 Found 105 hits with Last Name = 'textor' and Initial = 'gp'
Found 105 hits with Last Name = 'textor' and Initial = 'gp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317108
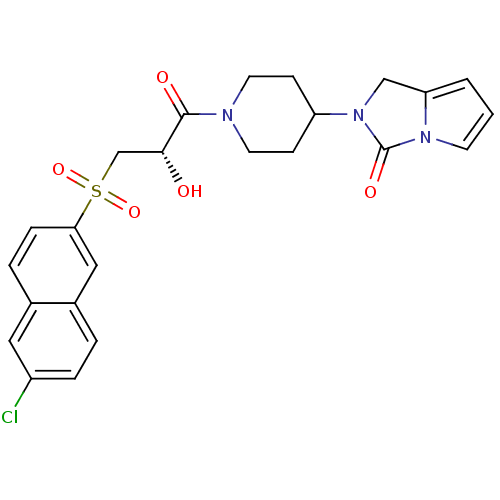
(2-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1Cc2cccn2C1=O |r| Show InChI InChI=1S/C24H24ClN3O5S/c25-18-5-3-17-13-21(6-4-16(17)12-18)34(32,33)15-22(29)23(30)26-10-7-19(8-11-26)28-14-20-2-1-9-27(20)24(28)31/h1-6,9,12-13,19,22,29H,7-8,10-11,14-15H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50304619
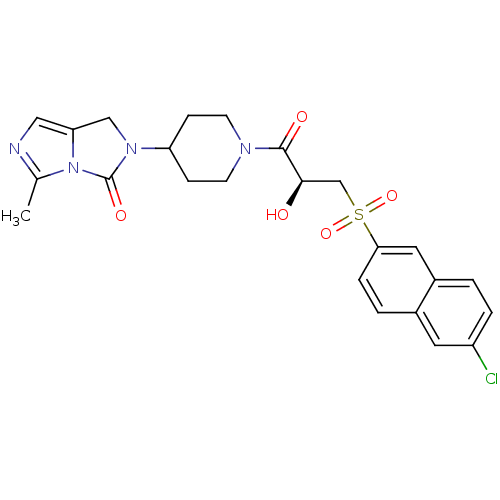
((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...)Show SMILES Cc1ncc2CN(C3CCN(CC3)C(=O)[C@H](O)CS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12 |r| Show InChI InChI=1S/C24H25ClN4O5S/c1-15-26-12-20-13-28(24(32)29(15)20)19-6-8-27(9-7-19)23(31)22(30)14-35(33,34)21-5-3-16-10-18(25)4-2-17(16)11-21/h2-5,10-12,19,22,30H,6-9,13-14H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317098
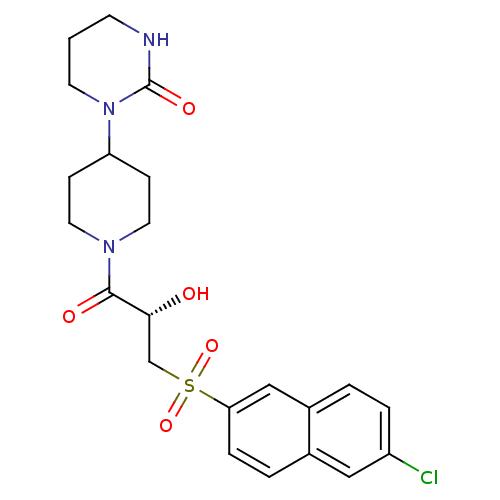
(1-(1-{(2S)-3-[(6-chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCNC1=O |r| Show InChI InChI=1S/C22H26ClN3O5S/c23-17-4-2-16-13-19(5-3-15(16)12-17)32(30,31)14-20(27)21(28)25-10-6-18(7-11-25)26-9-1-8-24-22(26)29/h2-5,12-13,18,20,27H,1,6-11,14H2,(H,24,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S2222 as substrate after 10 mins by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317107

