 Found 1381 hits with Last Name = 'somers' and Initial = 'do'
Found 1381 hits with Last Name = 'somers' and Initial = 'do' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442142
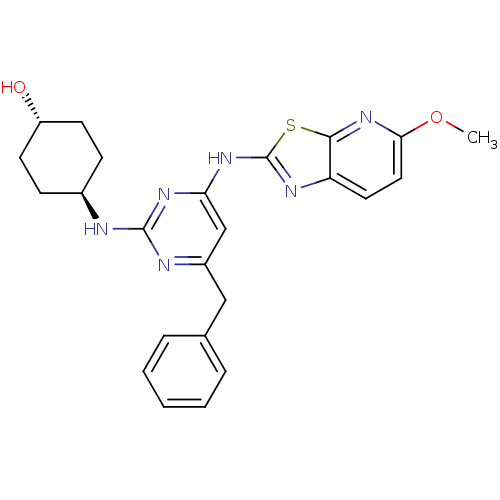
(CHEMBL2441275)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.29,-5.26,;33.51,-3.93,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C24H26N6O2S/c1-32-21-12-11-19-22(30-21)33-24(27-19)29-20-14-17(13-15-5-3-2-4-6-15)26-23(28-20)25-16-7-9-18(31)10-8-16/h2-6,11-12,14,16,18,31H,7-10,13H2,1H3,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442143
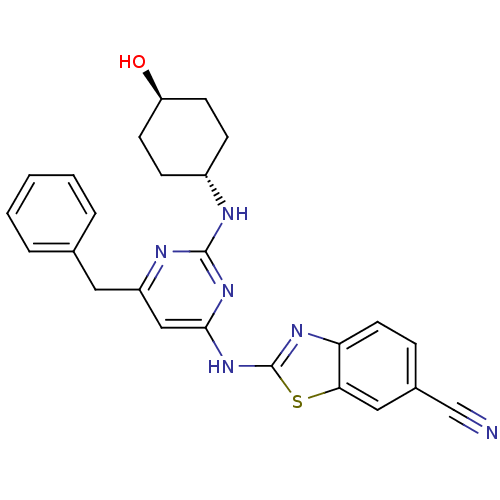
(CHEMBL2441274)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C#N)n1 |r,wU:1.0,wD:4.7,(23.68,-13.98,;22.35,-14.78,;22.37,-16.32,;21.05,-17.1,;19.71,-16.35,;19.69,-14.81,;21.01,-14.02,;18.39,-17.14,;17.04,-16.39,;15.72,-17.18,;14.38,-16.44,;13.04,-17.23,;11.71,-16.46,;11.71,-14.92,;10.38,-14.14,;9.04,-14.92,;9.04,-16.46,;10.38,-17.22,;14.35,-14.9,;15.67,-14.1,;15.65,-12.56,;16.85,-11.63,;16.82,-10.09,;18.27,-9.58,;18.87,-8.16,;20.4,-7.96,;21.33,-9.19,;20.73,-10.61,;19.2,-10.8,;18.33,-12.08,;22.85,-8.99,;24.37,-8.8,;17.02,-14.86,)| Show InChI InChI=1S/C25H24N6OS/c26-15-17-6-11-21-22(13-17)33-25(29-21)31-23-14-19(12-16-4-2-1-3-5-16)28-24(30-23)27-18-7-9-20(32)10-8-18/h1-6,11,13-14,18,20,32H,7-10,12H2,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442141

(CHEMBL2441276)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3cccnc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C23H24N6OS/c30-18-10-8-16(9-11-18)25-22-26-17(13-15-5-2-1-3-6-15)14-20(28-22)29-23-27-19-7-4-12-24-21(19)31-23/h1-7,12,14,16,18,30H,8-11,13H2,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442145

(CHEMBL2441271)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(Cl)cc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;33.51,-3.74,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H24ClN5OS/c25-16-6-11-20-21(13-16)32-24(28-20)30-22-14-18(12-15-4-2-1-3-5-15)27-23(29-22)26-17-7-9-19(31)10-8-17/h1-6,11,13-14,17,19,31H,7-10,12H2,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442146
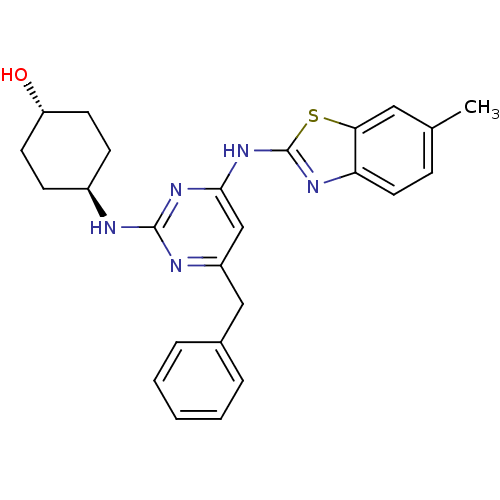
(CHEMBL2441270)Show SMILES Cc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:24.25,wD:21.21,(33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H27N5OS/c1-16-7-12-21-22(13-16)32-25(28-21)30-23-15-19(14-17-5-3-2-4-6-17)27-24(29-23)26-18-8-10-20(31)11-9-18/h2-7,12-13,15,18,20,31H,8-11,14H2,1H3,(H2,26,27,28,29,30)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263211
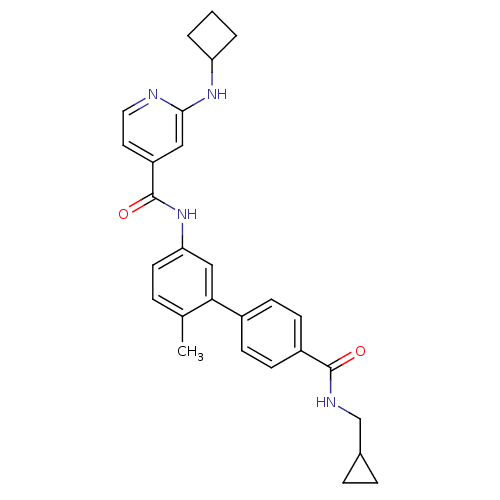
