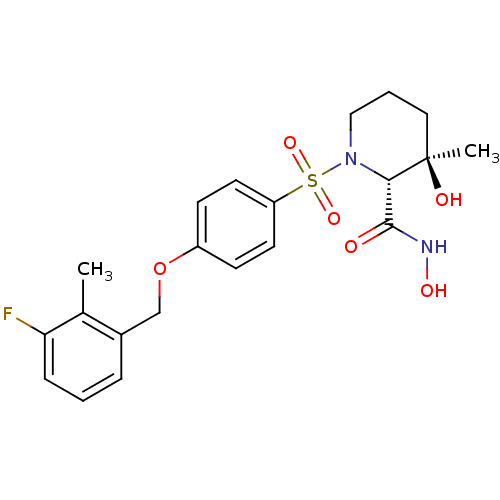
((2R,3R)-1-[4-(3-Fluoro-2-methyl-benzyloxy)-benzene...)Show SMILES Cc1c(F)cccc1COc1ccc(cc1)S(=O)(=O)N1CCC[C@@](C)(O)[C@@H]1C(=O)NO Show InChI InChI=1S/C21H25FN2O6S/c1-14-15(5-3-6-18(14)22)13-30-16-7-9-17(10-8-16)31(28,29)24-12-4-11-21(2,26)19(24)20(25)23-27/h3,5-10,19,26-27H,4,11-13H2,1-2H3,(H,23,25)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Rattus norvegicus) | BDBM50168758

((2R,3S)-3-Ethyl-1-[4-(4-fluoro-benzyloxy)-benzenes...)Show SMILES CC[C@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C21H25FN2O6S/c1-2-21(26)12-3-13-24(19(21)20(25)23-27)31(28,29)18-10-8-17(9-11-18)30-14-15-4-6-16(22)7-5-15/h4-11,19,26-27H,2-3,12-14H2,1H3,(H,23,25)/t19-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against rat TACE |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168738
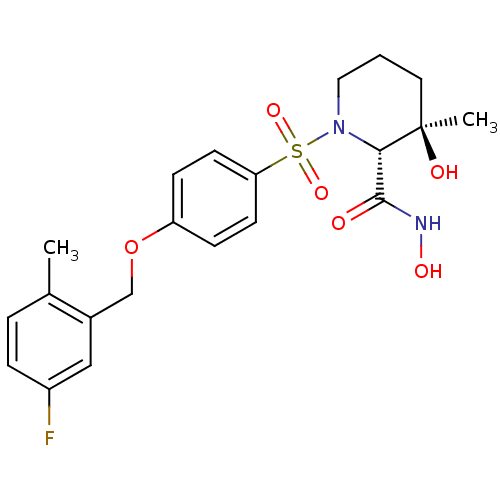
((2R,3R)-1-[4-(5-Fluoro-2-methyl-benzyloxy)-benzene...)Show SMILES Cc1ccc(F)cc1COc1ccc(cc1)S(=O)(=O)N1CCC[C@@](C)(O)[C@@H]1C(=O)NO Show InChI InChI=1S/C21H25FN2O6S/c1-14-4-5-16(22)12-15(14)13-30-17-6-8-18(9-7-17)31(28,29)24-11-3-10-21(2,26)19(24)20(25)23-27/h4-9,12,19,26-27H,3,10-11,13H2,1-2H3,(H,23,25)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50167620
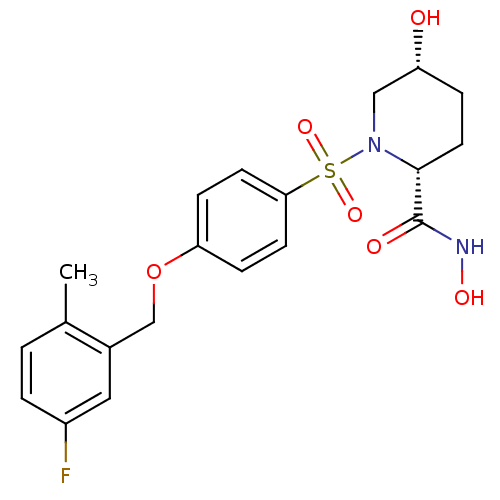
((2R,5R)-1-[4-(5-Fluoro-2-methyl-benzyloxy)-benzene...)Show SMILES Cc1ccc(F)cc1COc1ccc(cc1)S(=O)(=O)N1C[C@H](O)CC[C@@H]1C(=O)NO Show InChI InChI=1S/C20H23FN2O6S/c1-13-2-3-15(21)10-14(13)12-29-17-5-7-18(8-6-17)30(27,28)23-11-16(24)4-9-19(23)20(25)22-26/h2-3,5-8,10,16,19,24,26H,4,9,11-12H2,1H3,(H,22,25)/t16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Groton Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 15: 2808-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.105
BindingDB Entry DOI: 10.7270/Q2BC3Z24 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168752
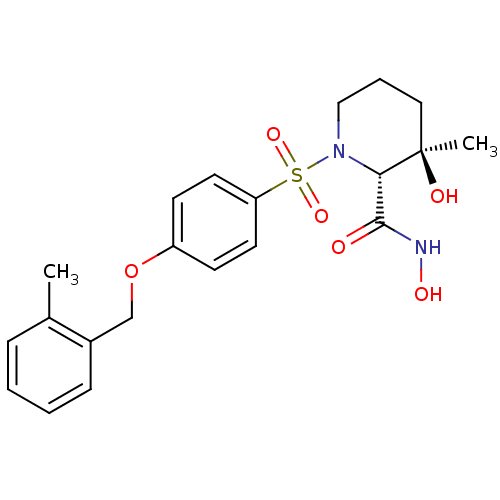
((2R,3R)-3-Hydroxy-3-methyl-1-[4-(2-methyl-benzylox...)Show SMILES Cc1ccccc1COc1ccc(cc1)S(=O)(=O)N1CCC[C@@](C)(O)[C@@H]1C(=O)NO Show InChI InChI=1S/C21H26N2O6S/c1-15-6-3-4-7-16(15)14-29-17-8-10-18(11-9-17)30(27,28)23-13-5-12-21(2,25)19(23)20(24)22-26/h3-4,6-11,19,25-26H,5,12-14H2,1-2H3,(H,22,24)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151746