(2-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1Cc2ccccc2C1=O |r| Show InChI InChI=1S/C26H25ClN2O5S/c27-20-7-5-18-14-22(8-6-17(18)13-20)35(33,34)16-24(30)26(32)28-11-9-21(10-12-28)29-15-19-3-1-2-4-23(19)25(29)31/h1-8,13-14,21,24,30H,9-12,15-16H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317098

(1-(1-{(2S)-3-[(6-chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCNC1=O |r| Show InChI InChI=1S/C22H26ClN3O5S/c23-17-4-2-16-13-19(5-3-15(16)12-17)32(30,31)14-20(27)21(28)25-10-6-18(7-11-25)26-9-1-8-24-22(26)29/h2-5,12-13,18,20,27H,1,6-11,14H2,(H,24,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317096
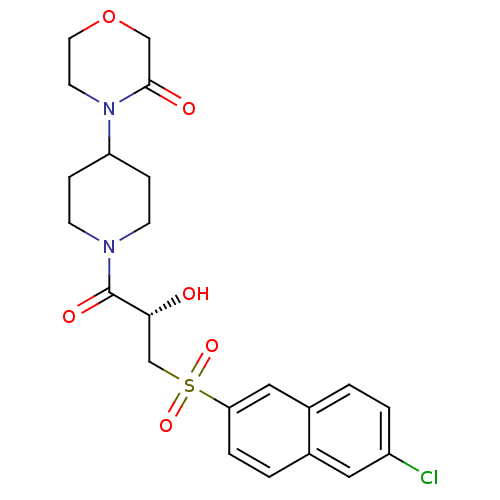
(4-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCOCC1=O |r| Show InChI InChI=1S/C22H25ClN2O6S/c23-17-3-1-16-12-19(4-2-15(16)11-17)32(29,30)14-20(26)22(28)24-7-5-18(6-8-24)25-9-10-31-13-21(25)27/h1-4,11-12,18,20,26H,5-10,13-14H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50270656
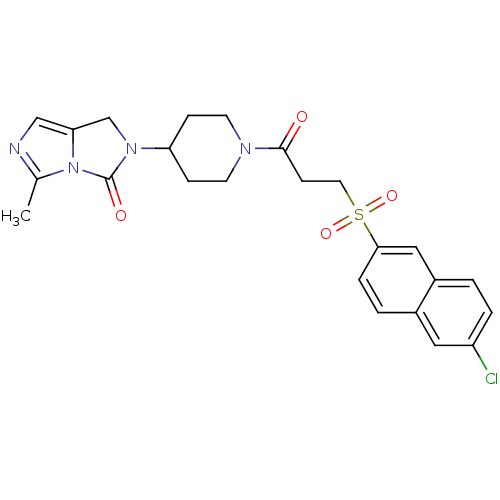
(2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...)Show SMILES Cc1ncc2CN(C3CCN(CC3)C(=O)CCS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12 Show InChI InChI=1S/C24H25ClN4O4S/c1-16-26-14-21-15-28(24(31)29(16)21)20-6-9-27(10-7-20)23(30)8-11-34(32,33)22-5-3-17-12-19(25)4-2-18(17)13-22/h2-5,12-14,20H,6-11,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50304620
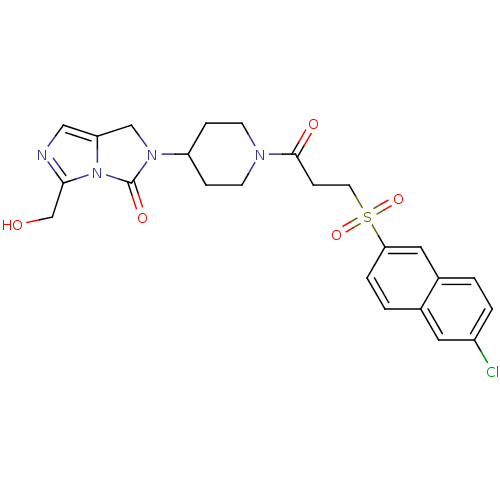