(2-Cyclobutylamino-N-[4'-(cyclopropylmethyl-carbamo...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CCC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-18-5-12-24(32-28(34)22-13-14-29-26(15-22)31-23-3-2-4-23)16-25(18)20-8-10-21(11-9-20)27(33)30-17-19-6-7-19/h5,8-16,19,23H,2-4,6-7,17H2,1H3,(H,29,31)(H,30,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442139
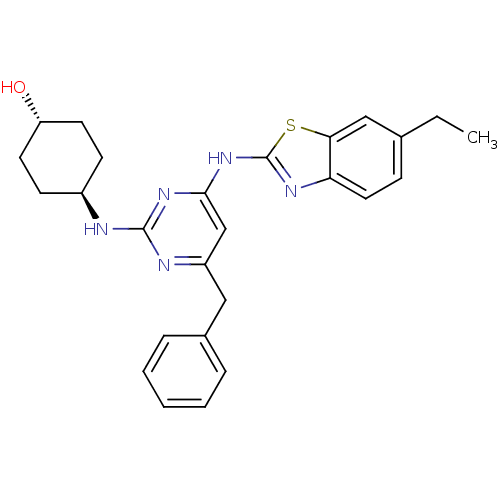
(CHEMBL2441273)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C26H29N5OS/c1-2-17-8-13-22-23(15-17)33-26(29-22)31-24-16-20(14-18-6-4-3-5-7-18)28-25(30-24)27-19-9-11-21(32)12-10-19/h3-8,13,15-16,19,21,32H,2,9-12,14H2,1H3,(H2,27,28,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50264756
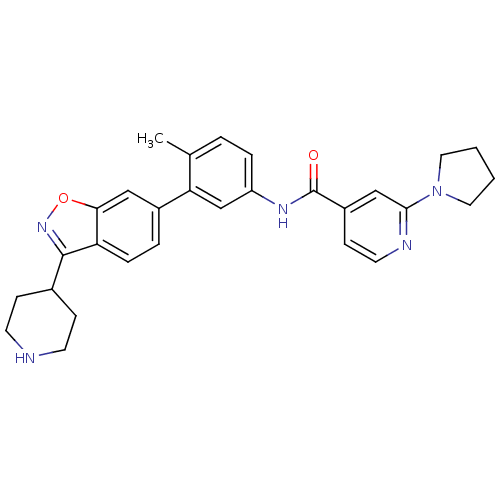
(CHEMBL485286 | N-(4-methyl-3-(3-(piperidin-4-yl)be...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc2c(noc2c1)C1CCNCC1 Show InChI InChI=1S/C29H31N5O2/c1-19-4-6-23(32-29(35)22-10-13-31-27(17-22)34-14-2-3-15-34)18-25(19)21-5-7-24-26(16-21)36-33-28(24)20-8-11-30-12-9-20/h4-7,10,13,16-18,20,30H,2-3,8-9,11-12,14-15H2,1H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442147
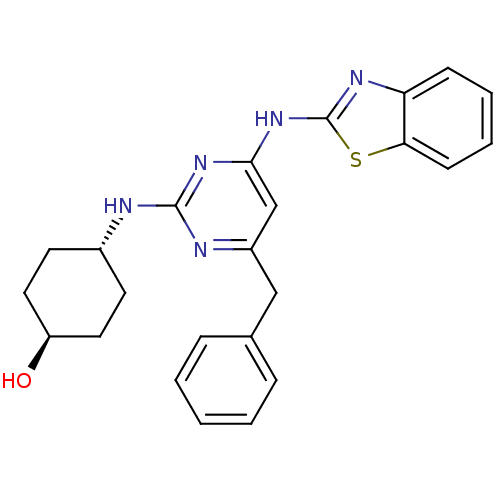
(CHEMBL2441269)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccccc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H25N5OS/c30-19-12-10-17(11-13-19)25-23-26-18(14-16-6-2-1-3-7-16)15-22(28-23)29-24-27-20-8-4-5-9-21(20)31-24/h1-9,15,17,19,30H,10-14H2,(H2,25,26,27,28,29)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50264993
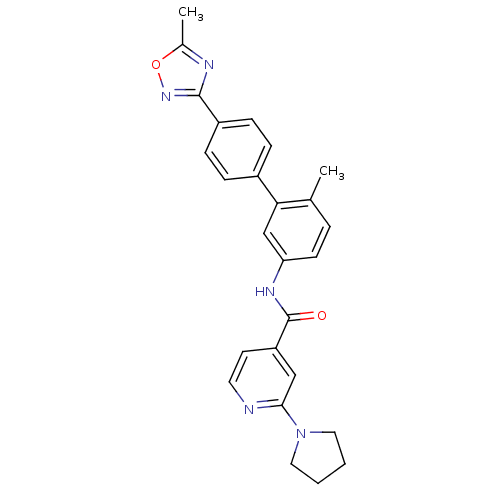
(CHEMBL521722 | N-(6-methyl-4'-(5-methyl-1,2,4-oxad...)Show SMILES Cc1nc(no1)-c1ccc(cc1)-c1cc(NC(=O)c2ccnc(c2)N2CCCC2)ccc1C Show InChI InChI=1S/C26H25N5O2/c1-17-5-10-22(29-26(32)21-11-12-27-24(15-21)31-13-3-4-14-31)16-23(17)19-6-8-20(9-7-19)25-28-18(2)33-30-25/h5-12,15-16H,3-4,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50264755

(4-methyl-N-(3-morpholin-4-ylphenyl)-3-(3-piperidin...)Show SMILES Cc1ccc(cc1-c1ccc2c(noc2c1)C1CCNCC1)C(=O)Nc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C30H32N4O3/c1-20-5-6-23(30(35)32-24-3-2-4-25(19-24)34-13-15-36-16-14-34)17-27(20)22-7-8-26-28(18-22)37-33-29(26)21-9-11-31-12-10-21/h2-8,17-19,21,31H,9-16H2,1H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263261
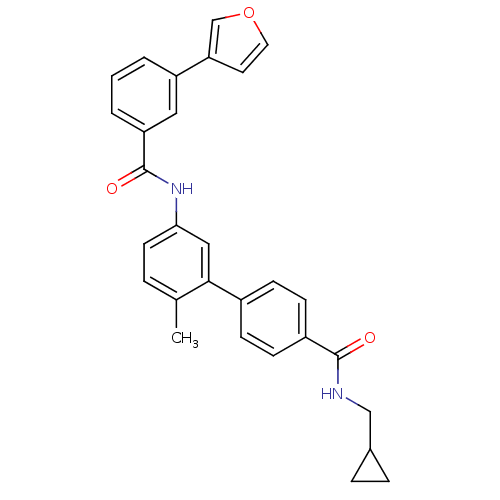
(5'-(3-Furan-3-yl-benzoylamino)-2'-methyl-biphenyl-...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)-c2ccoc2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C29H26N2O3/c1-19-5-12-26(31-29(33)24-4-2-3-23(15-24)25-13-14-34-18-25)16-27(19)21-8-10-22(11-9-21)28(32)30-17-20-6-7-20/h2-5,8-16,18,20H,6-7,17H2,1H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442140
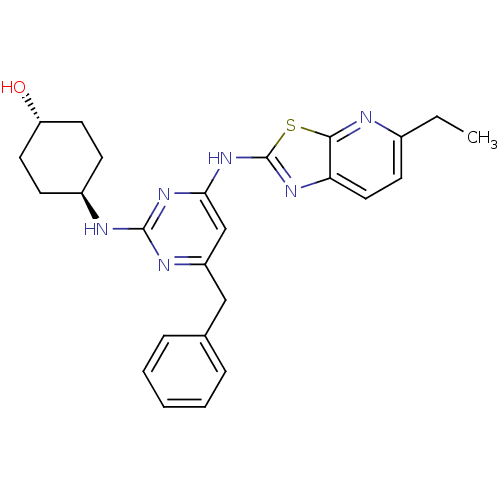
