 Found 600 hits with Last Name = 'tai' and Initial = 'k'
Found 600 hits with Last Name = 'tai' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50116105

(3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1cnc(cc1C)N(C)C |(-1.91,-13.39,;-3.24,-14.16,;-4.58,-13.4,;-5.91,-14.18,;-7.24,-13.41,;-8.57,-14.19,;-9.91,-13.42,;-5.9,-15.72,;-7.22,-16.49,;-7.23,-18.03,;-8.56,-18.8,;-5.9,-18.8,;-4.55,-18.03,;-3.07,-18.5,;-2.16,-17.24,;-.62,-17.23,;-3.09,-15.99,;-4.56,-16.48,;-2.58,-19.96,;-3.61,-21.11,;-3.13,-22.57,;-1.62,-22.88,;-.59,-21.72,;-1.08,-20.27,;-.06,-19.11,;-1.13,-24.34,;.38,-24.65,;-2.15,-25.5,)| Show InChI InChI=1S/C22H32N6/c1-8-10-27(11-9-2)20-13-16(4)24-22-21(17(5)25-28(20)22)18-14-23-19(26(6)7)12-15(18)3/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420920
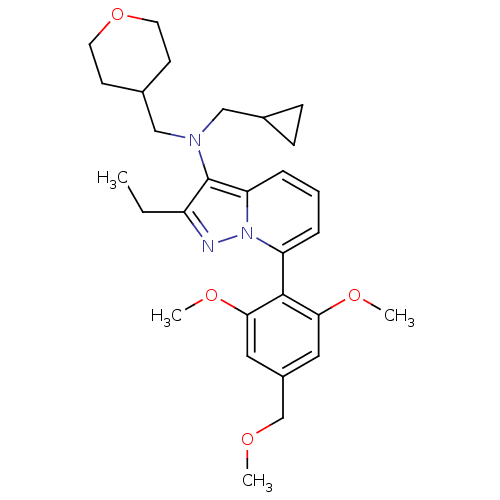
(CHEMBL2087549 | CHEMBL2087567)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(COC)cc1OC |(-4.48,2.67,;-3.74,1.32,;-2.2,1.29,;-1.32,.02,;.16,.47,;1.48,-.33,;2.82,.42,;2.86,1.96,;1.54,2.75,;.19,2.01,;-1.27,2.51,;-1.71,3.99,;-.66,5.11,;.86,4.84,;2.3,5.36,;2.03,3.85,;-3.21,4.34,;-3.58,5.83,;-5.06,6.26,;-5.43,7.76,;-4.32,8.82,;-2.84,8.39,;-2.47,6.9,;1.44,-1.87,;2.76,-2.66,;4.11,-1.92,;5.43,-2.72,;2.73,-4.2,;1.38,-4.95,;1.35,-6.49,;2.67,-7.28,;2.64,-8.82,;.07,-4.15,;.1,-2.61,;-1.22,-1.81,;-2.57,-2.56,)| Show InChI InChI=1S/C29H39N3O4/c1-5-23-29(31(17-20-9-10-20)18-21-11-13-36-14-12-21)25-8-6-7-24(32(25)30-23)28-26(34-3)15-22(19-33-2)16-27(28)35-4/h6-8,15-16,20-21H,5,9-14,17-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50022831
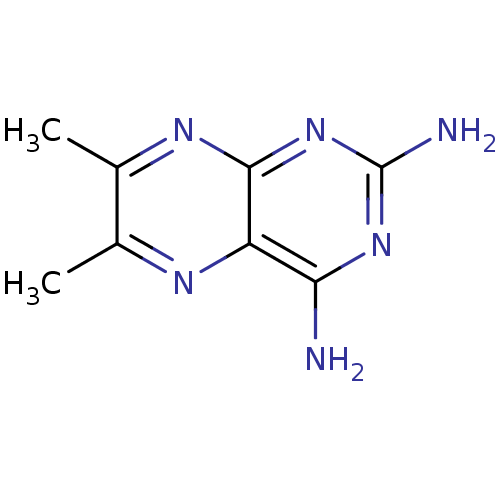
(6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143)Show InChI InChI=1S/C8H10N6/c1-3-4(2)12-7-5(11-3)6(9)13-8(10)14-7/h1-2H3,(H4,9,10,12,13,14) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 8 |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50022831