(2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...)Show SMILES OCc1ncc2CN(C3CCN(CC3)C(=O)CCS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12 Show InChI InChI=1S/C24H25ClN4O5S/c25-18-3-1-17-12-21(4-2-16(17)11-18)35(33,34)10-7-23(31)27-8-5-19(6-9-27)28-14-20-13-26-22(15-30)29(20)24(28)32/h1-4,11-13,19,30H,5-10,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317095
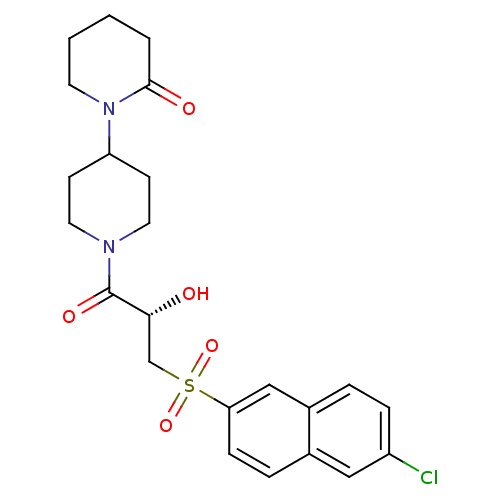
(1'-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2-h...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCCC1=O |r| Show InChI InChI=1S/C23H27ClN2O5S/c24-18-6-4-17-14-20(7-5-16(17)13-18)32(30,31)15-21(27)23(29)25-11-8-19(9-12-25)26-10-2-1-3-22(26)28/h4-7,13-14,19,21,27H,1-3,8-12,15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317092
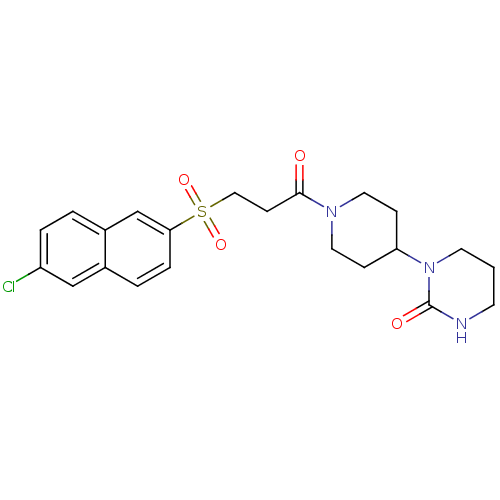
(1-(1-{3-[(6-Chloronaphthalen-2-yl)sulfonyl]propano...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)CCC(=O)N1CCC(CC1)N1CCCNC1=O Show InChI InChI=1S/C22H26ClN3O4S/c23-18-4-2-17-15-20(5-3-16(17)14-18)31(29,30)13-8-21(27)25-11-6-19(7-12-25)26-10-1-9-24-22(26)28/h2-5,14-15,19H,1,6-13H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299079
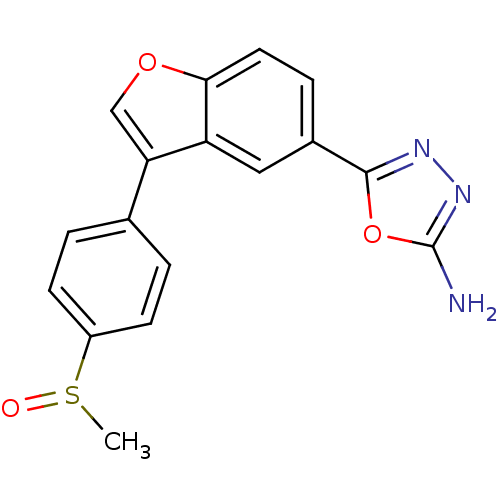
(5-{3-[4-(Methylsulfinyl)phenyl]-1-benzofuran-5-yl}...)Show SMILES CS(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1nnc(N)o1 Show InChI InChI=1S/C17H13N3O3S/c1-24(21)12-5-2-10(3-6-12)14-9-22-15-7-4-11(8-13(14)15)16-19-20-17(18)23-16/h2-9H,1H3,(H2,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317101
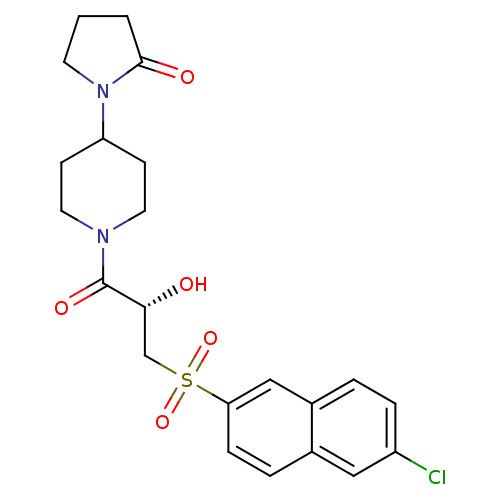