(CHEMBL2441277)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.5,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H28N6OS/c1-2-17-10-13-21-23(26-17)33-25(29-21)31-22-15-19(14-16-6-4-3-5-7-16)28-24(30-22)27-18-8-11-20(32)12-9-18/h3-7,10,13,15,18,20,32H,2,8-9,11-12,14H2,1H3,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265035
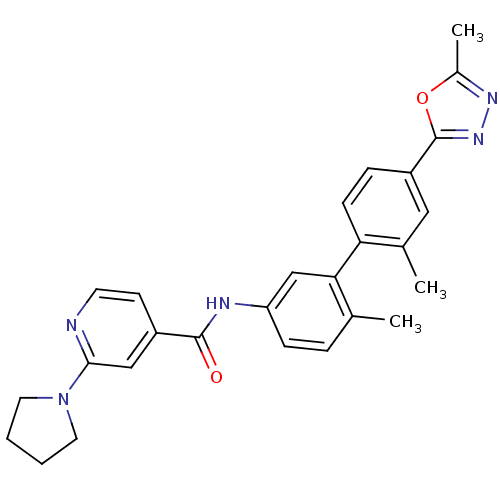
(CHEMBL495729 | N-(2',6-dimethyl-4'-(5-methyl-1,3,4...)Show SMILES Cc1nnc(o1)-c1ccc(c(C)c1)-c1cc(NC(=O)c2ccnc(c2)N2CCCC2)ccc1C Show InChI InChI=1S/C27H27N5O2/c1-17-6-8-22(29-26(33)20-10-11-28-25(15-20)32-12-4-5-13-32)16-24(17)23-9-7-21(14-18(23)2)27-31-30-19(3)34-27/h6-11,14-16H,4-5,12-13H2,1-3H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263129
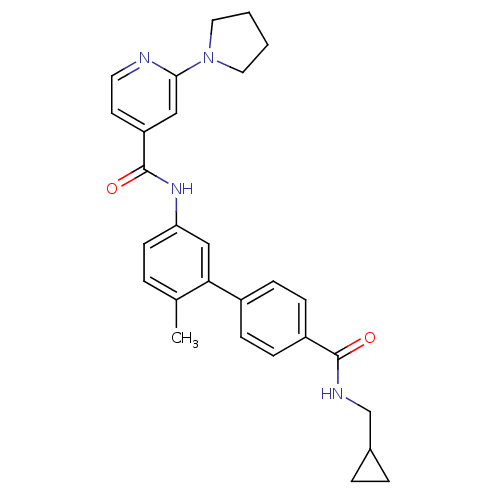
(CHEMBL477182 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-19-4-11-24(31-28(34)23-12-13-29-26(16-23)32-14-2-3-15-32)17-25(19)21-7-9-22(10-8-21)27(33)30-18-20-5-6-20/h4,7-13,16-17,20H,2-3,5-6,14-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263295
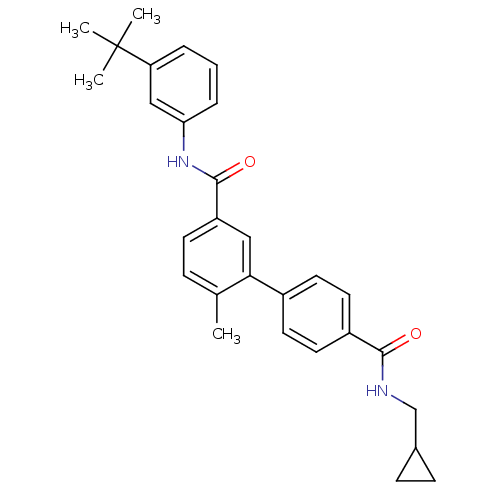
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-[(3-ter...)Show SMILES Cc1ccc(cc1-c1ccc(cc1)C(=O)NCC1CC1)C(=O)Nc1cccc(c1)C(C)(C)C Show InChI InChI=1S/C29H32N2O2/c1-19-8-11-23(28(33)31-25-7-5-6-24(17-25)29(2,3)4)16-26(19)21-12-14-22(15-13-21)27(32)30-18-20-9-10-20/h5-8,11-17,20H,9-10,18H2,1-4H3,(H,30,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262918
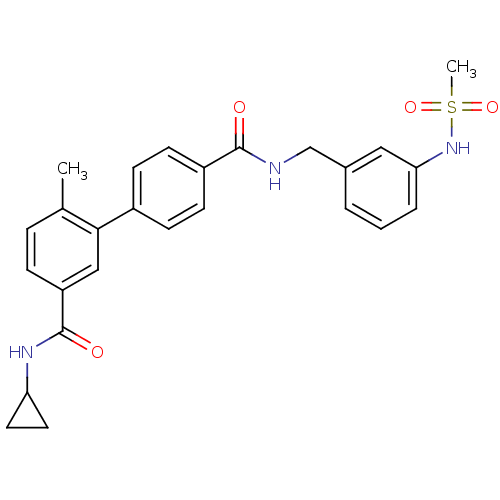
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...)Show SMILES Cc1ccc(cc1-c1ccc(cc1)C(=O)NCc1cccc(NS(C)(=O)=O)c1)C(=O)NC1CC1 Show InChI InChI=1S/C26H27N3O4S/c1-17-6-7-21(26(31)28-22-12-13-22)15-24(17)19-8-10-20(11-9-19)25(30)27-16-18-4-3-5-23(14-18)29-34(2,32)33/h3-11,14-15,22,29H,12-13,16H2,1-2H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263260
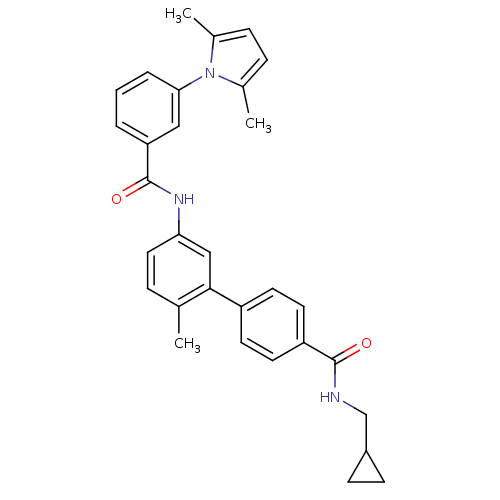
(5'-[3-(2,5-Dimethyl-pyrrol-1-yl)-benzoylamino]-2'-...)Show SMILES Cc1ccc(C)n1-c1cccc(c1)C(=O)Nc1ccc(C)c(c1)-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C31H31N3O2/c1-20-7-16-27(18-29(20)24-12-14-25(15-13-24)30(35)32-19-23-10-11-23)33-31(36)26-5-4-6-28(17-26)34-21(2)8-9-22(34)3/h4-9,12-18,23H,10-11,19H2,1-3H3,(H,32,35)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265257

(CHEMBL498390 | N-(4-methyl-3-(1-oxo-1,2-dihydroiso...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc2cc[nH]c(=O)c2c1 Show InChI InChI=1S/C26H24N4O2/c1-17-4-7-21(16-22(17)19-6-5-18-8-11-28-26(32)23(18)14-19)29-25(31)20-9-10-27-24(15-20)30-12-2-3-13-30/h4-11,14-16H,2-3,12-13H2,1H3,(H,28,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263167
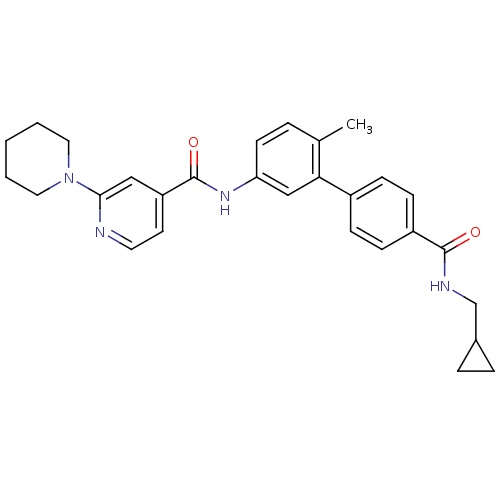