(4-[4-(3-Chloro-benzyloxy)-benzenesulfonyl]-3-hydro...)Show SMILES ONC(=O)[C@@]1(O)COCC[C@H]1S(=O)(=O)c1ccc(OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C19H20ClNO7S/c20-14-3-1-2-13(10-14)11-28-15-4-6-16(7-5-15)29(25,26)17-8-9-27-12-19(17,23)18(22)21-24/h1-7,10,17,23-24H,8-9,11-12H2,(H,21,22)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168741
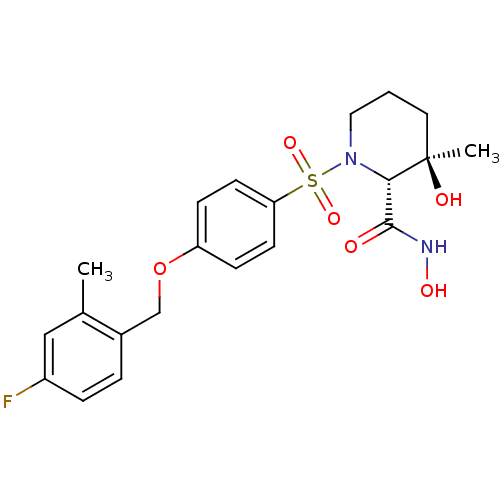
((2R,3R)-1-[4-(4-Fluoro-2-methyl-benzyloxy)-benzene...)Show SMILES Cc1cc(F)ccc1COc1ccc(cc1)S(=O)(=O)N1CCC[C@@](C)(O)[C@@H]1C(=O)NO Show InChI InChI=1S/C21H25FN2O6S/c1-14-12-16(22)5-4-15(14)13-30-17-6-8-18(9-7-17)31(28,29)24-11-3-10-21(2,26)19(24)20(25)23-27/h4-9,12,19,26-27H,3,10-11,13H2,1-2H3,(H,23,25)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168756

((2R,3R)-3-Hydroxy-1-[4-(isoquinolin-8-ylmethoxy)-b...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2cccc3ccncc23)cc1 Show InChI InChI=1S/C23H25N3O6S/c1-23(28)11-3-13-26(21(23)22(27)25-29)33(30,31)19-8-6-18(7-9-19)32-15-17-5-2-4-16-10-12-24-14-20(16)17/h2,4-10,12,14,21,28-29H,3,11,13,15H2,1H3,(H,25,27)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against TNF-alpha release in LPS treated whole blood |
Bioorg Med Chem Lett 15: 3385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.037
BindingDB Entry DOI: 10.7270/Q25D8RCC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50151733

