

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20461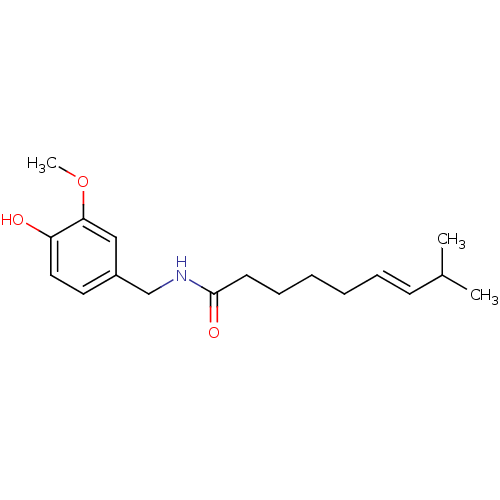 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human recombinant TRPV1 | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50052442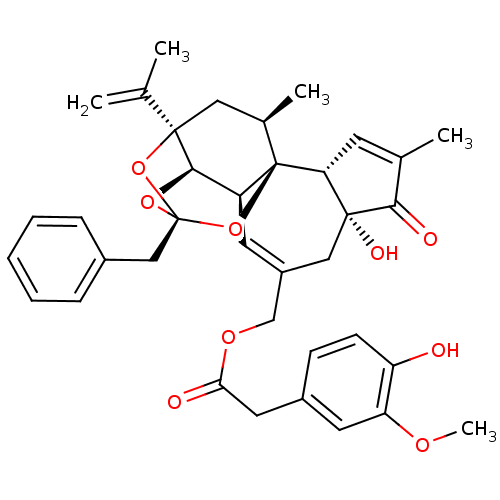 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human recombinant TRPV1 | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50318494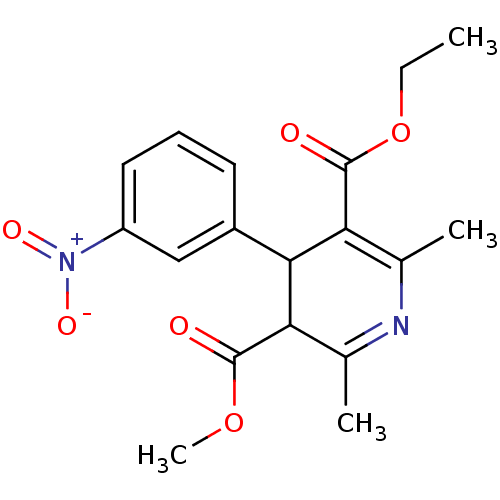 (3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228791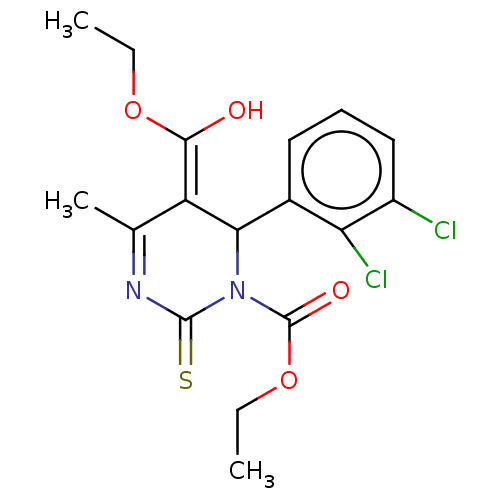 (CHEMBL329897) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228797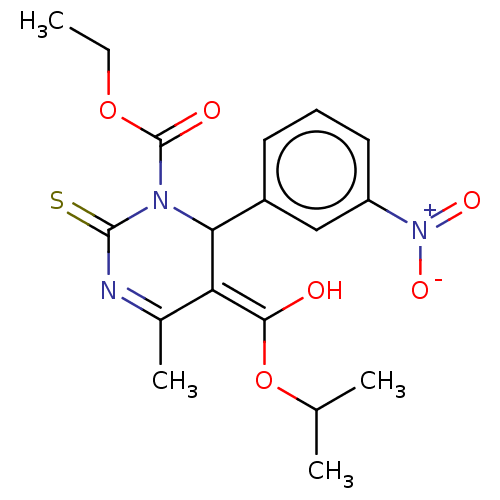 (CHEMBL89260) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228807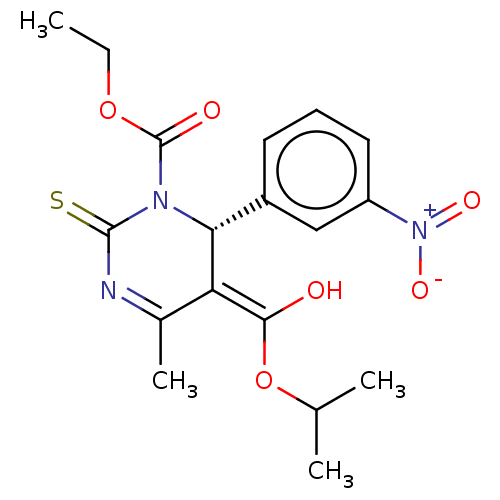 (CHEMBL2092901) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228801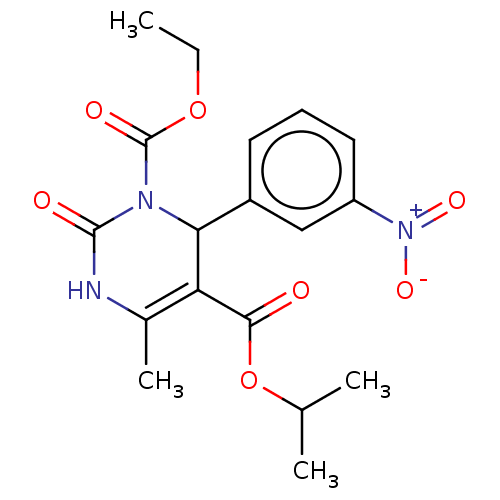 (CHEMBL89049) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228811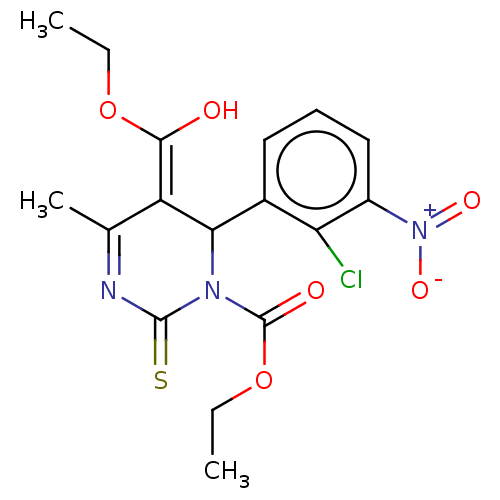 (CHEMBL84906) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325890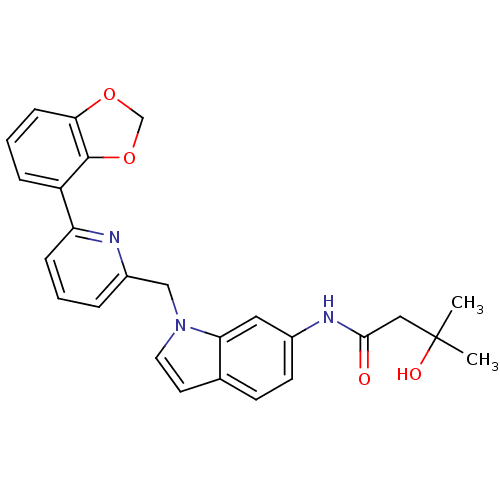 (CHEMBL1224536 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228808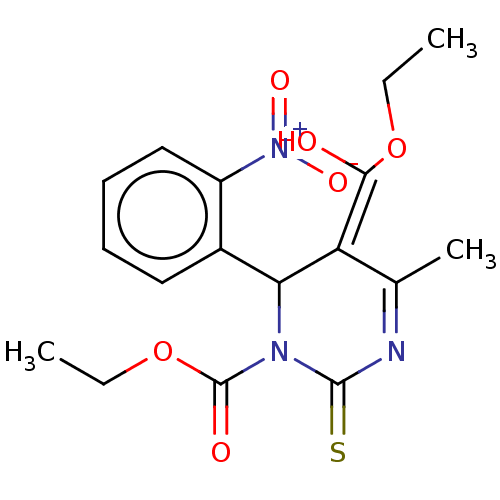 (CHEMBL86415) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228810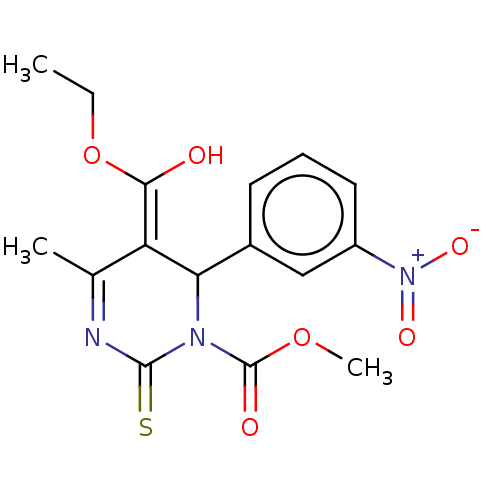 (CHEMBL89904) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227246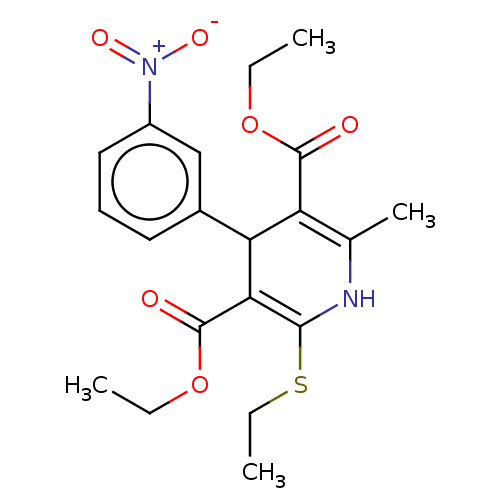 (CHEMBL3392282) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325894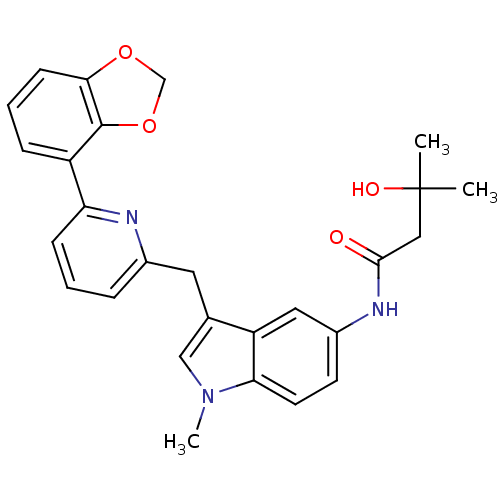 (CHEMBL1224615 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228804 (CHEMBL89175) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331116 (2-morpholino-N-(4-(trifluoromethyl)phenyl)-7-(3-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331116 (2-morpholino-N-(4-(trifluoromethyl)phenyl)-7-(3-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325888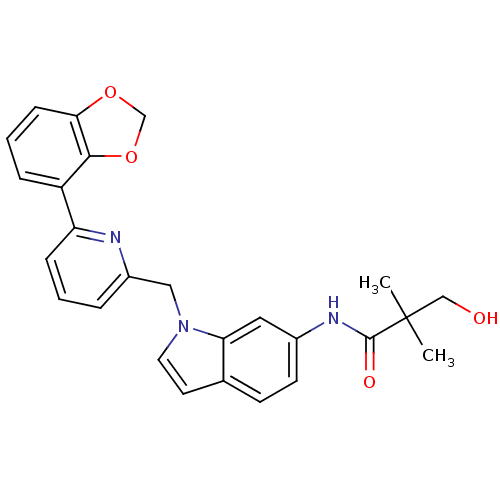 (CHEMBL1224534 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325892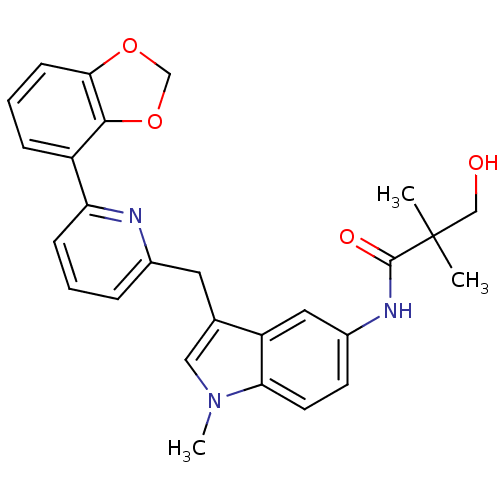 (CHEMBL1224613 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331150 (CHEMBL1290369 | N-(4-tert-butylphenyl)-7-(3-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228812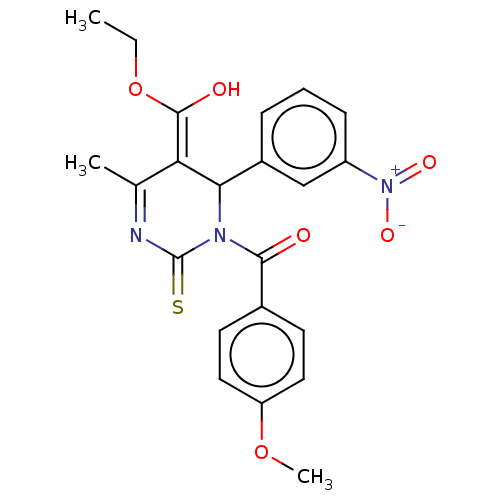 (CHEMBL315125) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50332848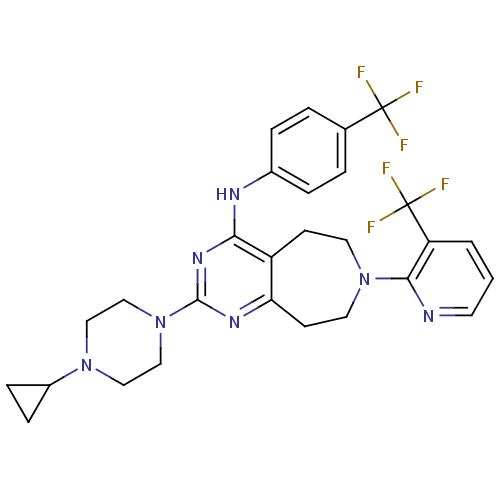 (2-(4-cyclopropylpiperazin-1-yl)-N-(4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50332846 (2-(4-isobutylpiperazin-1-yl)-N-(4-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228796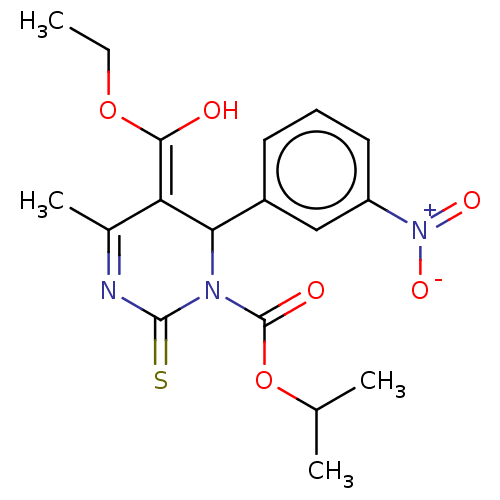 (CHEMBL330707) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50254114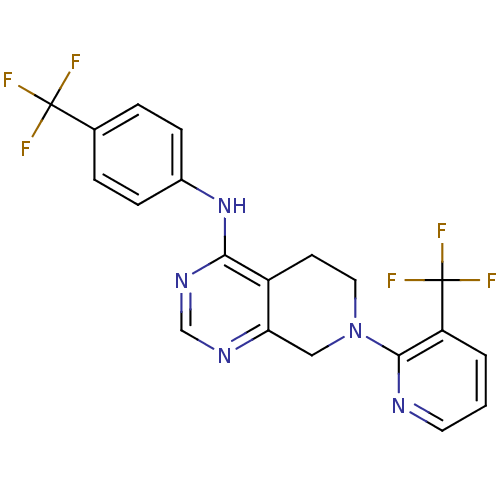 (CHEMBL461658 | N-(4-(trifluoromethyl)phenyl)-7-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50332850 (2-(4-cyclopentylpiperazin-1-yl)-N-(4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228795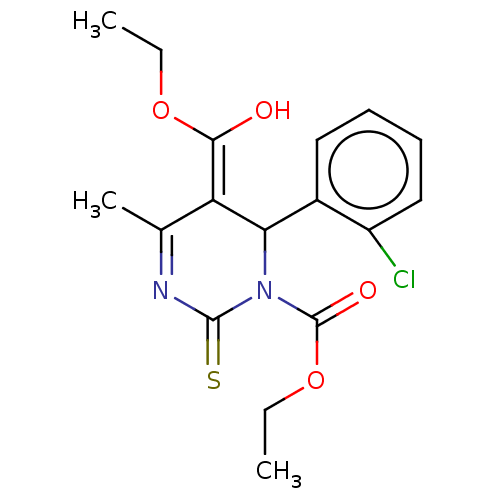 (CHEMBL315298) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (voltage-gated calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228790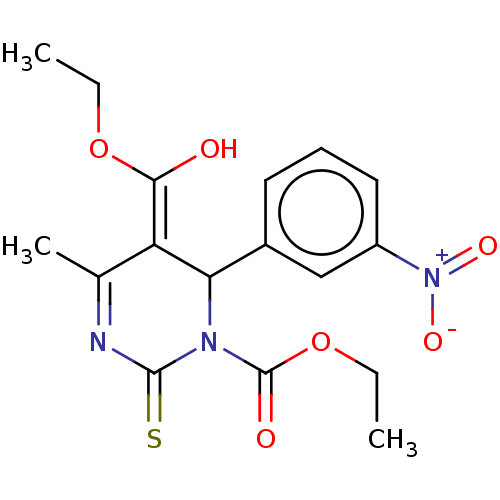 (CHEMBL440695) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331154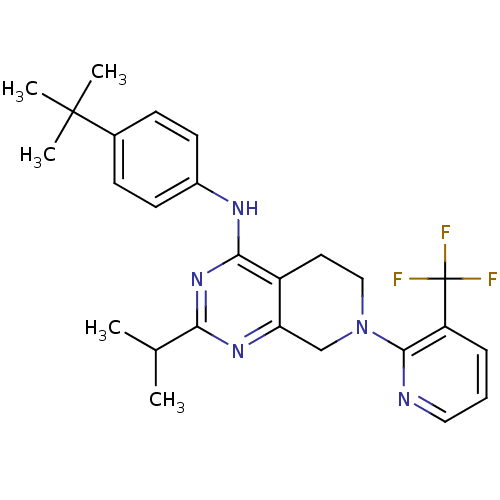 (CHEMBL1290584 | N-(4-tert-butylphenyl)-2-isopropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331131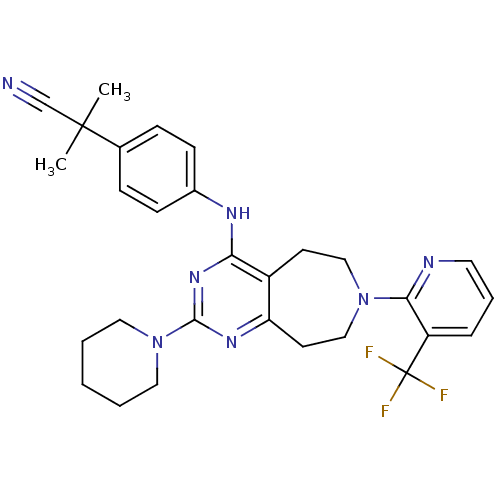 (2-methyl-2-(4-(2-(piperidin-1-yl)-7-(3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325895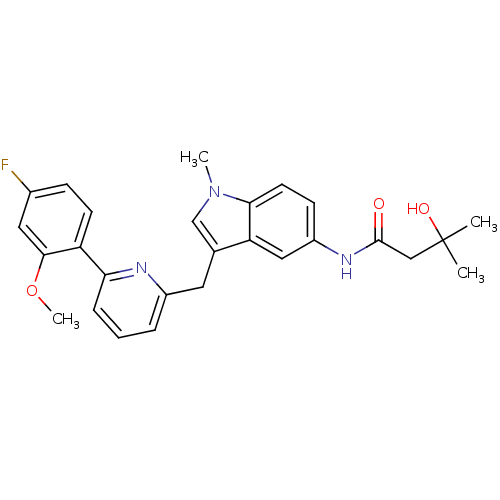 (CHEMBL1224667 | N-(3-((6-(4-fluoro-2-methoxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331117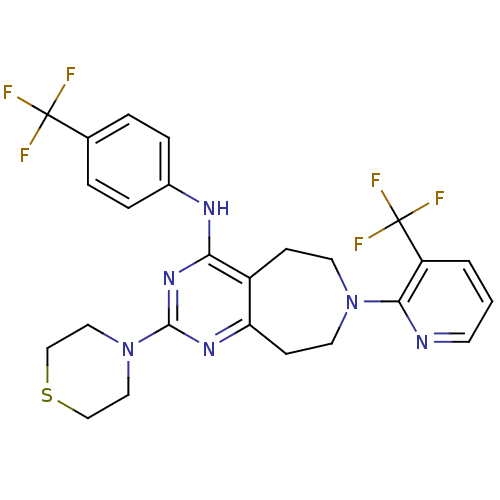 (2-thiomorpholino-N-(4-(trifluoromethyl)phenyl)-7-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20461 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat TRPV1 | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50331116 (2-morpholino-N-(4-(trifluoromethyl)phenyl)-7-(3-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331124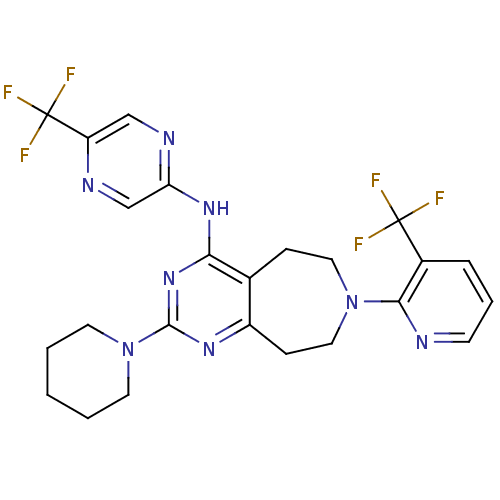 (2-(piperidin-1-yl)-N-(5-(trifluoromethyl)pyrazin-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325882 (CHEMBL1224373 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325891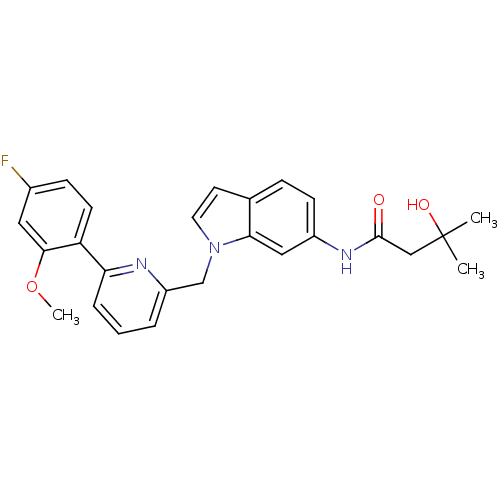 (CHEMBL1224612 | N-(1-((6-(4-fluoro-2-methoxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50332849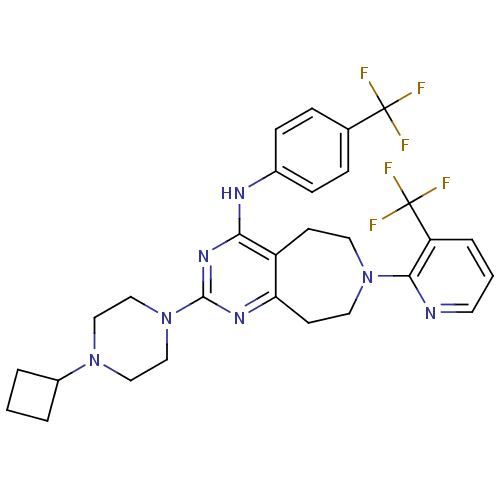 (2-(4-cyclobutylpiperazin-1-yl)-N-(4-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331137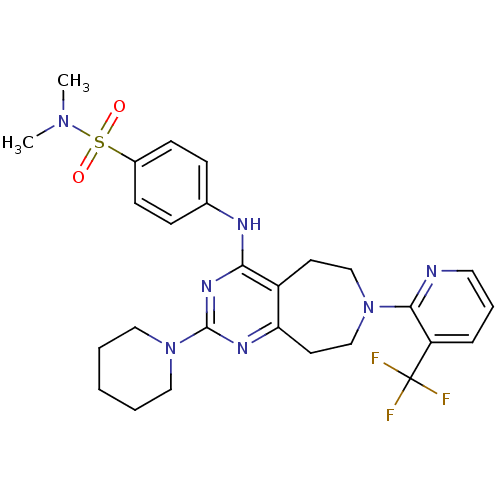 (CHEMBL1289818 | N,N-dimethyl-4-(2-(piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331157 (CHEMBL1290704 | N-(4-tert-butylphenyl)-7-(3-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50227344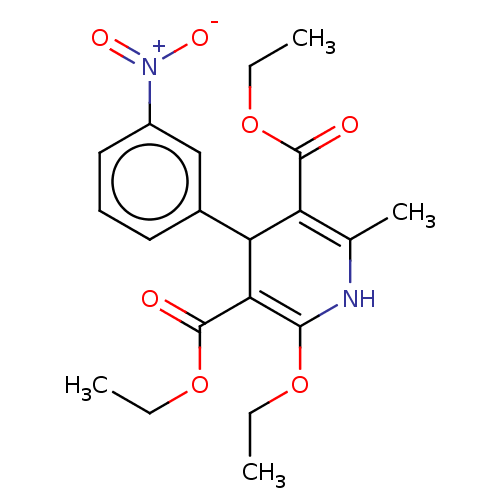 (CHEMBL3392280) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50228805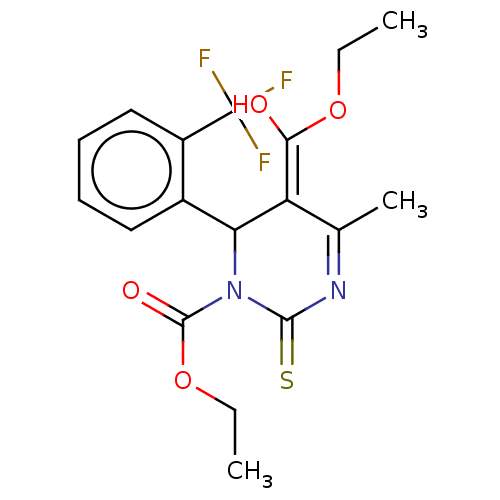 (CHEMBL87921) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Squibb Institute for Medical Research Curated by ChEMBL | Assay Description In vitro vasorelaxant activity (calcium channel blocking activity) was determined with potassium-depolarized rabbit thoracic aorta | J Med Chem 33: 2629-35 (1990) BindingDB Entry DOI: 