(3,4,5,6-Tetrahydro-2H-[1,2']bipyridinyl-4'-carboxy...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C29H32N4O2/c1-20-5-12-25(32-29(35)24-13-14-30-27(17-24)33-15-3-2-4-16-33)18-26(20)22-8-10-23(11-9-22)28(34)31-19-21-6-7-21/h5,8-14,17-18,21H,2-4,6-7,15-16,19H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265034
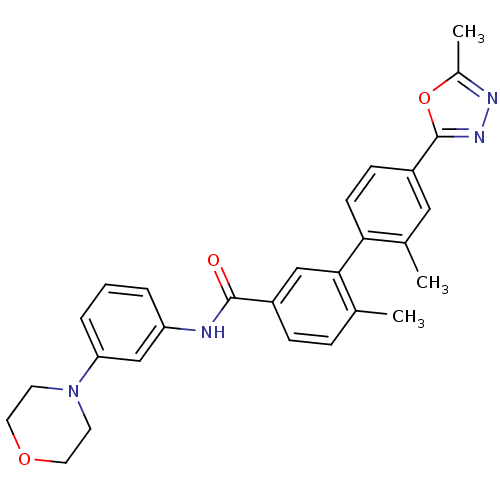
(2',6-dimethyl-4'-(5-methyl-1,3,4-oxadiazol-2-yl)-N...)Show SMILES Cc1nnc(o1)-c1ccc(c(C)c1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C28H28N4O3/c1-18-7-8-21(27(33)29-23-5-4-6-24(17-23)32-11-13-34-14-12-32)16-26(18)25-10-9-22(15-19(25)2)28-31-30-20(3)35-28/h4-10,15-17H,11-14H2,1-3H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265109

(CHEMBL525727 | N-cyclopropyl-4-methyl-3-(4-oxo-2-(...)Show SMILES Cc1ccc(cc1-c1ccc2c(c1)nc([nH]c2=O)-c1cccs1)C(=O)NC1CC1 Show InChI InChI=1S/C23H19N3O2S/c1-13-4-5-15(22(27)24-16-7-8-16)11-18(13)14-6-9-17-19(12-14)25-21(26-23(17)28)20-3-2-10-29-20/h2-6,9-12,16H,7-8H2,1H3,(H,24,27)(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263212
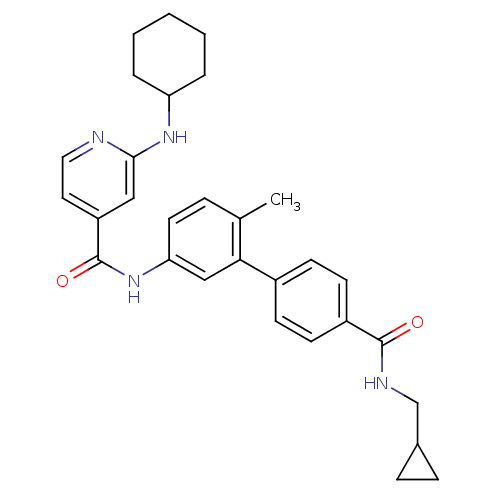
(2-Cyclohexylamino-N-[4'-(cyclopropylmethyl-carbamo...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CCCCC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C30H34N4O2/c1-20-7-14-26(18-27(20)22-10-12-23(13-11-22)29(35)32-19-21-8-9-21)34-30(36)24-15-16-31-28(17-24)33-25-5-3-2-4-6-25/h7,10-18,21,25H,2-6,8-9,19H2,1H3,(H,31,33)(H,32,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262974
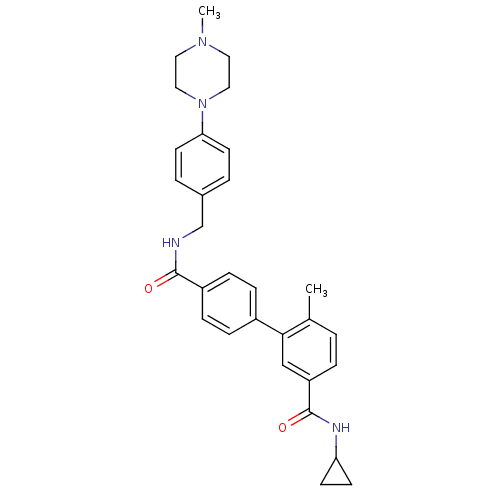
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...)Show SMILES CN1CCN(CC1)c1ccc(CNC(=O)c2ccc(cc2)-c2cc(ccc2C)C(=O)NC2CC2)cc1 Show InChI InChI=1S/C30H34N4O2/c1-21-3-6-25(30(36)32-26-11-12-26)19-28(21)23-7-9-24(10-8-23)29(35)31-20-22-4-13-27(14-5-22)34-17-15-33(2)16-18-34/h3-10,13-14,19,26H,11-12,15-18,20H2,1-2H3,(H,31,35)(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50418599
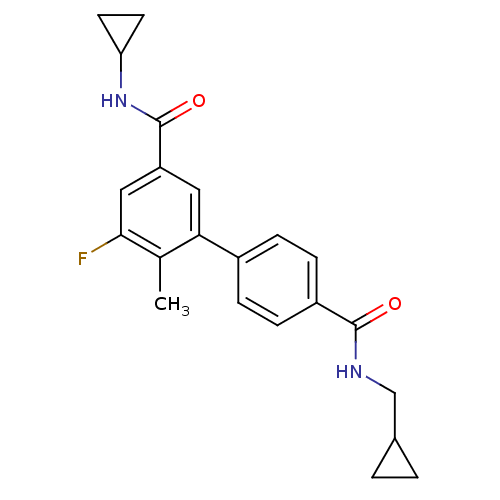
(CHEMBL1784164)Show SMILES Cc1c(F)cc(cc1-c1ccc(cc1)C(=O)NCC1CC1)C(=O)NC1CC1 Show InChI InChI=1S/C22H23FN2O2/c1-13-19(10-17(11-20(13)23)22(27)25-18-8-9-18)15-4-6-16(7-5-15)21(26)24-12-14-2-3-14/h4-7,10-11,14,18H,2-3,8-9,12H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p38alpha by fluorescence polarization method |
J Med Chem 52: 6257-69 (2009)
Article DOI: 10.1021/jm9004779
BindingDB Entry DOI: 10.7270/Q2J967NC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442150
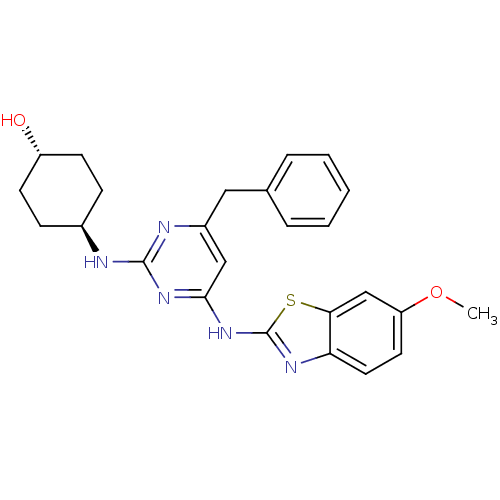