(6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143)Show InChI InChI=1S/C8H10N6/c1-3-4(2)12-7-5(11-3)6(9)13-8(10)14-7/h1-2H3,(H4,9,10,12,13,14) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Inhibition constant for Tyr31-dihydrofolate reductase (DHFR)-NADPH-Compound complex |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 9.5 |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.40E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Thermodynamic Dissociation Constant for compound-Val31-dihydrofolate reductase (DHFR) complex at pH 7 |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50022831

(6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143)Show InChI InChI=1S/C8H10N6/c1-3-4(2)12-7-5(11-3)6(9)13-8(10)14-7/h1-2H3,(H4,9,10,12,13,14) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Dissociation constant at(Koff) Tyr-31 of dihydrofolate reductase (DHFR) |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(Homo sapiens (Human)) | BDBM50420920

(CHEMBL2087549 | CHEMBL2087567)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(COC)cc1OC |(-4.48,2.67,;-3.74,1.32,;-2.2,1.29,;-1.32,.02,;.16,.47,;1.48,-.33,;2.82,.42,;2.86,1.96,;1.54,2.75,;.19,2.01,;-1.27,2.51,;-1.71,3.99,;-.66,5.11,;.86,4.84,;2.3,5.36,;2.03,3.85,;-3.21,4.34,;-3.58,5.83,;-5.06,6.26,;-5.43,7.76,;-4.32,8.82,;-2.84,8.39,;-2.47,6.9,;1.44,-1.87,;2.76,-2.66,;4.11,-1.92,;5.43,-2.72,;2.73,-4.2,;1.38,-4.95,;1.35,-6.49,;2.67,-7.28,;2.64,-8.82,;.07,-4.15,;.1,-2.61,;-1.22,-1.81,;-2.57,-2.56,)| Show InChI InChI=1S/C29H39N3O4/c1-5-23-29(31(17-20-9-10-20)18-21-11-13-36-14-12-21)25-8-6-7-24(32(25)30-23)28-26(34-3)15-22(19-33-2)16-27(28)35-4/h6-8,15-16,20-21H,5,9-14,17-19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF2 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University
Curated by ChEMBL
| Assay Description
Thermodynamic Dissociation Constant for compound-Phe31-dihydrofolate reductase (DHFR) complex at pH 8.5 |
J Med Chem 31: 129-37 (1988)
BindingDB Entry DOI: 10.7270/Q2MP53VF |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084833

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27NO2/c1-24(2)13-14-25(3,4)20-15-18(9-10-19(20)24)22-12-11-21(26-22)16-5-7-17(8-6-16)23(27)28/h5-12,15,26H,13-14H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor gamma |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against Retinoic acid receptor gamma |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor beta |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor beta |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084832
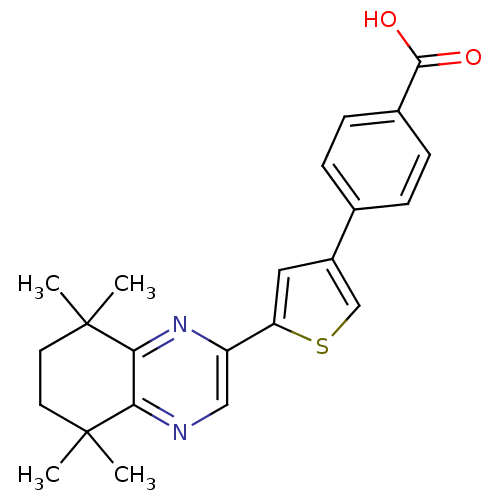
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084835