((3S,4R)-4-[4-(2-Chloro-benzyloxy)-benzenesulfonyl]...)Show SMILES ONC(=O)[C@@]1(O)COCC[C@H]1S(=O)(=O)c1ccc(OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C19H20ClNO7S/c20-16-4-2-1-3-13(16)11-28-14-5-7-15(8-6-14)29(25,26)17-9-10-27-12-19(17,23)18(22)21-24/h1-8,17,23-24H,9-12H2,(H,21,22)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 14: 4727-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.081
BindingDB Entry DOI: 10.7270/Q29S1QGP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50167621

((2R,5R)-5-Hydroxy-3,3-dimethyl-1-[4-(3-methyl-benz...)Show SMILES Cc1cccc(COc2ccc(cc2)S(=O)(=O)N2C[C@H](O)CC(C)(C)[C@@H]2C(=O)NO)c1 Show InChI InChI=1S/C22H28N2O6S/c1-15-5-4-6-16(11-15)14-30-18-7-9-19(10-8-18)31(28,29)24-13-17(25)12-22(2,3)20(24)21(26)23-27/h4-11,17,20,25,27H,12-14H2,1-3H3,(H,23,26)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Groton Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 15: 2808-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.105
BindingDB Entry DOI: 10.7270/Q2BC3Z24 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172252

(CHEMBL3809526)Show SMILES CC(=O)Nc1cc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)ccc1N1CCC[C@H](N)C1 |r| Show InChI InChI=1S/C23H27N9O/c1-14(33)27-19-9-18(6-7-20(19)31-8-2-3-16(25)13-31)28-21-10-22(29-17-4-5-17)32-23(30-21)15(11-24)12-26-32/h6-7,9-10,12,16-17,29H,2-5,8,13,25H2,1H3,(H,27,33)(H,28,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172322

(CHEMBL3809288)Show SMILES CC(=O)Nc1cc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)ccc1N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H25N9O/c1-13(32)26-18-8-17(4-5-19(18)30-7-6-15(24)12-30)27-20-9-21(28-16-2-3-16)31-22(29-20)14(10-23)11-25-31/h4-5,8-9,11,15-16,28H,2-3,6-7,12,24H2,1H3,(H,26,32)(H,27,29)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172323
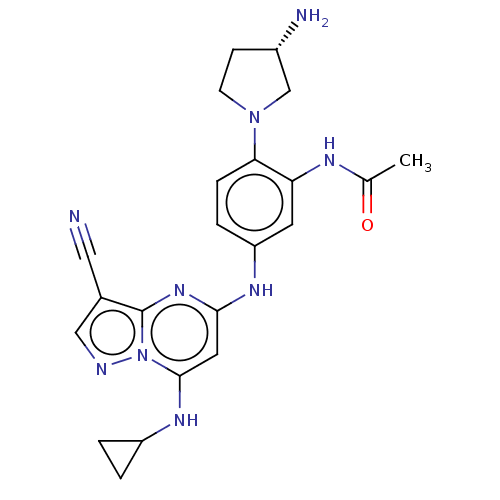
(CHEMBL3810252)Show SMILES CC(=O)Nc1cc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)ccc1N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H25N9O/c1-13(32)26-18-8-17(4-5-19(18)30-7-6-15(24)12-30)27-20-9-21(28-16-2-3-16)31-22(29-20)14(10-23)11-25-31/h4-5,8-9,11,15-16,28H,2-3,6-7,12,24H2,1H3,(H,26,32)(H,27,29)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24899

