

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095997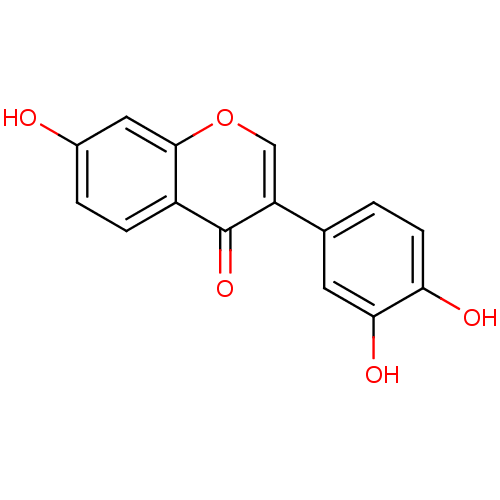 (3',4',7-trihydroxyisoflavone | CHEMBL13486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096004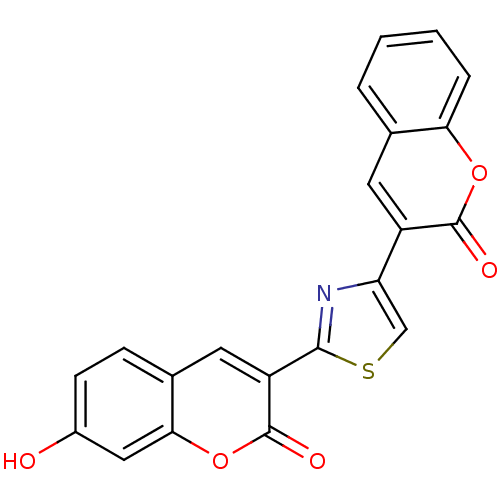 (7-Hydroxy-3-[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096003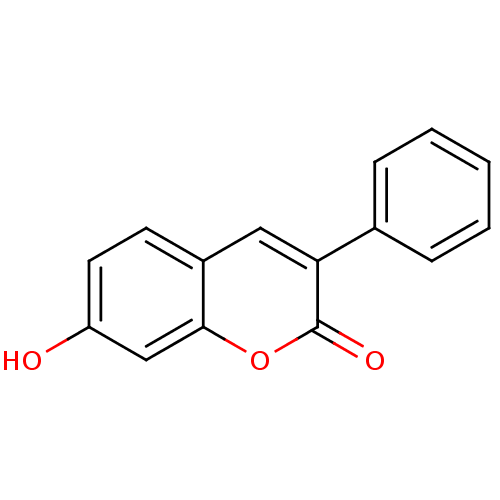 (7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096001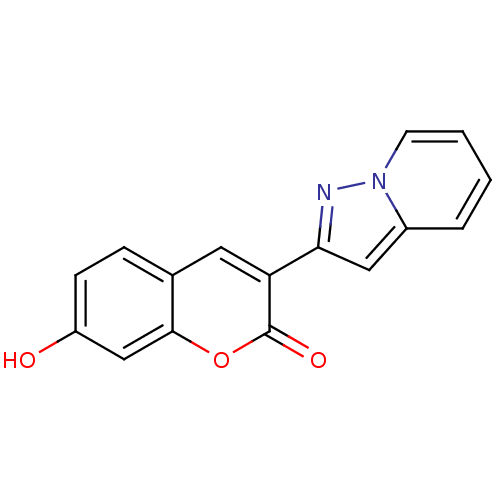 (7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095993 (7-Hydroxy-2-oxo-2H-chromene-3-carbothioic acid ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096002 (3-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-7-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096007 (2-Oxo-2H-chromene-3-carboxylic acid 2-oxo-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370676 (CHEMBL607907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human galactosyltransferase using UDP-Gal | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096006 (7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096000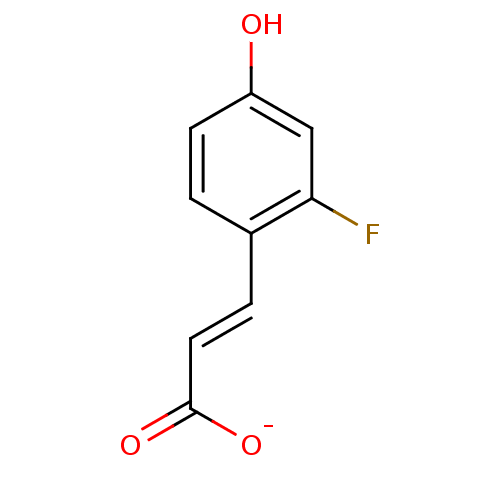 (3-(2-Fluoro-4-hydroxy-phenyl)-acrylic acid anion) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095994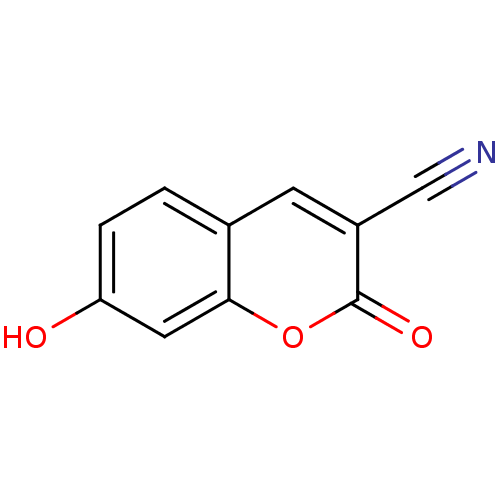 (3-Cyano-7-hydroxycoumarin (2) | 7-Hydroxy-2-oxo-2H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096005 (7-Hydroxy-3-(4-methyl-thiazol-2-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096008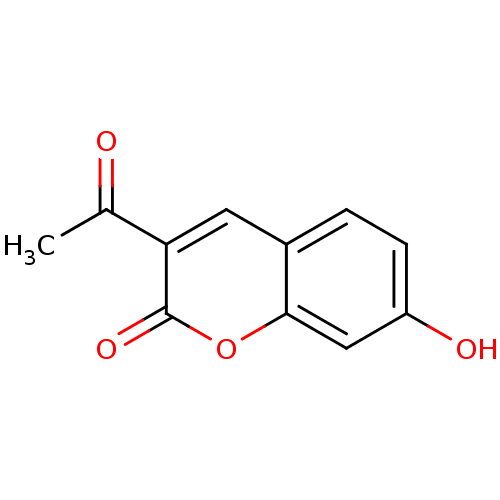 (3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370674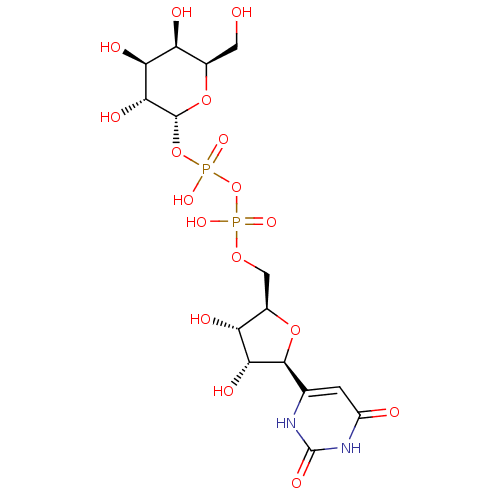 (UDP-GALACTOSE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095996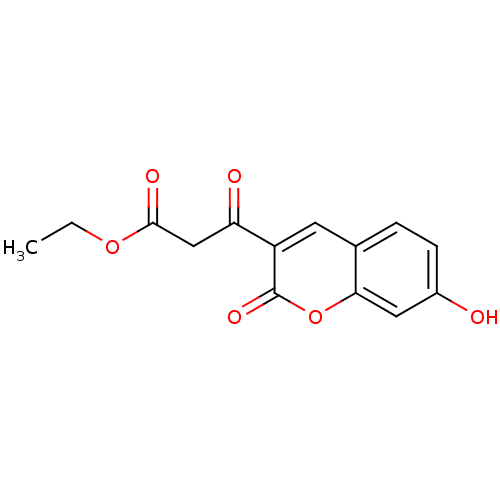 (3-(7-Hydroxy-2-oxo-2H-chromen-3-yl)-3-oxo-propioni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096009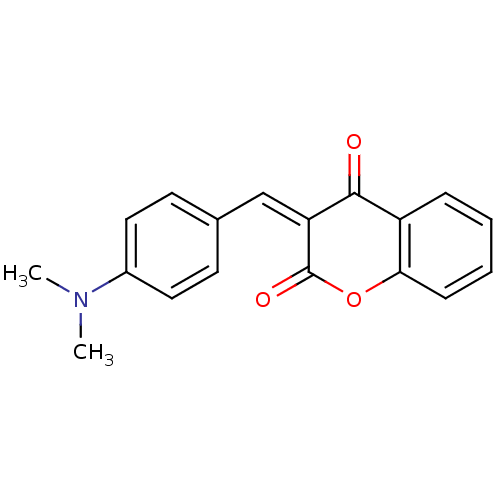 (3-(4-Dimethylamino-benzylidene)-chroman-2,4-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095995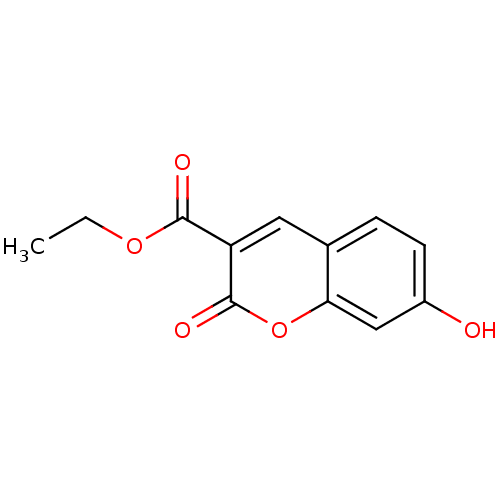 (7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370675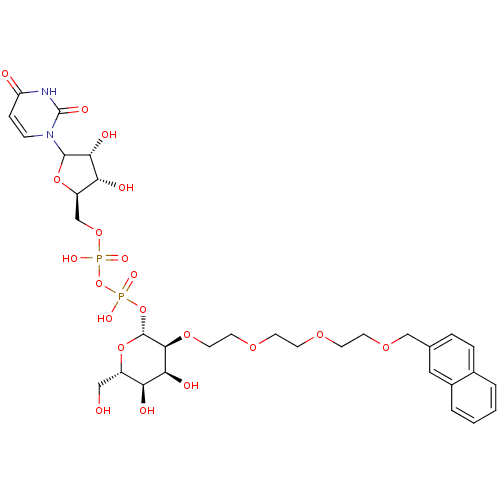 (CHEMBL607908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370678 (CHEMBL611116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370677 (CHEMBL609634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50173721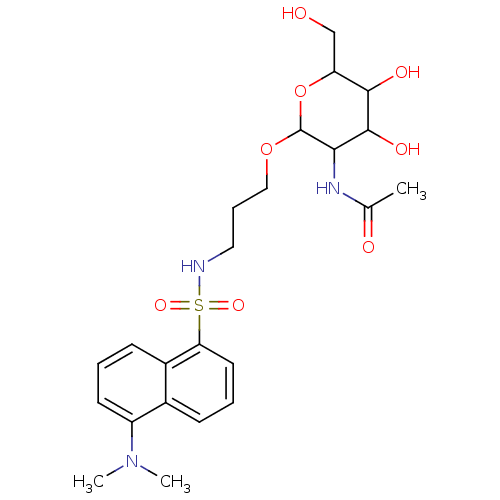 (CHEMBL196432 | Uridine-5'-diphosphogalactose deriv...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human Beta-1,4-galactosyltransferase I using UDP-Gal (0-160 uM) | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1,4-galactosyltransferase 1 (Homo sapiens (Human)) | BDBM50370679 (CHEMBL611112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I | J Med Chem 48: 6054-65 (2005) Article DOI: 10.1021/jm0504297 BindingDB Entry DOI: 10.7270/Q2JW8FPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264847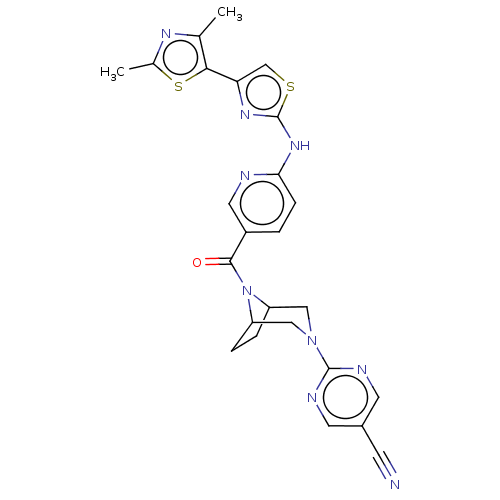 (CHEMBL4096902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50256209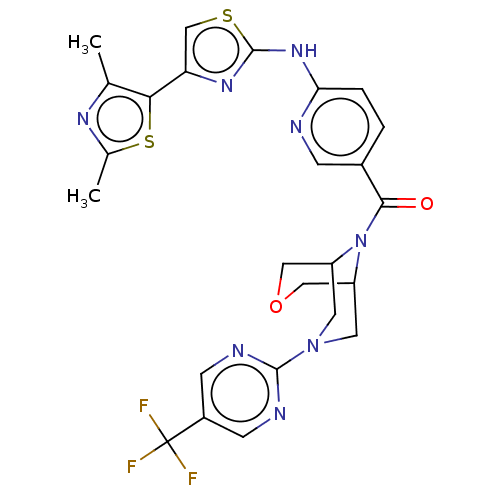 (CHEMBL4101768) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264846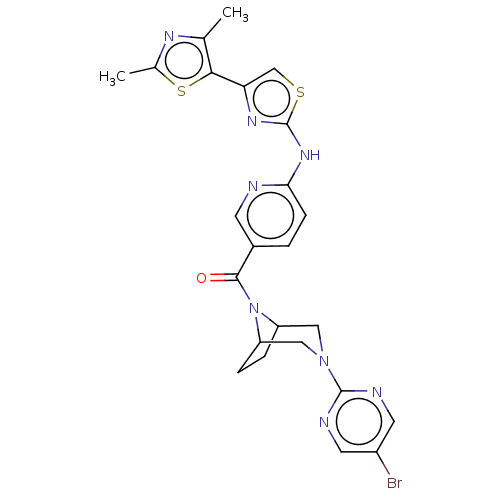 (CHEMBL4066531) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809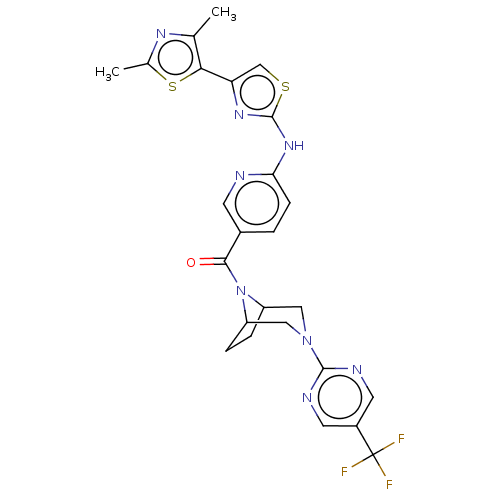 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118179 (US8653125, Ia-40) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50256228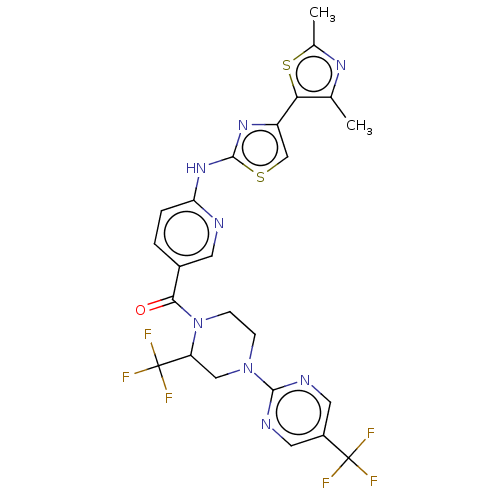 (CHEMBL4099293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192811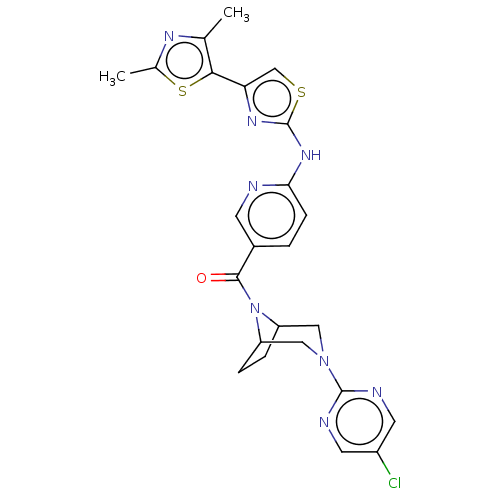 (CHEMBL3971502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192811 (CHEMBL3971502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264858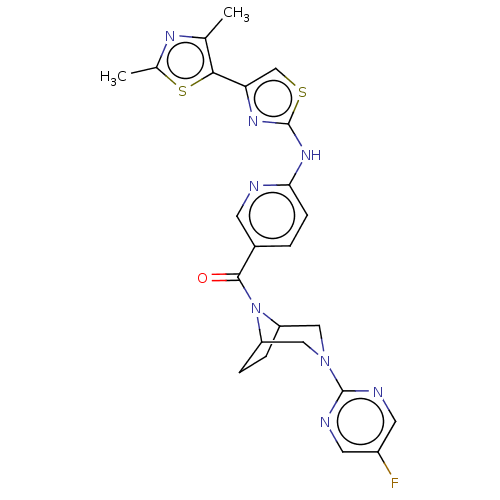 (CHEMBL4077638) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192807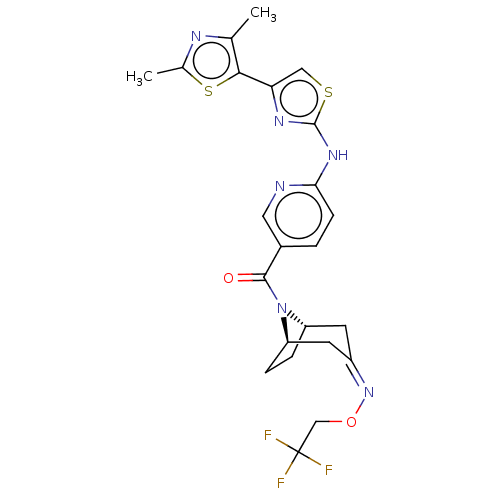 (CHEMBL3984947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264859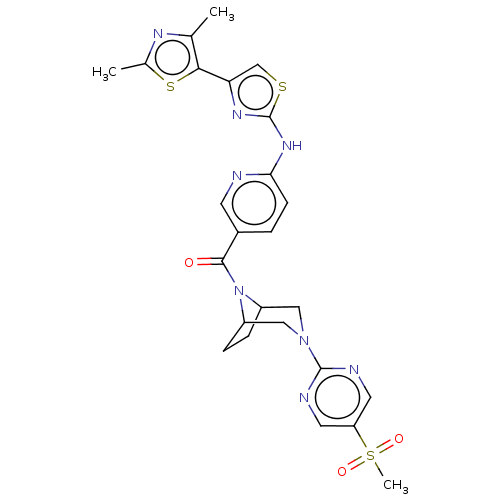 (CHEMBL4078117) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of