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25N3O2/c1-22(2)11-12-23(3,4)20-19(22)24-13-18(26-20)17-10-9-16(25-17)14-5-7-15(8-6-14)21(27)28/h5-10,13,25H,11-12H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084832

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084834
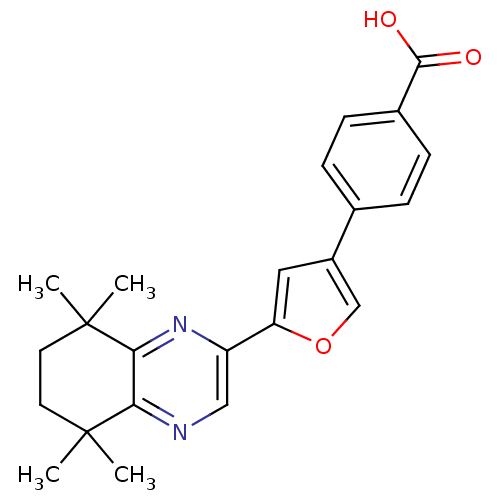
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161301
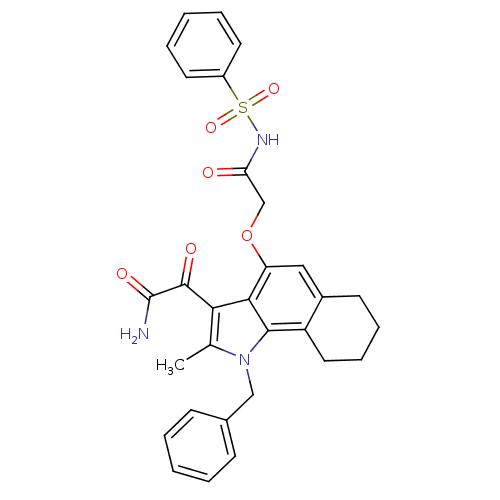
(2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C30H29N3O6S/c1-19-26(29(35)30(31)36)27-24(39-18-25(34)32-40(37,38)22-13-6-3-7-14-22)16-21-12-8-9-15-23(21)28(27)33(19)17-20-10-4-2-5-11-20/h2-7,10-11,13-14,16H,8-9,12,15,17-18H2,1H3,(H2,31,36)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084830
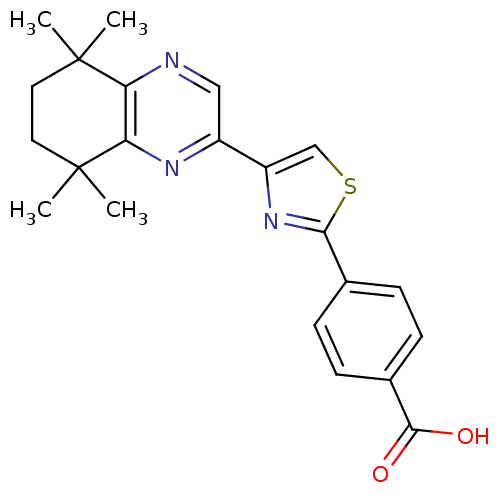
(4-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1csc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H23N3O2S/c1-21(2)9-10-22(3,4)18-17(21)23-11-15(24-18)16-12-28-19(25-16)13-5-7-14(8-6-13)20(26)27/h5-8,11-12H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50116105