(3-{[1-(4-chlorophenyl)ethyl]sulfanyl}-5-[(methylca...)Show InChI InChI=1S/C14H15ClN4O2S2/c1-7(8-3-5-9(15)6-4-8)22-13-10(11(16)20)12(23-19-13)18-14(21)17-2/h3-7H,1-2H3,(H2,16,20)(H2,17,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172327
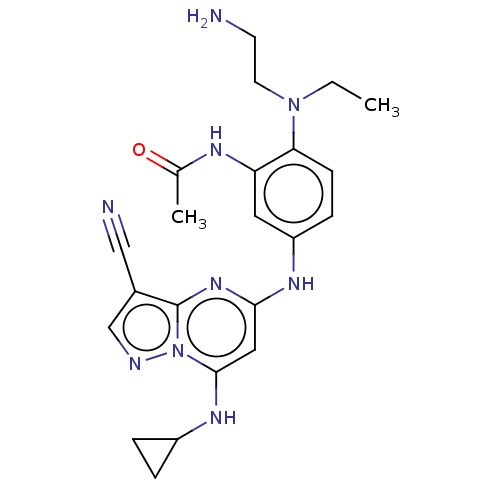
(CHEMBL3808860)Show SMILES CCN(CCN)c1ccc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)cc1NC(C)=O Show InChI InChI=1S/C22H27N9O/c1-3-30(9-8-23)19-7-6-17(10-18(19)26-14(2)32)27-20-11-21(28-16-4-5-16)31-22(29-20)15(12-24)13-25-31/h6-7,10-11,13,16,28H,3-5,8-9,23H2,1-2H3,(H,26,32)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172243
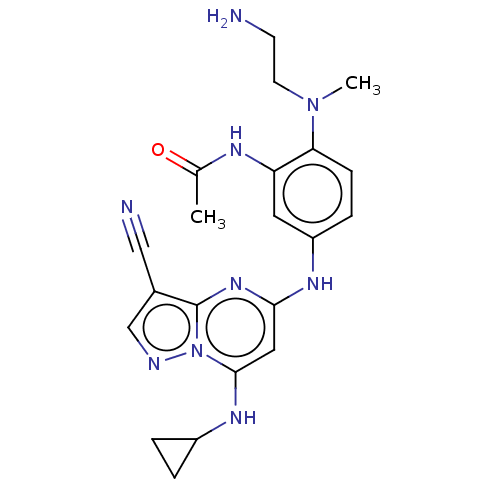
(CHEMBL3103192)Show SMILES CN(CCN)c1ccc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)cc1NC(C)=O Show InChI InChI=1S/C21H25N9O/c1-13(31)25-17-9-16(5-6-18(17)29(2)8-7-22)26-19-10-20(27-15-3-4-15)30-21(28-19)14(11-23)12-24-30/h5-6,9-10,12,15,27H,3-4,7-8,22H2,1-2H3,(H,25,31)(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172250
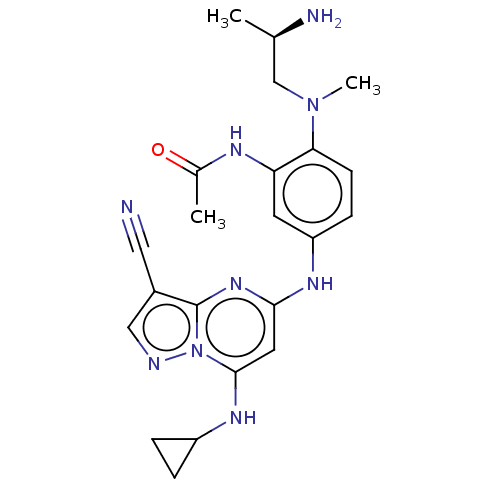
(CHEMBL3809024)Show SMILES C[C@@H](N)CN(C)c1ccc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)cc1NC(C)=O |r| Show InChI InChI=1S/C22H27N9O/c1-13(24)12-30(3)19-7-6-17(8-18(19)26-14(2)32)27-20-9-21(28-16-4-5-16)31-22(29-20)15(10-23)11-25-31/h6-9,11,13,16,28H,4-5,12,24H2,1-3H3,(H,26,32)(H,27,29)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50172249
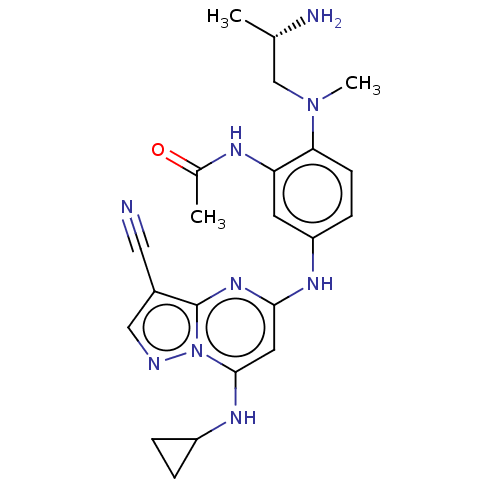
(CHEMBL3809915)Show SMILES C[C@H](N)CN(C)c1ccc(Nc2cc(NC3CC3)n3ncc(C#N)c3n2)cc1NC(C)=O |r| Show InChI InChI=1S/C22H27N9O/c1-13(24)12-30(3)19-7-6-17(8-18(19)26-14(2)32)27-20-9-21(28-16-4-5-16)31-22(29-20)15(10-23)11-25-31/h6-9,11,13,16,28H,4-5,12,24H2,1-3H3,(H,26,32)(H,27,29)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6xHis-tagged recombinant human CK2alpha expressed in fall armyworm Sf21 cells using BODIPY-FL-RRRDDDSDDD-CONH2 a... |
ACS Med Chem Lett 7: 300-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00452
BindingDB Entry DOI: 10.7270/Q2C53NRM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data