(CHEMBL2441112)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(39.46,-21.76,;38.71,-20.41,;37.17,-20.38,;36.42,-19.04,;34.89,-19.01,;34.11,-20.32,;32.6,-20.62,;32.41,-22.14,;31.06,-22.88,;31.04,-24.42,;29.69,-25.17,;29.66,-26.7,;28.32,-27.45,;26.94,-26.74,;25.65,-27.57,;24.28,-26.86,;24.21,-25.33,;25.51,-24.5,;26.87,-25.2,;30.98,-27.5,;32.32,-26.75,;33.65,-27.55,;33.62,-29.09,;32.27,-29.83,;32.24,-31.37,;33.56,-32.17,;33.53,-33.71,;34.9,-31.42,;34.93,-29.88,;32.35,-25.21,;33.8,-22.8,;34.85,-21.67,;36.38,-21.7,)| Show InChI InChI=1S/C25H27N5O2S/c1-32-20-11-12-21-22(15-20)33-25(28-21)30-23-14-18(13-16-5-3-2-4-6-16)27-24(29-23)26-17-7-9-19(31)10-8-17/h2-6,11-12,14-15,17,19,31H,7-10,13H2,1H3,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265371
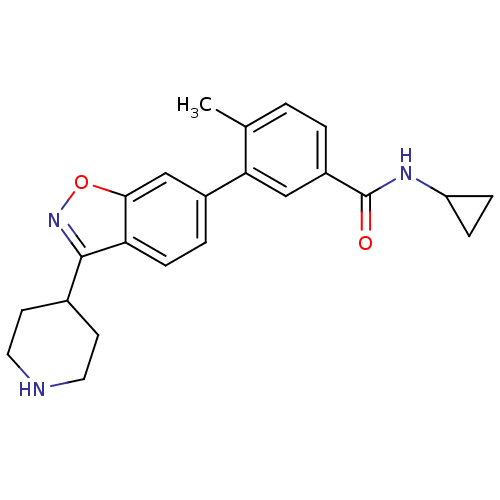
(CHEMBL497563 | N-cyclopropyl-4-methyl-3-(3-(piperi...)Show SMILES Cc1ccc(cc1-c1ccc2c(noc2c1)C1CCNCC1)C(=O)NC1CC1 Show InChI InChI=1S/C23H25N3O2/c1-14-2-3-17(23(27)25-18-5-6-18)12-20(14)16-4-7-19-21(13-16)28-26-22(19)15-8-10-24-11-9-15/h2-4,7,12-13,15,18,24H,5-6,8-11H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262917
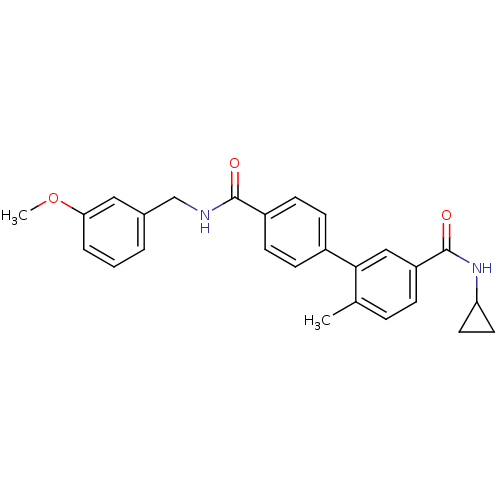
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 3-cyclopr...)Show SMILES COc1cccc(CNC(=O)c2ccc(cc2)-c2cc(ccc2C)C(=O)NC2CC2)c1 Show InChI InChI=1S/C26H26N2O3/c1-17-6-7-21(26(30)28-22-12-13-22)15-24(17)19-8-10-20(11-9-19)25(29)27-16-18-4-3-5-23(14-18)31-2/h3-11,14-15,22H,12-13,16H2,1-2H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263168

(CHEMBL476351 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCOCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O3/c1-19-2-9-24(31-28(34)23-10-11-29-26(16-23)32-12-14-35-15-13-32)17-25(19)21-5-7-22(8-6-21)27(33)30-18-20-3-4-20/h2,5-11,16-17,20H,3-4,12-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265260
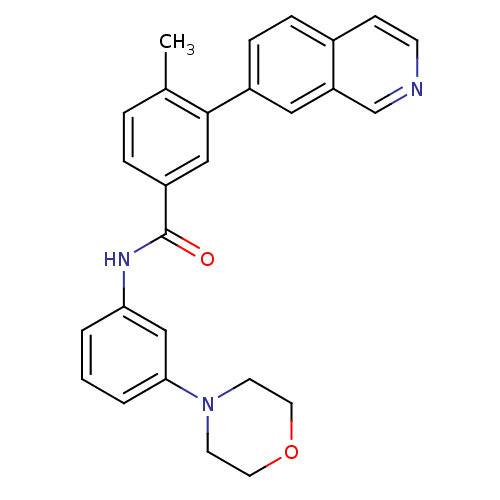
(3-(isoquinolin-7-yl)-4-methyl-N-(3-morpholinopheny...)Show SMILES Cc1ccc(cc1-c1ccc2ccncc2c1)C(=O)Nc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C27H25N3O2/c1-19-5-6-22(16-26(19)21-8-7-20-9-10-28-18-23(20)15-21)27(31)29-24-3-2-4-25(17-24)30-11-13-32-14-12-30/h2-10,15-18H,11-14H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263210
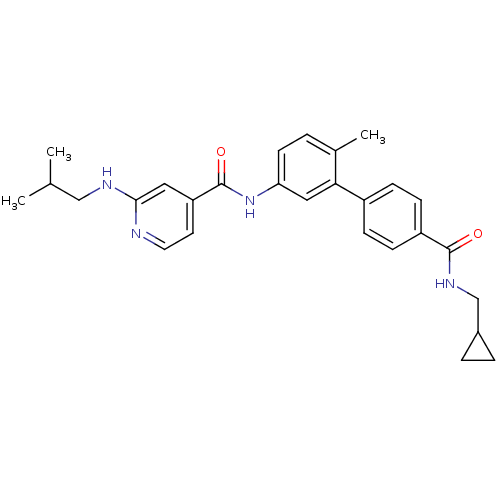
(CHEMBL478234 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES CC(C)CNc1cc(ccn1)C(=O)Nc1ccc(C)c(c1)-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H32N4O2/c1-18(2)16-30-26-14-23(12-13-29-26)28(34)32-24-11-4-19(3)25(15-24)21-7-9-22(10-8-21)27(33)31-17-20-5-6-20/h4,7-15,18,20H,5-6,16-17H2,1-3H3,(H,29,30)(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265071
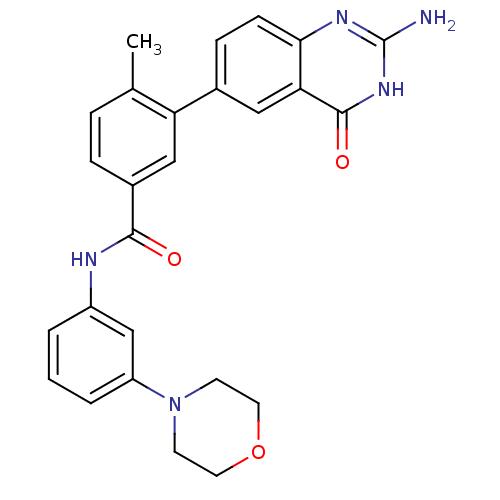
(3-(2-amino-4-oxo-3,4-dihydroquinazolin-6-yl)-4-met...)Show SMILES Cc1ccc(cc1-c1ccc2nc(N)[nH]c(=O)c2c1)C(=O)Nc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C26H25N5O3/c1-16-5-6-18(14-21(16)17-7-8-23-22(13-17)25(33)30-26(27)29-23)24(32)28-19-3-2-4-20(15-19)31-9-11-34-12-10-31/h2-8,13-15H,9-12H2,1H3,(H,28,32)(H3,27,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442153