(3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1cnc(cc1C)N(C)C |(-1.91,-13.39,;-3.24,-14.16,;-4.58,-13.4,;-5.91,-14.18,;-7.24,-13.41,;-8.57,-14.19,;-9.91,-13.42,;-5.9,-15.72,;-7.22,-16.49,;-7.23,-18.03,;-8.56,-18.8,;-5.9,-18.8,;-4.55,-18.03,;-3.07,-18.5,;-2.16,-17.24,;-.62,-17.23,;-3.09,-15.99,;-4.56,-16.48,;-2.58,-19.96,;-3.61,-21.11,;-3.13,-22.57,;-1.62,-22.88,;-.59,-21.72,;-1.08,-20.27,;-.06,-19.11,;-1.13,-24.34,;.38,-24.65,;-2.15,-25.5,)| Show InChI InChI=1S/C22H32N6/c1-8-10-27(11-9-2)20-13-16(4)24-22-21(17(5)25-28(20)22)18-14-23-19(26(6)7)12-15(18)3/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... |
J Med Chem 55: 8450-63 (2012)
Article DOI: 10.1021/jm300864p
BindingDB Entry DOI: 10.7270/Q2KD202P |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161305
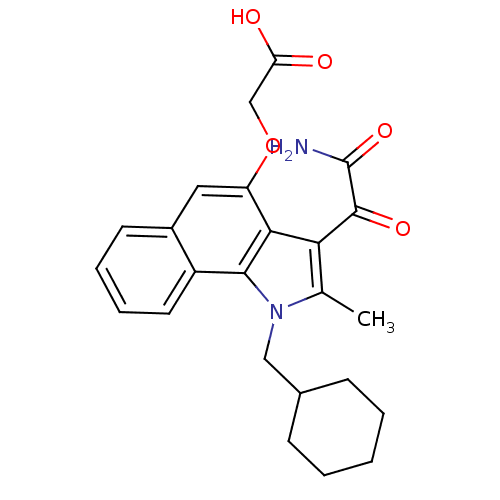
((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1CC1CCCCC1 Show InChI InChI=1S/C24H26N2O5/c1-14-20(23(29)24(25)30)21-18(31-13-19(27)28)11-16-9-5-6-10-17(16)22(21)26(14)12-15-7-3-2-4-8-15/h5-6,9-11,15H,2-4,7-8,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084832

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084831

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(o1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161293
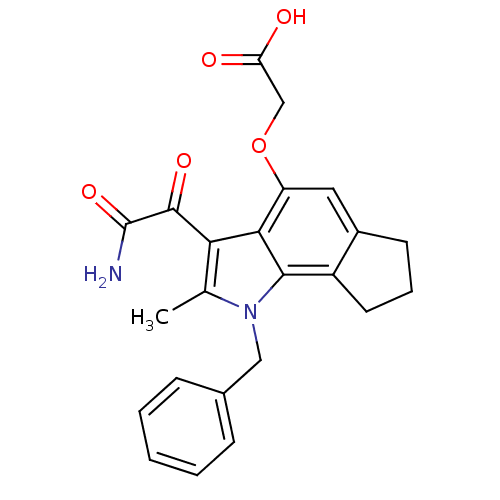
((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C23H22N2O5/c1-13-19(22(28)23(24)29)20-17(30-12-18(26)27)10-15-8-5-9-16(15)21(20)25(13)11-14-6-3-2-4-7-14/h2-4,6-7,10H,5,8-9,11-12H2,1H3,(H2,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161299

((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H24N2O5/c1-2-17-20(23(29)24(25)30)21-18(31-13-19(27)28)11-15-9-6-10-16(15)22(21)26(17)12-14-7-4-3-5-8-14/h3-5,7-8,11H,2,6,9-10,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161301