hypotonicity-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192809 (CHEMBL3941914) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118190 (US8653125, Ic-16) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264845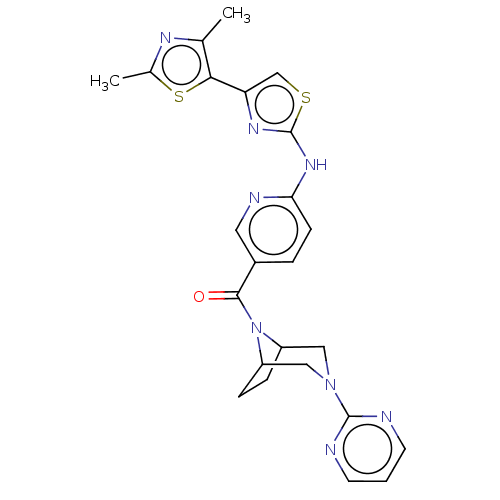 (CHEMBL4095552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118188 (US8653125, Ic-13) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50192804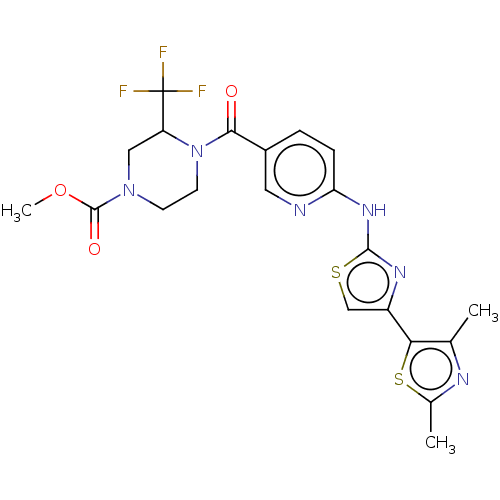 (CHEMBL3933401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation | Bioorg Med Chem Lett 26: 4936-4941 (2016) Article DOI: 10.1016/j.bmcl.2016.09.014 BindingDB Entry DOI: 10.7270/Q2154K06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118192 (US8653125, Id-8) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50256212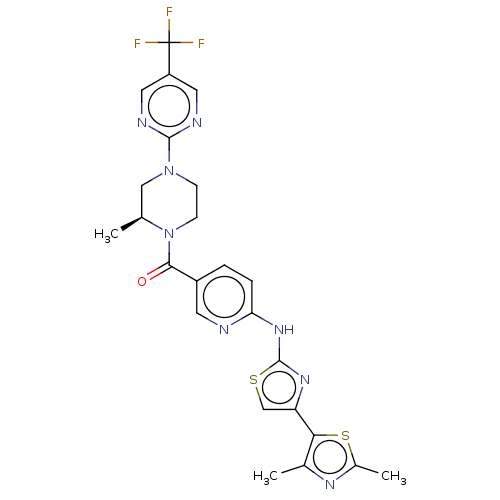 (CHEMBL4060956) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118178 (US8653125, Ia-35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264848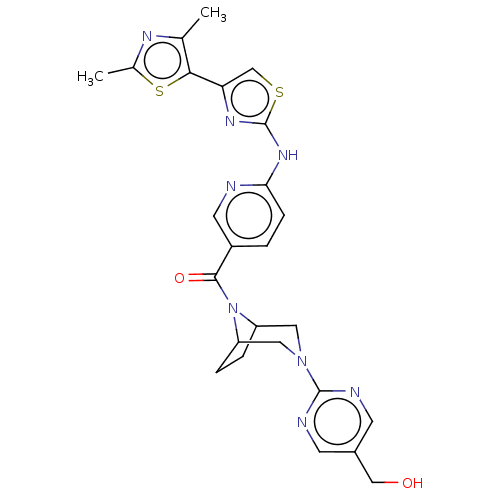 (CHEMBL4062935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118189 (US8653125, Ic-14) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118191 (US8653125, Ic-17) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Mus musculus (Mouse)) | BDBM118176 (US8653125, Ia-30) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Shionogi Co., Ltd. US Patent | Assay Description cDNA sequence encoding mouse NPY Y5 receptor (Biochem. Biophys. Acta 1328: 83-89, 1997) was cloned in the expression vector (pME18S, Takebe et al. Mo... | US Patent US8653125 (2014) BindingDB Entry DOI: 10.7270/Q2VQ31BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264857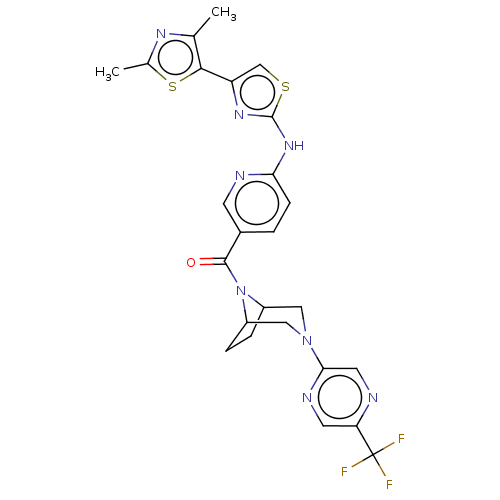 (CHEMBL4090714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264856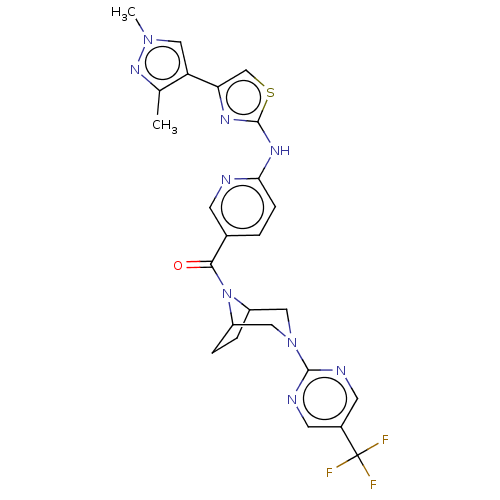 (CHEMBL4084992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50608178 (CHEMBL5268044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 260 total ) | Next | Last >> |