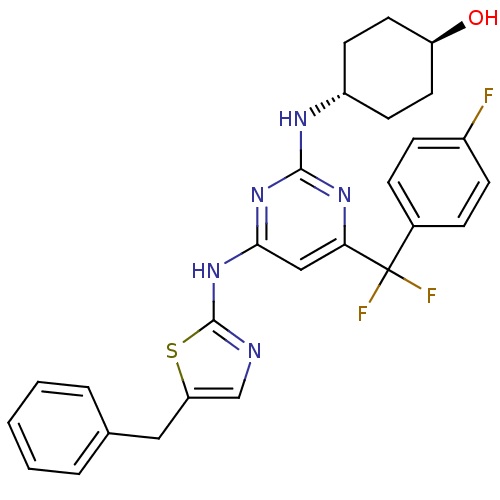
(CHEMBL2441283)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ncc(Cc3ccccc3)s2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(16.64,-34.58,;16.67,-33.04,;15.34,-32.24,;15.37,-30.7,;16.72,-29.96,;18.04,-30.75,;18.01,-32.29,;16.75,-28.42,;15.43,-27.62,;15.46,-26.08,;14.14,-25.29,;14.17,-23.75,;15.51,-23,;15.7,-21.49,;17.21,-21.19,;17.96,-22.54,;19.49,-22.73,;20.42,-21.5,;21.94,-21.69,;22.87,-20.46,;22.27,-19.04,;20.73,-18.86,;19.81,-20.09,;16.91,-23.66,;12.79,-26.03,;12.76,-27.57,;14.08,-28.37,;11.42,-28.31,;10.23,-29.3,;12.4,-29.5,;10.05,-27.6,;8.75,-28.44,;7.38,-27.73,;7.31,-26.19,;5.94,-25.48,;8.61,-25.36,;9.97,-26.06,)| Show InChI InChI=1S/C27H26F3N5OS/c28-19-8-6-18(7-9-19)27(29,30)23-15-24(34-25(33-23)32-20-10-12-21(36)13-11-20)35-26-31-16-22(37-26)14-17-4-2-1-3-5-17/h1-9,15-16,20-21,36H,10-14H2,(H2,31,32,33,34,35)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262915
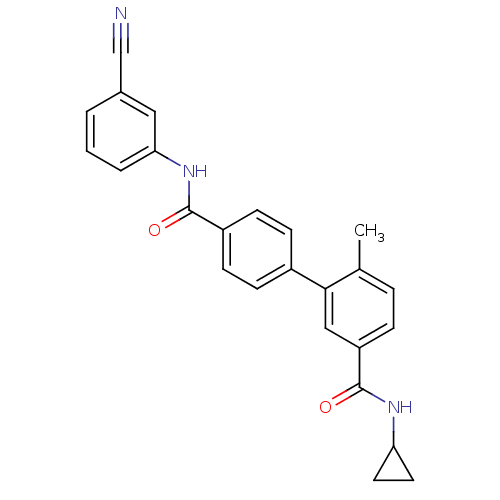
(6-Methyl-biphenyl-3,4'-dicarboxylic acid 4'-[(3-cy...)Show SMILES Cc1ccc(cc1-c1ccc(cc1)C(=O)Nc1cccc(c1)C#N)C(=O)NC1CC1 Show InChI InChI=1S/C25H21N3O2/c1-16-5-6-20(25(30)27-21-11-12-21)14-23(16)18-7-9-19(10-8-18)24(29)28-22-4-2-3-17(13-22)15-26/h2-10,13-14,21H,11-12H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262867
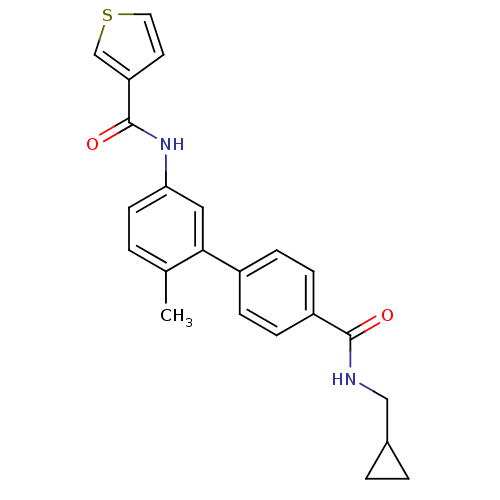
(CHEMBL476542 | Thiophene-3-carboxylic acid [4'-(cy...)Show SMILES Cc1ccc(NC(=O)c2ccsc2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C23H22N2O2S/c1-15-2-9-20(25-23(27)19-10-11-28-14-19)12-21(15)17-5-7-18(8-6-17)22(26)24-13-16-3-4-16/h2,5-12,14,16H,3-4,13H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263170
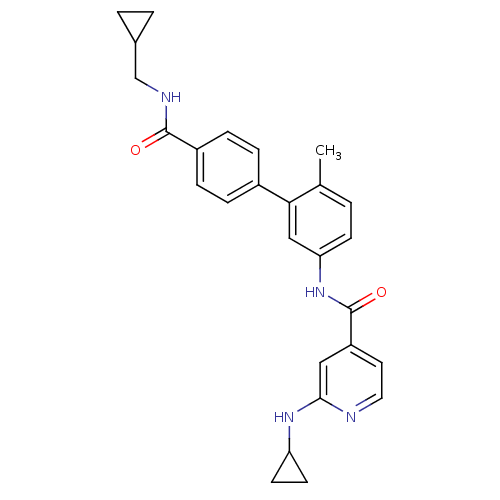
(2-Cyclopropylamino-N-[4'-(cyclopropylmethyl-carbam...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NC3CC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C27H28N4O2/c1-17-2-9-23(31-27(33)21-12-13-28-25(14-21)30-22-10-11-22)15-24(17)19-5-7-20(8-6-19)26(32)29-16-18-3-4-18/h2,5-9,12-15,18,22H,3-4,10-11,16H2,1H3,(H,28,30)(H,29,32)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263168

(CHEMBL476351 | N-[4'-(Cyclopropylmethyl-carbamoyl)...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCOCC2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O3/c1-19-2-9-24(31-28(34)23-10-11-29-26(16-23)32-12-14-35-15-13-32)17-25(19)21-5-7-22(8-6-21)27(33)30-18-20-3-4-20/h2,5-11,16-17,20H,3-4,12-15,18H2,1H3,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated ATF2 phosphorylation by TR-FRET assay |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262868
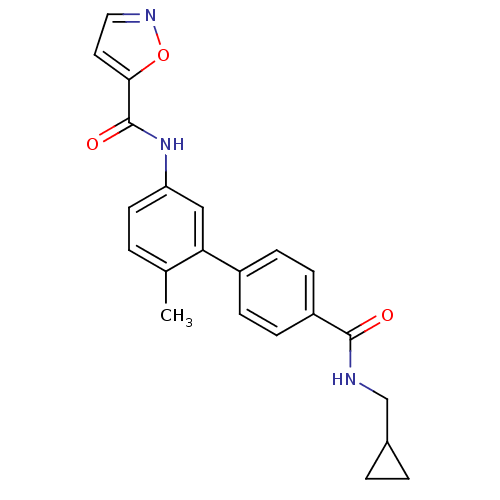