(2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C30H29N3O6S/c1-19-26(29(35)30(31)36)27-24(39-18-25(34)32-40(37,38)22-13-6-3-7-14-22)16-21-12-8-9-15-23(21)28(27)33(19)17-20-10-4-2-5-11-20/h2-7,10-11,13-14,16H,8-9,12,15,17-18H2,1H3,(H2,31,36)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50398565
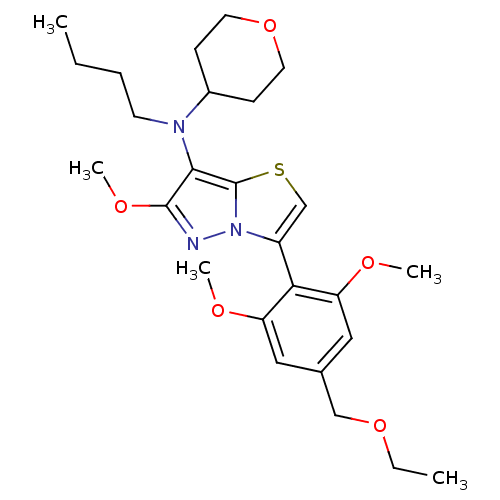
(CHEMBL2179195)Show SMILES CCCCN(C1CCOCC1)c1c(OC)nn2c(csc12)-c1c(OC)cc(COCC)cc1OC |(2.51,-41.6,;3.29,-42.93,;4.83,-42.92,;5.59,-41.58,;7.13,-41.58,;7.85,-40.22,;9.39,-40.17,;10.11,-38.82,;9.3,-37.51,;7.76,-37.56,;7.03,-38.92,;7.67,-43.03,;6.79,-44.29,;5.26,-44.32,;4.52,-45.67,;7.72,-45.51,;9.18,-45.01,;10.66,-45.45,;11.53,-44.19,;10.6,-42.96,;9.15,-43.47,;11.4,-46.8,;10.62,-48.12,;9.08,-48.09,;8.28,-49.41,;11.36,-49.46,;12.9,-49.49,;13.65,-50.84,;15.19,-50.87,;15.93,-52.21,;17.47,-52.24,;13.69,-48.17,;12.94,-46.82,;13.74,-45.5,;12.99,-44.16,)| Show InChI InChI=1S/C26H37N3O5S/c1-6-8-11-28(19-9-12-34-13-10-19)24-25(32-5)27-29-20(17-35-26(24)29)23-21(30-3)14-18(16-33-7-2)15-22(23)31-4/h14-15,17,19H,6-13,16H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... |
J Med Chem 55: 8450-63 (2012)
Article DOI: 10.1021/jm300864p
BindingDB Entry DOI: 10.7270/Q2KD202P |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161308
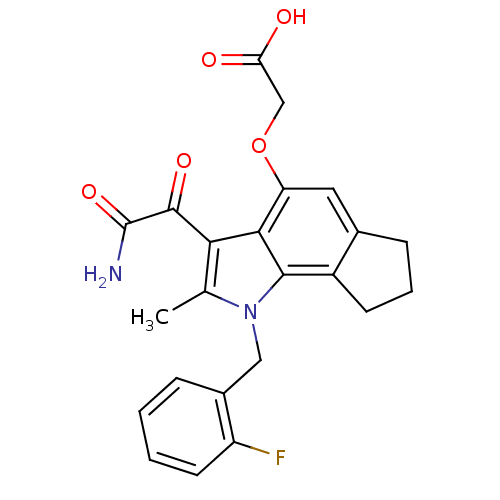
(CHEMBL179118 | [3-Aminooxalyl-1-(2-fluoro-benzyl)-...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1F Show InChI InChI=1S/C23H21FN2O5/c1-12-19(22(29)23(25)30)20-17(31-11-18(27)28)9-13-6-4-7-15(13)21(20)26(12)10-14-5-2-3-8-16(14)24/h2-3,5,8-9H,4,6-7,10-11H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420904
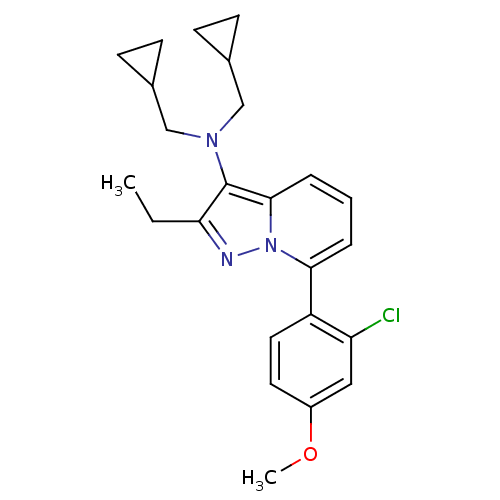
(CHEMBL2087550)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(OC)cc1Cl Show InChI InChI=1S/C24H28ClN3O/c1-3-21-24(27(14-16-7-8-16)15-17-9-10-17)23-6-4-5-22(28(23)26-21)19-12-11-18(29-2)13-20(19)25/h4-6,11-13,16-17H,3,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161293

