

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50440737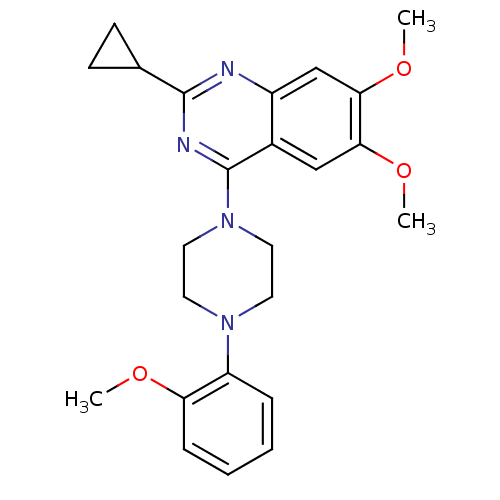 (CHEMBL2431120) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT3 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50440737 (CHEMBL2431120) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to PBR receptor (unknown origin) | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314930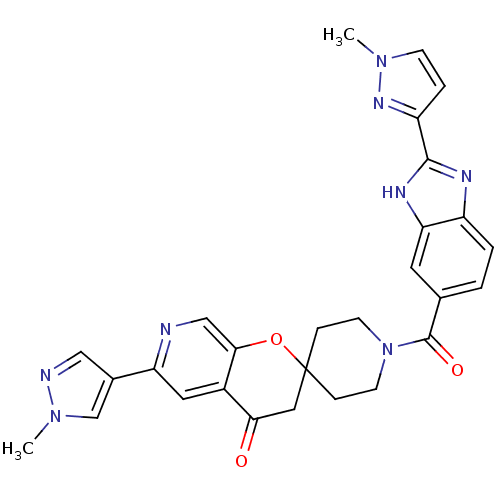 (1-(2-(1-methyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50440738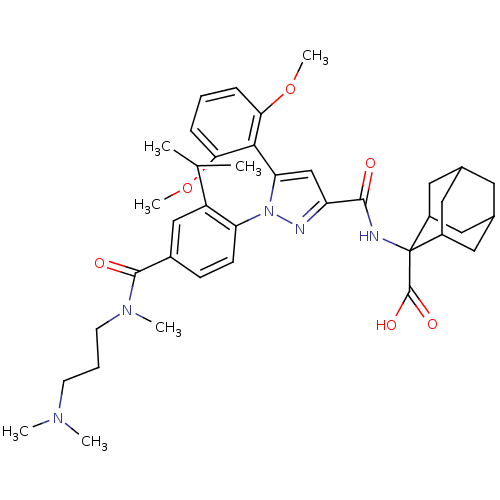 (CHEMBL2431105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics at Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]-neurotensin from NTR1 in HUVEC after 1 hr by gamma counting analysis | ACS Med Chem Lett 4: 846-851 (2013) Article DOI: 10.1021/ml400176n BindingDB Entry DOI: 10.7270/Q2959JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314931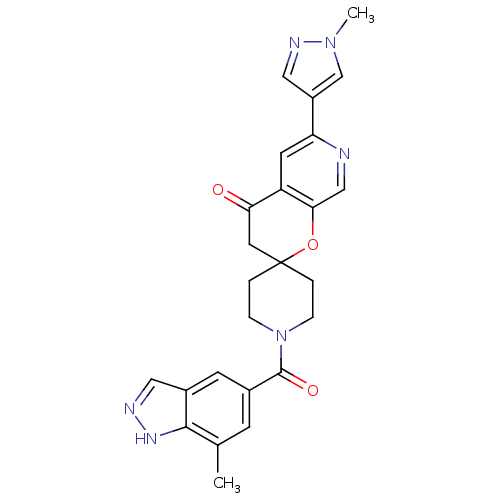 (1-(7-methyl-1H-indazole-5-carbonyl)-6'-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314932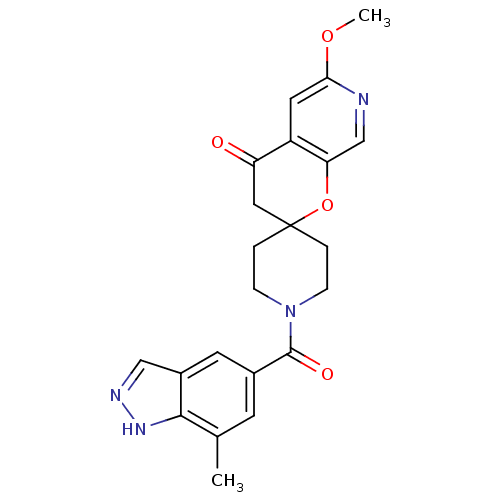 (6'-methoxy-1-(7-methyl-1H-indazole-5-carbonyl)spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314938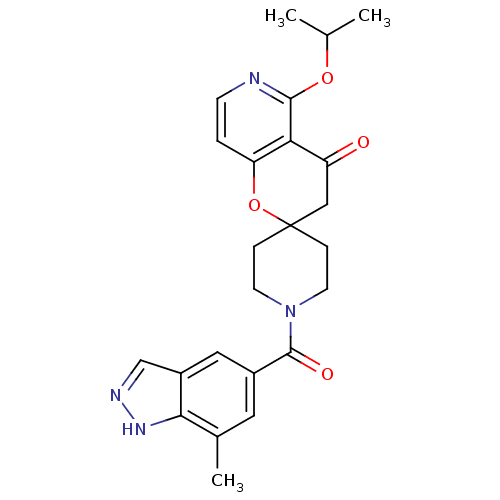 (5'-isopropoxy-1-(7-methyl-1H-indazole-5-carbonyl)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314905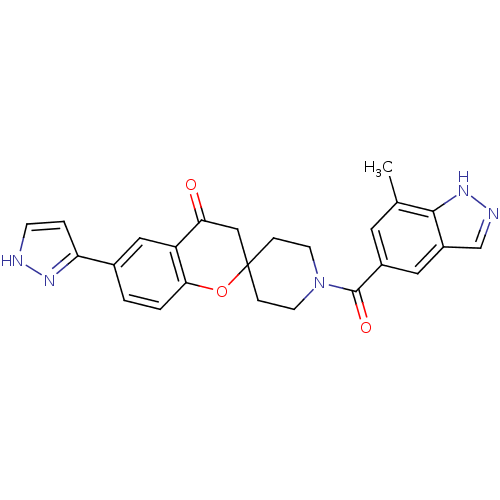 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1H-pyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314906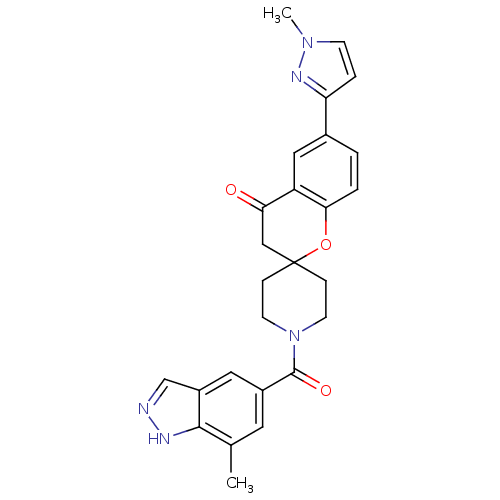 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314929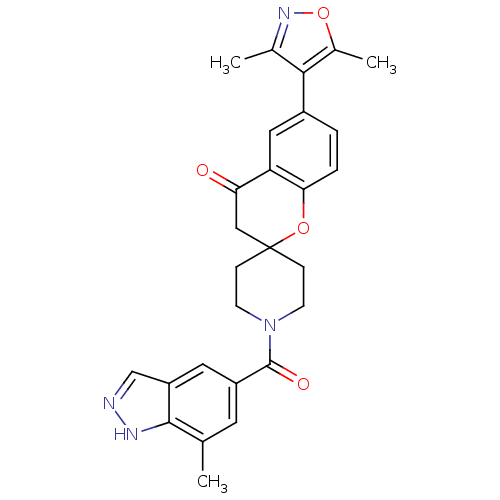 (6-(3,5-dimethylisoxazol-4-yl)-1'-(7-methyl-1H-inda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314925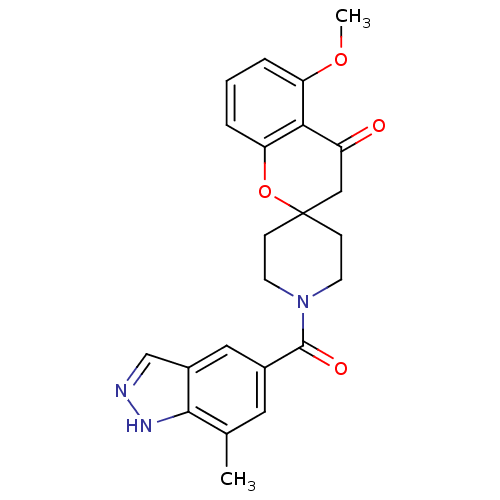 (5-methoxy-1'-(7-methyl-1H-indazole-5-carbonyl)spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365293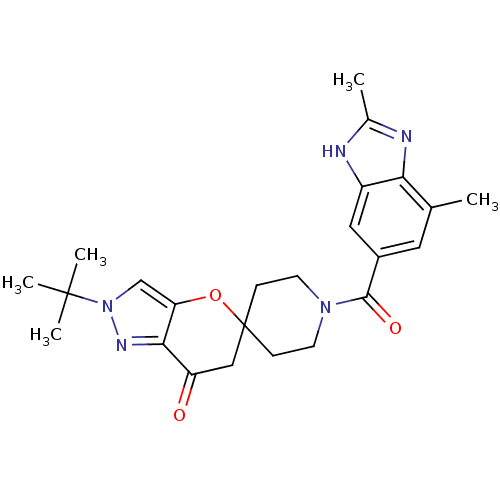 (CHEMBL1958374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365279 (CHEMBL1958360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365279 (CHEMBL1958360) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365292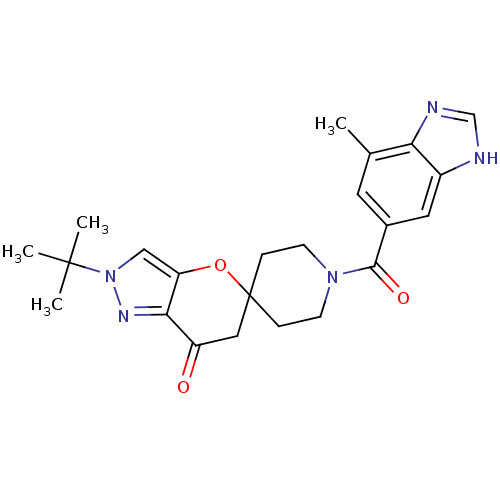 (CHEMBL1958373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314903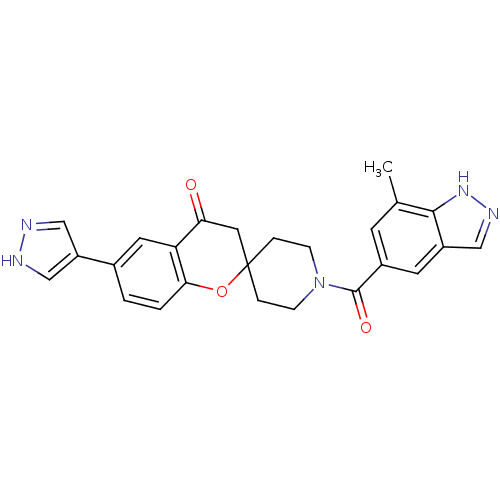 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1H-pyrazol...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314904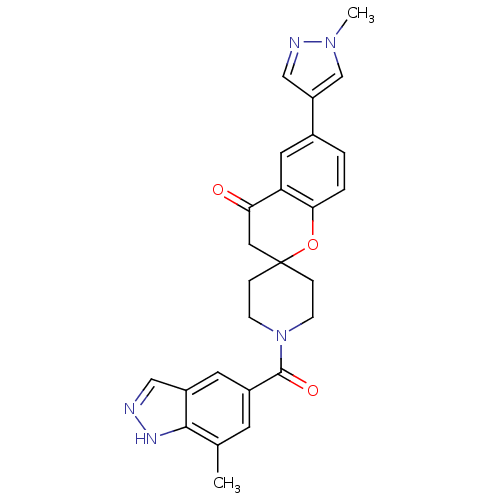 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1-methyl-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314907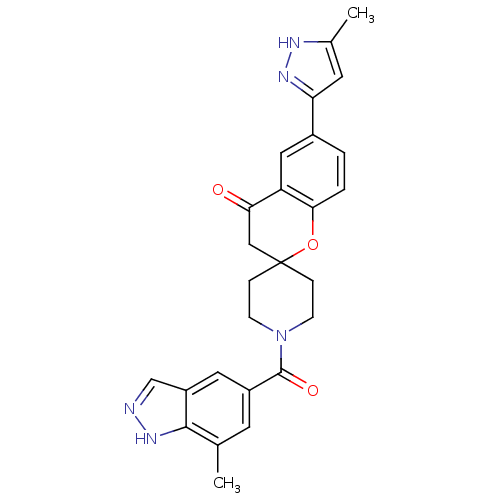 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(5-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50355142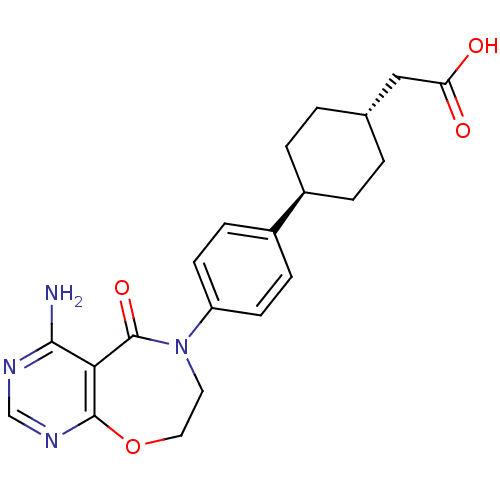 (CHEMBL1835919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting | ACS Med Chem Lett 2: 407-412 (2011) Article DOI: 10.1021/ml200051p BindingDB Entry DOI: 10.7270/Q2MW2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365287 (CHEMBL1958368) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365277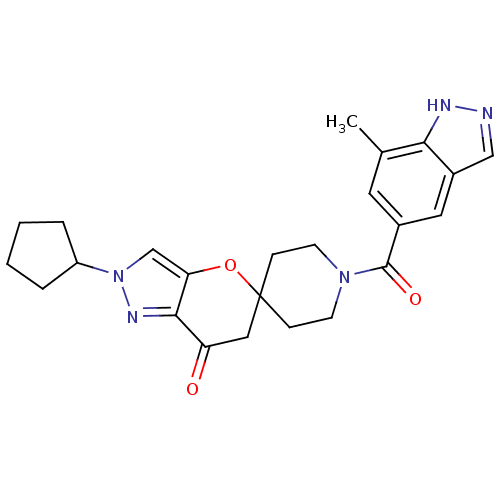 (CHEMBL1958359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365292 (CHEMBL1958373) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365293 (CHEMBL1958374) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314894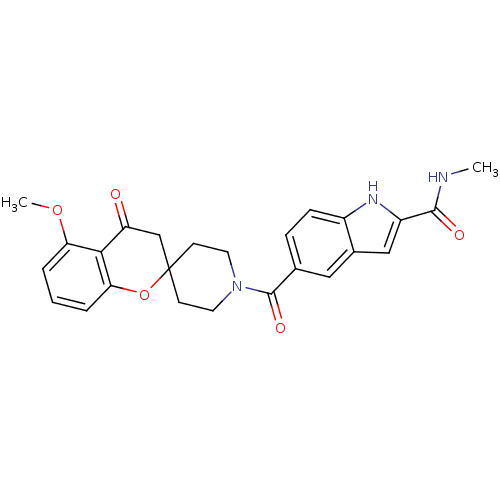 (5-(5-methoxy-4-oxospiro[chroman-2,4'-piperidine]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365295 (CHEMBL1955896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314888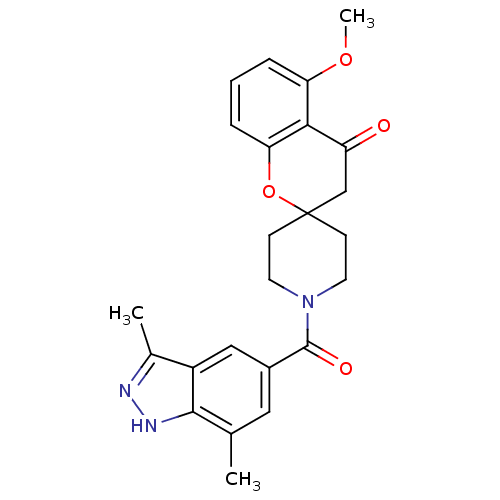 (1'-(3,7-dimethyl-1H-indazole-5-carbonyl)-5-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365284 (CHEMBL1958365) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314943 (1-(3,7-dimethyl-1H-indazole-5-carbonyl)-5'-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365290 (CHEMBL1958371) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314897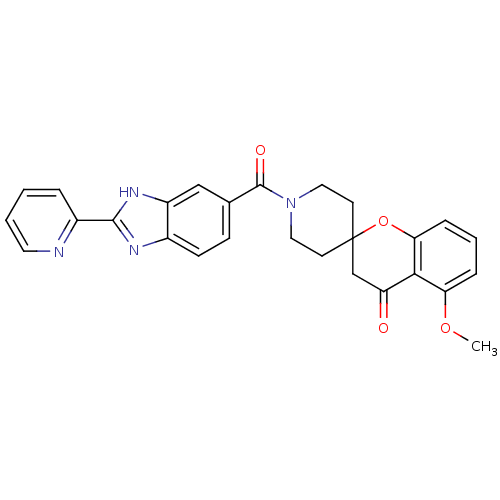 (5-methoxy-1'-(2-(pyridin-2-yl)-1H-benzo[d]imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365281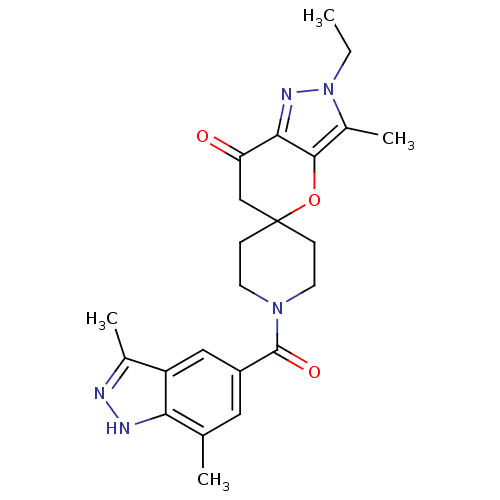 (CHEMBL1958362) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314929 (6-(3,5-dimethylisoxazol-4-yl)-1'-(7-methyl-1H-inda...