(CHEMBL476543 | Isoxazole-5-carboxylic acid [4'-(cy...)Show SMILES Cc1ccc(NC(=O)c2ccno2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C22H21N3O3/c1-14-2-9-18(25-22(27)20-10-11-24-28-20)12-19(14)16-5-7-17(8-6-16)21(26)23-13-15-3-4-15/h2,5-12,15H,3-4,13H2,1H3,(H,23,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labelled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]pheny... |
Bioorg Med Chem Lett 18: 4428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.048
BindingDB Entry DOI: 10.7270/Q2CR5T5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265154
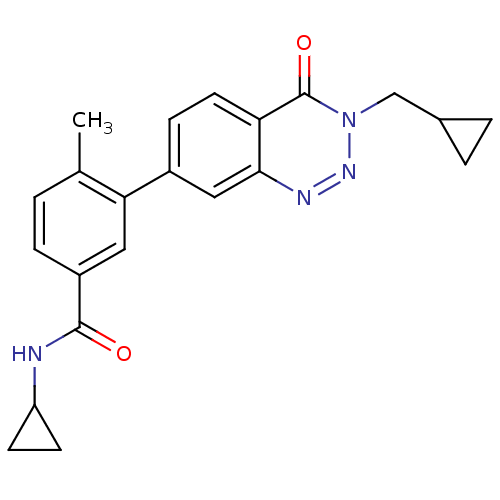
(CHEMBL521734 | N-cyclopropyl-3-(3-(cyclopropylmeth...)Show SMILES Cc1ccc(cc1-c1ccc2c(c1)nnn(CC1CC1)c2=O)C(=O)NC1CC1 Show InChI InChI=1S/C22H22N4O2/c1-13-2-5-16(21(27)23-17-7-8-17)10-19(13)15-6-9-18-20(11-15)24-25-26(22(18)28)12-14-3-4-14/h2,5-6,9-11,14,17H,3-4,7-8,12H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50262865
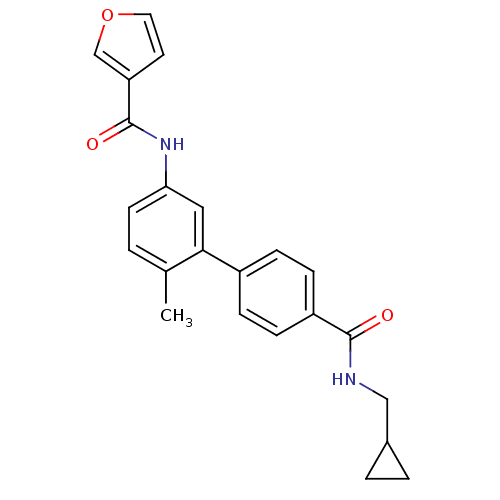
(CHEMBL477583 | Furan-3-carboxylic acid [4'-(cyclop...)Show SMILES Cc1ccc(NC(=O)c2ccoc2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C23H22N2O3/c1-15-2-9-20(25-23(27)19-10-11-28-14-19)12-21(15)17-5-7-18(8-6-17)22(26)24-13-16-3-4-16/h2,5-12,14,16H,3-4,13H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated ATF2 phosphorylation by TR-FRET assay |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442160
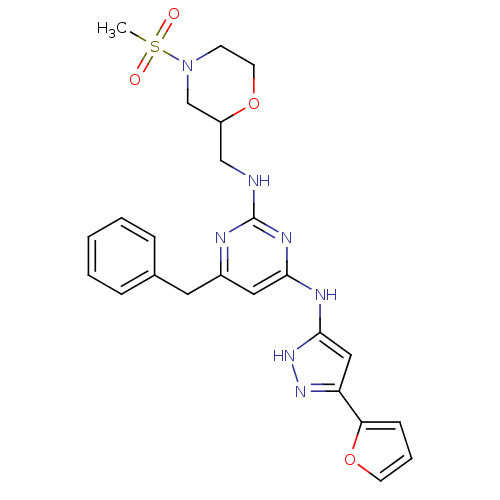
(CHEMBL2441279)Show SMILES CS(=O)(=O)N1CCOC(CNc2nc(Cc3ccccc3)cc(Nc3cc(n[nH]3)-c3ccco3)n2)C1 Show InChI InChI=1S/C24H27N7O4S/c1-36(32,33)31-9-11-34-19(16-31)15-25-24-26-18(12-17-6-3-2-4-7-17)13-22(28-24)27-23-14-20(29-30-23)21-8-5-10-35-21/h2-8,10,13-14,19H,9,11-12,15-16H2,1H3,(H3,25,26,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50263209
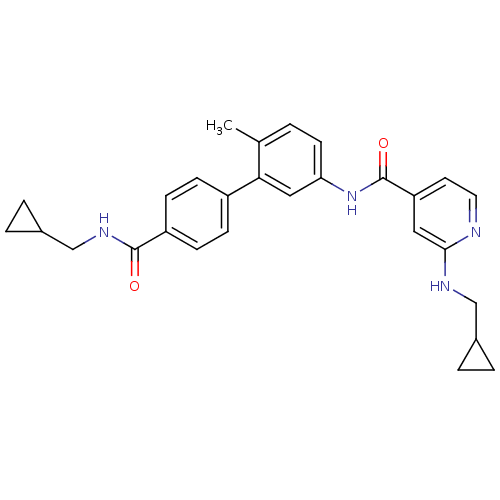
(2-(Cyclopropylmethyl-amino)-N-[4'-(cyclopropylmeth...)Show SMILES Cc1ccc(NC(=O)c2ccnc(NCC3CC3)c2)cc1-c1ccc(cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C28H30N4O2/c1-18-2-11-24(32-28(34)23-12-13-29-26(14-23)30-16-19-3-4-19)15-25(18)21-7-9-22(10-8-21)27(33)31-17-20-5-6-20/h2,7-15,19-20H,3-6,16-17H2,1H3,(H,29,30)(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442155
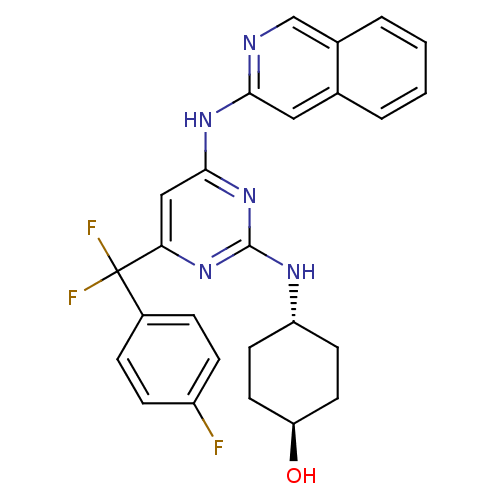
(CHEMBL2441281)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cc3ccccc3cn2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(43.15,-16.86,;43.18,-15.32,;41.86,-14.53,;41.89,-12.99,;43.24,-12.24,;44.55,-13.04,;44.52,-14.57,;43.27,-10.7,;41.95,-9.91,;41.97,-8.37,;40.65,-7.57,;40.68,-6.03,;41.99,-5.22,;43.35,-5.94,;44.66,-5.12,;46.01,-5.85,;47.32,-5.03,;47.26,-3.49,;45.91,-2.77,;44.61,-3.58,;43.23,-2.86,;41.93,-3.68,;39.3,-8.32,;39.28,-9.86,;40.6,-10.65,;37.93,-10.6,;36.74,-11.58,;38.91,-11.79,;36.57,-9.89,;35.27,-10.72,;33.9,-10.01,;33.83,-8.48,;32.46,-7.77,;35.13,-7.65,;36.49,-8.35,)| Show