((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C23H22N2O5/c1-13-19(22(28)23(24)29)20-17(30-12-18(26)27)10-15-8-5-9-16(15)21(20)25(13)11-14-6-3-2-4-7-14/h2-4,6-7,10H,5,8-9,11-12H2,1H3,(H2,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161298

((3-Aminooxalyl-1-benzyl-2-methyl-2,3,6,7,8,9-hexah...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H24N2O5/c1-14-20(23(29)24(25)30)21-18(31-13-19(27)28)11-16-9-5-6-10-17(16)22(21)26(14)12-15-7-3-2-4-8-15/h2-4,7-8,11H,5-6,9-10,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161294

(CHEMBL179966 | [3-Aminooxalyl-1-(3-fluoro-benzyl)-...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1cccc(F)c1 Show InChI InChI=1S/C23H21FN2O5/c1-12-19(22(29)23(25)30)20-17(31-11-18(27)28)9-14-5-3-7-16(14)21(20)26(12)10-13-4-2-6-15(24)8-13/h2,4,6,8-9H,3,5,7,10-11H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161305

((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1CC1CCCCC1 Show InChI InChI=1S/C24H26N2O5/c1-14-20(23(29)24(25)30)21-18(31-13-19(27)28)11-16-9-5-6-10-17(16)22(21)26(14)12-15-7-3-2-4-8-15/h5-6,9-11,15H,2-4,7-8,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161296
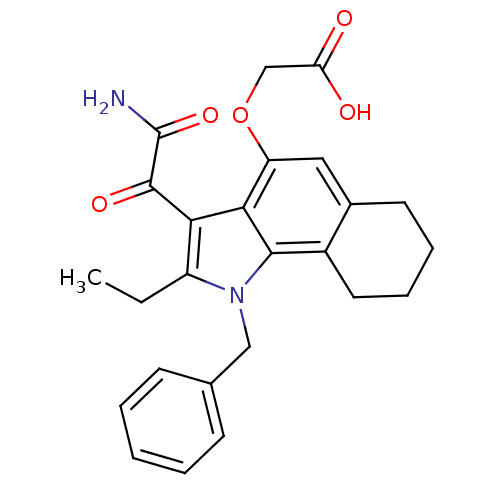
((3-Aminooxalyl-1-benzyl-2-ethyl-2,3,6,7,8,9-hexahy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H26N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-5,8-9,12H,2,6-7,10-11,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420906

(CHEMBL2087552)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(C)cc1OC Show InChI InChI=1S/C25H31N3O/c1-4-21-25(27(15-18-9-10-18)16-19-11-12-19)23-7-5-6-22(28(23)26-21)20-13-8-17(2)14-24(20)29-3/h5-8,13-14,18-19H,4,9-12,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161299