10.7270/Q2XD13XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325893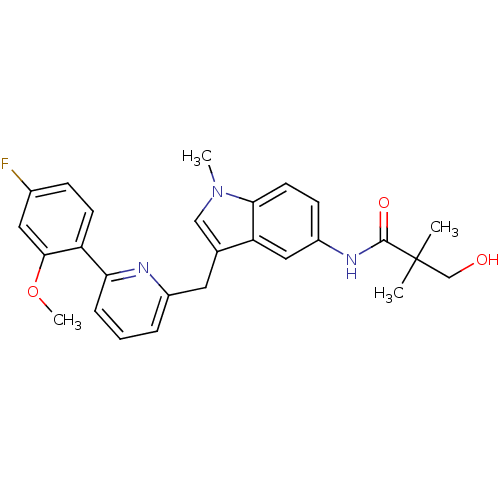 (CHEMBL1224614 | N-(3-((6-(4-fluoro-2-methoxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331160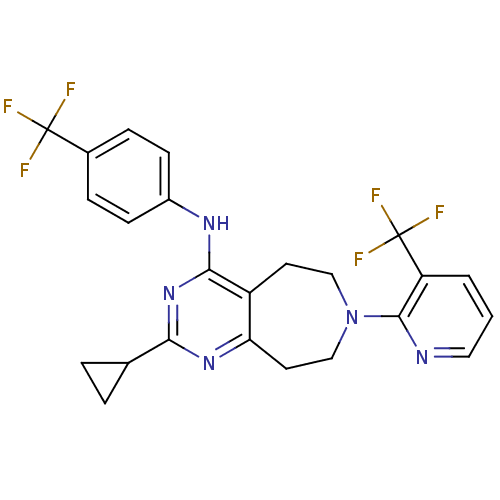 (2-cyclopropyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331111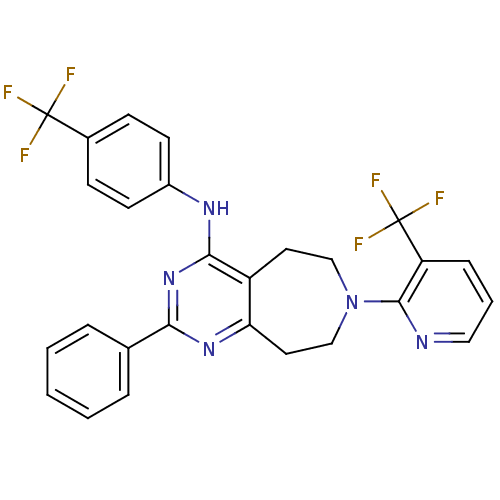 (2-phenyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50325886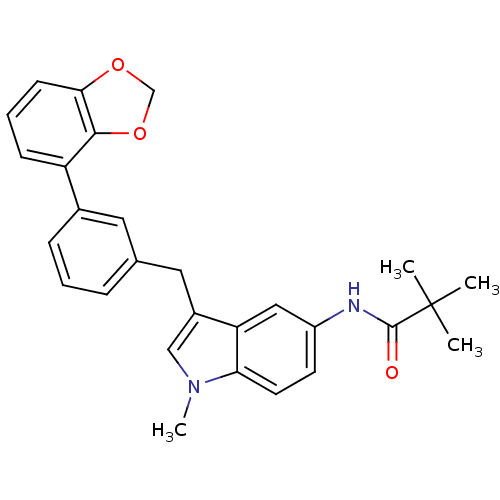 (CHEMBL1224460 | N-(3-(3-(benzo[d][1,3]dioxol-4-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 5449-53 (2010) Article DOI: 10.1016/j.bmcl.2010.07.091 BindingDB Entry DOI: 10.7270/Q2SN0958 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331132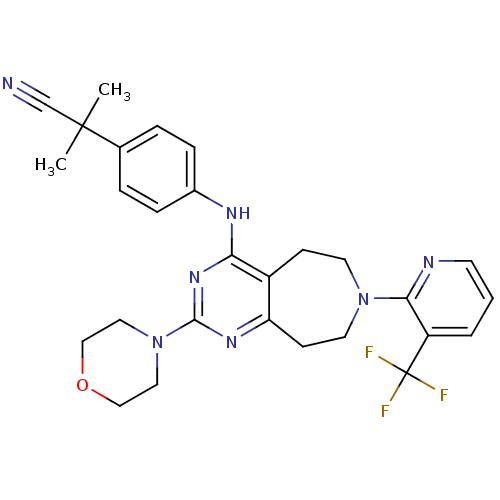 (2-methyl-2-(4-(2-morpholino-7-(3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331122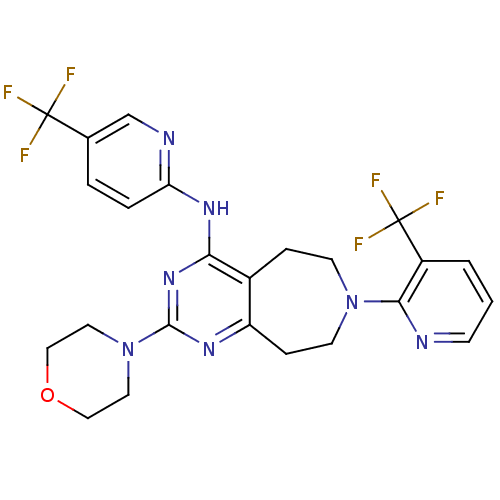 (2-morpholino-7-(3-(trifluoromethyl)pyridin-2-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50332847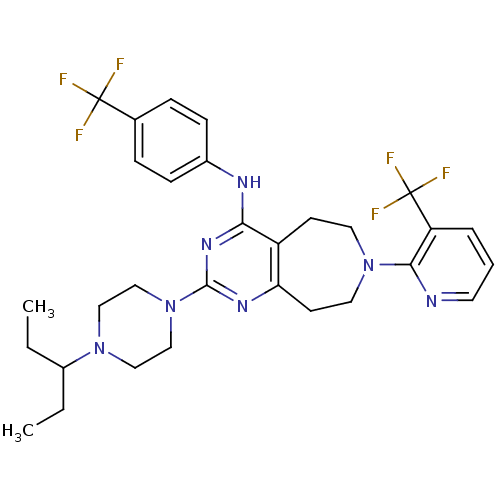 (2-(4-(pentan-3-yl)piperazin-1-yl)-N-(4-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50331121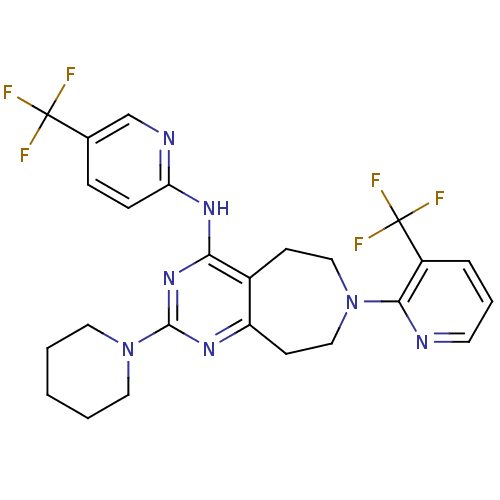 (2-(piperidin-1-yl)-7-(3-(trifluoromethyl)pyridin-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at human recombinant TRPV1 assessed as inhibition of capsaicin-induced in intracellular calcium levels by cell-based FLIPR assay | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50332848 (2-(4-cyclopropylpiperazin-1-yl)-N-(4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced intracellular Ca2+ level by FLIPR assay | Bioorg Med Chem Lett 20: 7142-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.006 BindingDB Entry DOI: 10.7270/Q26Q1XH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 165 total ) | Next | Last >> |