InChI InChI=1S/C26H24F3N5O/c27-19-7-5-18(6-8-19)26(28,29)22-14-24(33-23-13-16-3-1-2-4-17(16)15-30-23)34-25(32-22)31-20-9-11-21(35)12-10-20/h1-8,13-15,20-21,35H,9-12H2,(H2,30,31,32,33,34)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50418612
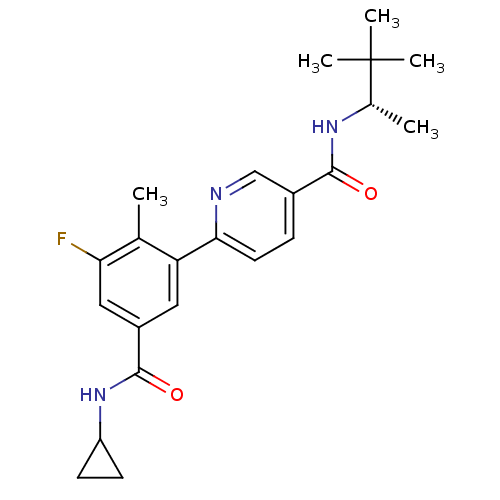
(CHEMBL1784177)Show SMILES C[C@H](NC(=O)c1ccc(nc1)-c1cc(cc(F)c1C)C(=O)NC1CC1)C(C)(C)C |r| Show InChI InChI=1S/C23H28FN3O2/c1-13-18(10-16(11-19(13)24)22(29)27-17-7-8-17)20-9-6-15(12-25-20)21(28)26-14(2)23(3,4)5/h6,9-12,14,17H,7-8H2,1-5H3,(H,26,28)(H,27,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p38alpha by fluorescence polarization method |
J Med Chem 52: 6257-69 (2009)
Article DOI: 10.1021/jm9004779
BindingDB Entry DOI: 10.7270/Q2J967NC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of rhodamine-green labeled (2-(6-amino-3-imino-3H-xanthen-9-yl)-5-{[({4-[4-(4-Cl-3-hydroxyphenyl)-5-(4-pyridinyl)-1H-imidazol-2yl]phenyl... |
Bioorg Med Chem Lett 18: 4433-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.028
BindingDB Entry DOI: 10.7270/Q2JH3M0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265155
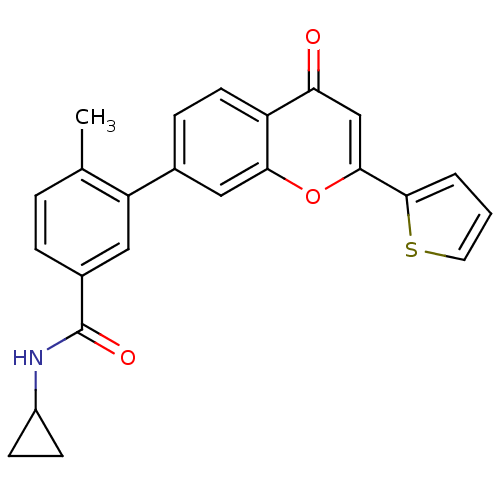
(CHEMBL496528 | N-cyclopropyl-4-methyl-3-(4-oxo-2-(...)Show SMILES Cc1ccc(cc1-c1ccc2c(c1)oc(cc2=O)-c1cccs1)C(=O)NC1CC1 Show InChI InChI=1S/C24H19NO3S/c1-14-4-5-16(24(27)25-17-7-8-17)11-19(14)15-6-9-18-20(26)13-22(28-21(18)12-15)23-3-2-10-29-23/h2-6,9-13,17H,7-8H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50264958
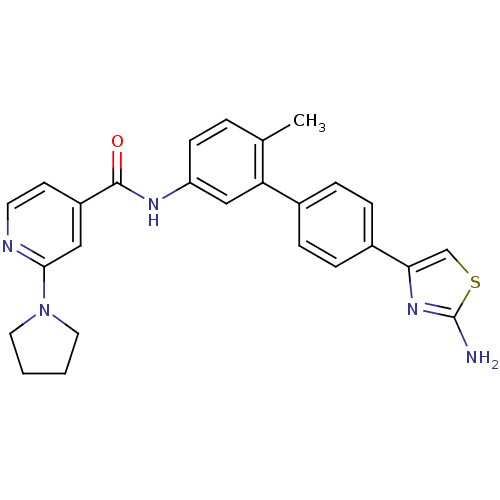
(CHEMBL526452 | N-(4'-(2-aminothiazol-4-yl)-6-methy...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc(cc1)-c1csc(N)n1 Show InChI InChI=1S/C26H25N5OS/c1-17-4-9-21(29-25(32)20-10-11-28-24(14-20)31-12-2-3-13-31)15-22(17)18-5-7-19(8-6-18)23-16-33-26(27)30-23/h4-11,14-16H,2-3,12-13H2,1H3,(H2,27,30)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50265072
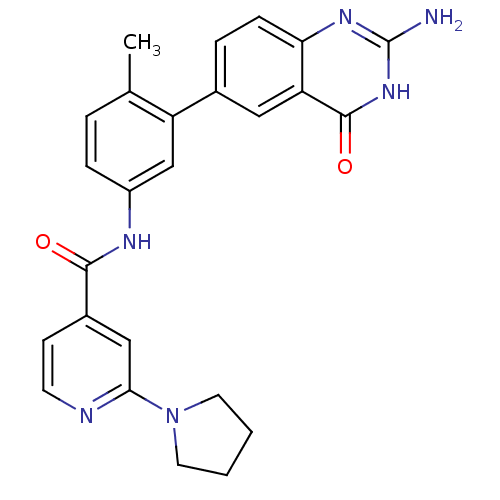
(CHEMBL521548 | N-(3-(2-amino-4-oxo-3,4-dihydroquin...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCCC2)cc1-c1ccc2nc(N)[nH]c(=O)c2c1 Show InChI InChI=1S/C25H24N6O2/c1-15-4-6-18(28-23(32)17-8-9-27-22(13-17)31-10-2-3-11-31)14-19(15)16-5-7-21-20(12-16)24(33)30-25(26)29-21/h4-9,12-14H,2-3,10-11H2,1H3,(H,28,32)(H3,26,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of fluorescent ATP competitive ligand from p38alpha |
Bioorg Med Chem Lett 18: 5285-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.051
BindingDB Entry DOI: 10.7270/Q28K7B0R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data