((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H24N2O5/c1-2-17-20(23(29)24(25)30)21-18(31-13-19(27)28)11-15-9-6-10-16(15)22(21)26(17)12-14-7-4-3-5-8-14/h3-5,7-8,11H,2,6,9-10,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420908
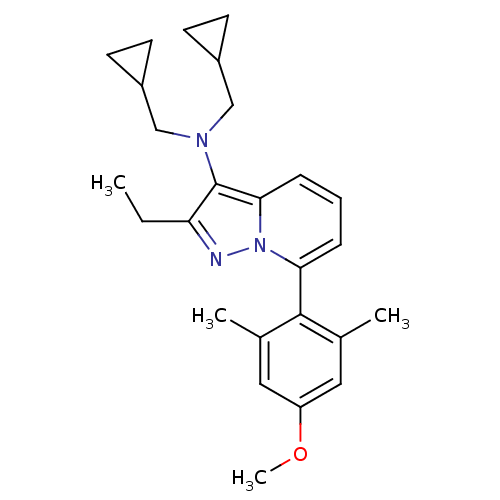
(CHEMBL2087554)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1c(C)cc(OC)cc1C |(-4.21,2.14,;-3.44,.81,;-1.9,.81,;-.99,-.43,;.47,.05,;1.81,-.72,;3.14,.05,;3.13,1.59,;1.8,2.36,;.47,1.59,;-1,2.06,;-1.48,3.53,;-2.98,3.84,;-3.38,5.33,;-2.99,6.82,;-4.48,6.42,;-.45,4.67,;-.85,6.16,;-.45,7.65,;-1.94,7.25,;1.81,-2.26,;3.14,-3.03,;4.48,-2.25,;3.15,-4.57,;1.81,-5.34,;1.82,-6.88,;3.15,-7.65,;.48,-4.57,;.48,-3.03,;-.86,-2.26,)| Show InChI InChI=1S/C26H33N3O/c1-5-22-26(28(15-19-9-10-19)16-20-11-12-20)24-8-6-7-23(29(24)27-22)25-17(2)13-21(30-4)14-18(25)3/h6-8,13-14,19-20H,5,9-12,15-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420909
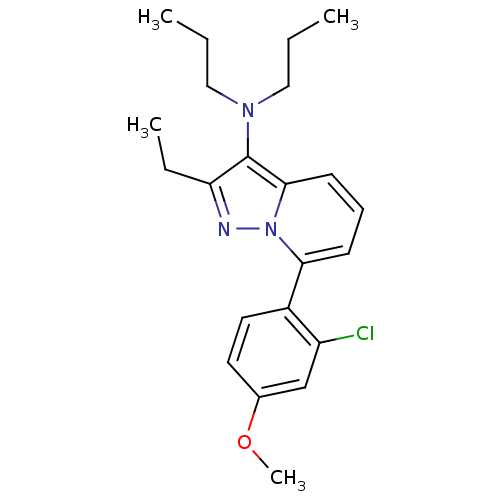
(CHEMBL2087555)Show SMILES CCCN(CCC)c1c(CC)nn2c(cccc12)-c1ccc(OC)cc1Cl Show InChI InChI=1S/C22H28ClN3O/c1-5-13-25(14-6-2)22-19(7-3)24-26-20(9-8-10-21(22)26)17-12-11-16(27-4)15-18(17)23/h8-12,15H,5-7,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161298

((3-Aminooxalyl-1-benzyl-2-methyl-2,3,6,7,8,9-hexah...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H24N2O5/c1-14-20(23(29)24(25)30)21-18(31-13-19(27)28)11-16-9-5-6-10-17(16)22(21)26(14)12-15-7-3-2-4-8-15/h2-4,7-8,11H,5-6,9-10,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420905
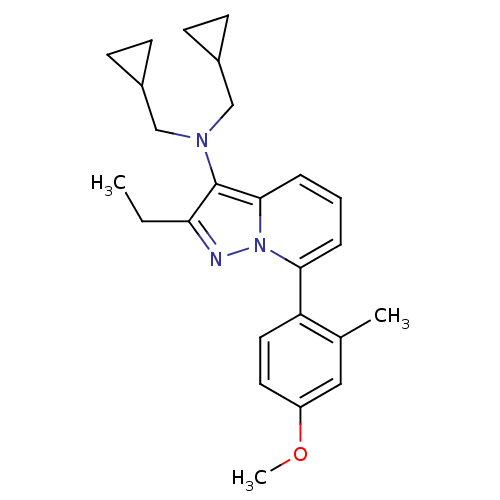
(CHEMBL2087551)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(OC)cc1C Show InChI InChI=1S/C25H31N3O/c1-4-22-25(27(15-18-8-9-18)16-19-10-11-19)24-7-5-6-23(28(24)26-22)21-13-12-20(29-3)14-17(21)2/h5-7,12-14,18-19H,4,8-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161296