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365285 (CHEMBL1958366) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365281 (CHEMBL1958362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365294 (CHEMBL1955895) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365277 (CHEMBL1958359) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365276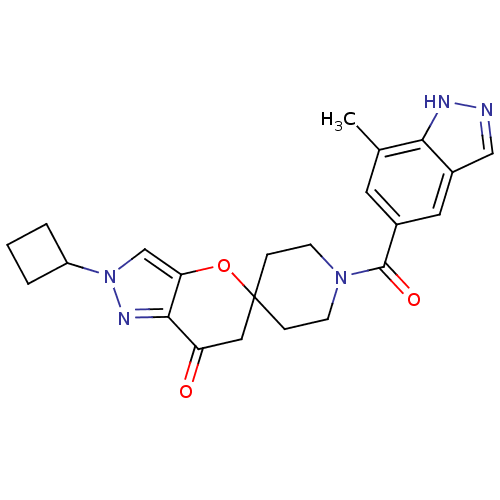 (CHEMBL1958358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365290 (CHEMBL1958371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365276 (CHEMBL1958358) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314896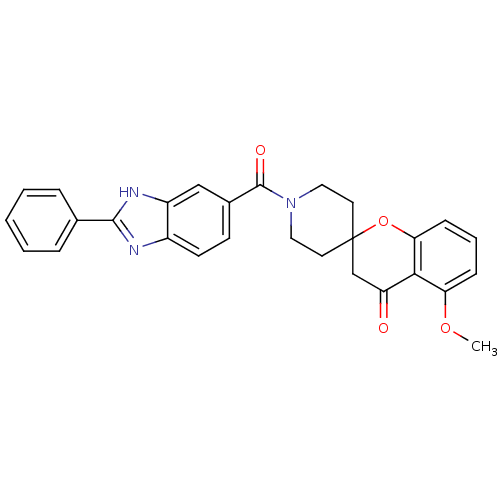 (5-methoxy-1'-(2-phenyl-1H-benzo[d]imidazole-5-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314895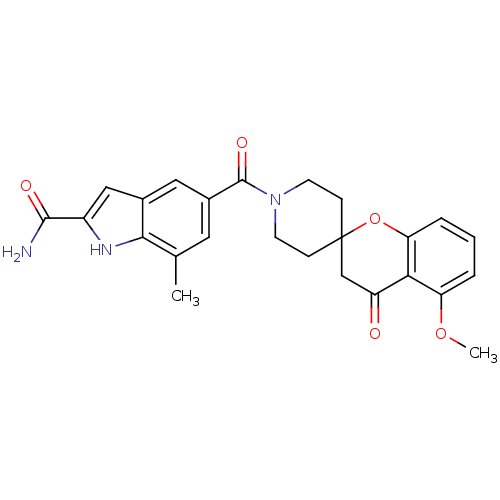 (5-(5-methoxy-4-oxospiro[chroman-2,4'-piperidine]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314896 (5-methoxy-1'-(2-phenyl-1H-benzo[d]imidazole-5-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314889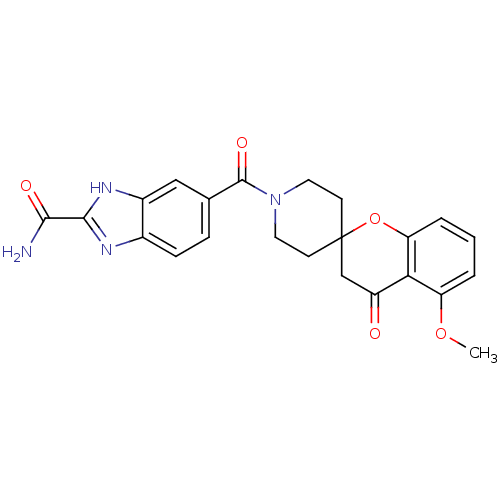 (5-(5-methoxy-4-oxospiro[chroman-2,4'-piperidine]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM27947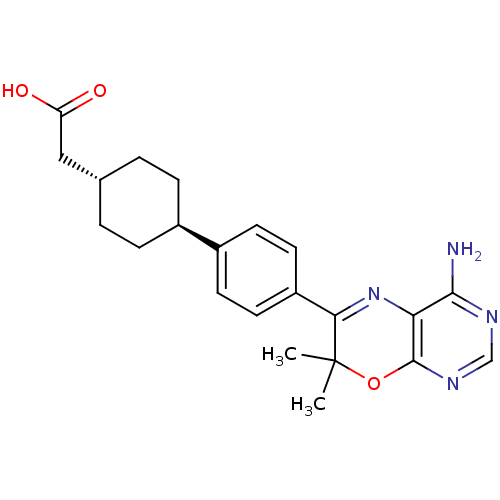 (2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting | ACS Med Chem Lett 2: 407-412 (2011) Article DOI: 10.1021/ml200051p BindingDB Entry DOI: 10.7270/Q2MW2HM6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365295 (CHEMBL1955896) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314928 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(2-methylox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314886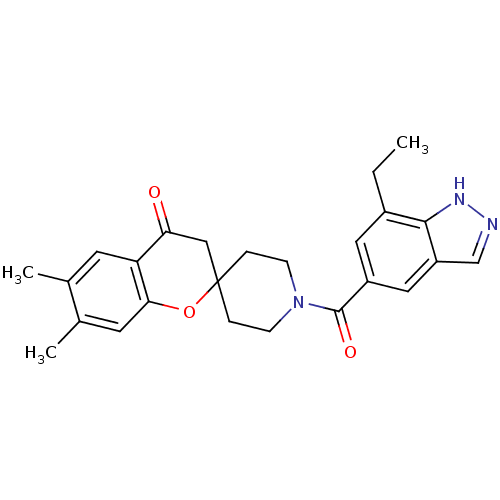 (1'-(7-ethyl-1H-indazole-5-carbonyl)-6,7-dimethylsp...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50314905 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1H-pyrazol...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 after 7 mins by liquid scintillation counter | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 675 total ) | Next | Last >> |