((3-Aminooxalyl-1-benzyl-2-ethyl-2,3,6,7,8,9-hexahy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H26N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-5,8-9,12H,2,6-7,10-11,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084829
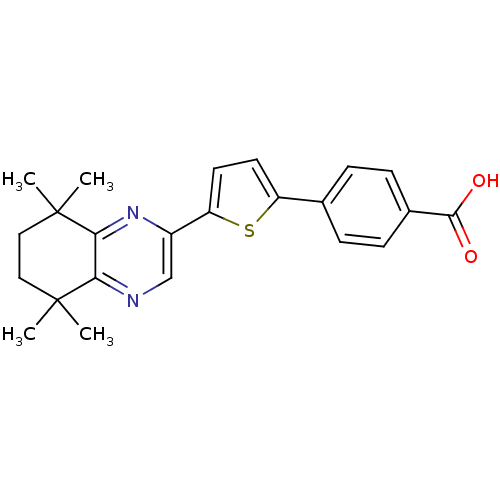
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084834

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420916
(CHEMBL2087562)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(C)cc1OC |(-4.48,1.41,;-3.7,.09,;-2.16,.1,;-1.25,-1.14,;.21,-.65,;1.55,-1.41,;2.88,-.63,;2.87,.91,;1.53,1.67,;.2,.89,;-1.27,1.35,;-1.75,2.81,;-.73,3.96,;.79,3.73,;2.22,4.3,;2,2.77,;-3.26,3.12,;-3.67,4.6,;-5.16,4.99,;-5.57,6.48,;-4.49,7.57,;-3,7.19,;-2.59,5.7,;1.57,-2.95,;2.91,-3.71,;4.23,-2.93,;5.57,-3.69,;2.92,-5.25,;1.59,-6.03,;1.6,-7.57,;.25,-5.27,;.24,-3.73,;-1.1,-2.97,;-2.43,-3.75,)| Show InChI InChI=1S/C28H37N3O3/c1-5-22-28(30(17-20-9-10-20)18-21-11-13-34-14-12-21)24-8-6-7-23(31(24)29-22)27-25(32-3)15-19(2)16-26(27)33-4/h6-8,15-16,20-21H,5,9-14,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in HEK293 cells assessed as inhibition of CRF-stimulated cAMP accumulation after 30 mins by fluo... |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420912
(CHEMBL2087558)Show SMILES CCc1nn2c(cccc2c1N(CCCF)CC1CC1)-c1c(C)cc(C)cc1OC |(-4.75,1.19,;-3.97,-.14,;-2.43,-.13,;-1.52,-1.37,;-.06,-.88,;1.28,-1.65,;2.61,-.87,;2.6,.67,;1.26,1.43,;-.07,.66,;-1.54,1.12,;-2.02,2.58,;-1,3.74,;-1.56,5.17,;-.59,6.37,;-1.16,7.81,;-3.53,2.9,;-4.09,4.33,;-5.29,5.29,;-3.86,5.85,;1.29,-3.19,;-.04,-3.96,;-1.38,-3.2,;-.03,-5.5,;1.3,-6.27,;1.31,-7.81,;2.63,-5.49,;2.62,-3.95,;3.95,-3.17,;5.29,-3.93,)| Show InChI InChI=1S/C25H32FN3O/c1-5-20-25(28(13-7-12-26)16-19-10-11-19)22-9-6-8-21(29(22)27-20)24-18(3)14-17(2)15-23(24)30-4/h6,8-9,14-15,19H,5,7,10-13,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data