(1-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCC1=O |r| Show InChI InChI=1S/C22H25ClN2O5S/c23-17-5-3-16-13-19(6-4-15(16)12-17)31(29,30)14-20(26)22(28)24-10-7-18(8-11-24)25-9-1-2-21(25)27/h3-6,12-13,18,20,26H,1-2,7-11,14H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317097
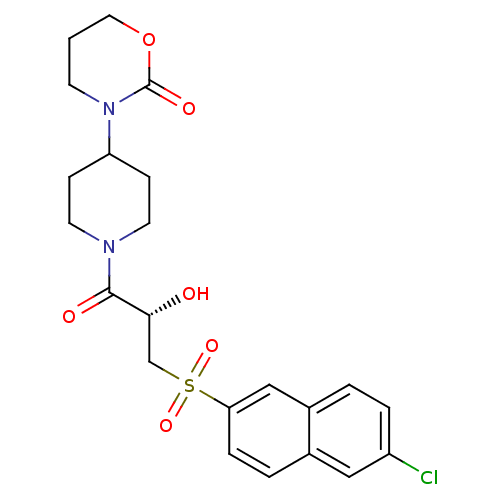
(3-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCOC1=O |r| Show InChI InChI=1S/C22H25ClN2O6S/c23-17-4-2-16-13-19(5-3-15(16)12-17)32(29,30)14-20(26)21(27)24-9-6-18(7-10-24)25-8-1-11-31-22(25)28/h2-5,12-13,18,20,26H,1,6-11,14H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299083
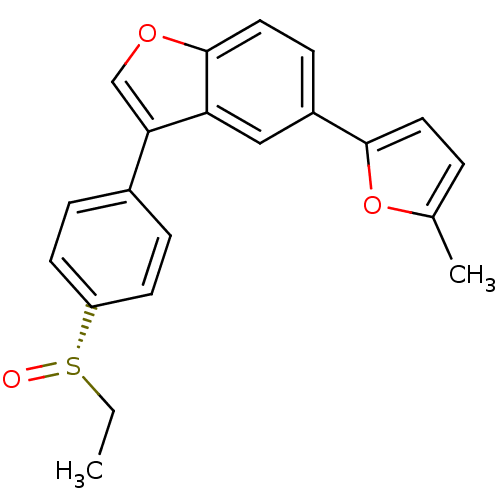
((S)-3-(4-(ethylsulfinyl)phenyl)-5-(5-methylfuran-2...)Show SMILES CC[S@](=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 |r| Show InChI InChI=1S/C21H18O3S/c1-3-25(22)17-8-5-15(6-9-17)19-13-23-21-11-7-16(12-18(19)21)20-10-4-14(2)24-20/h4-13H,3H2,1-2H3/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50304621
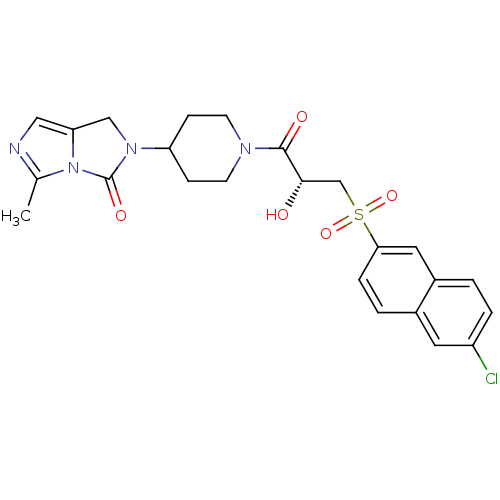
((R)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...)Show SMILES Cc1ncc2CN(C3CCN(CC3)C(=O)[C@@H](O)CS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12 |r| Show InChI InChI=1S/C24H25ClN4O5S/c1-15-26-12-20-13-28(24(32)29(15)20)19-6-8-27(9-7-19)23(31)22(30)14-35(33,34)21-5-3-16-10-18(25)4-2-17(16)11-21/h2-5,10-12,19,22,30H,6-9,13-14H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317104
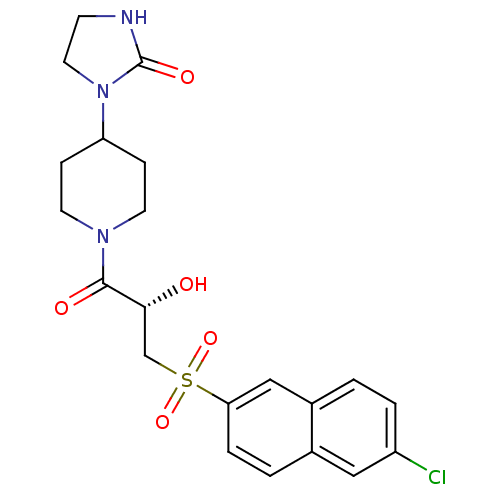
(1-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCNC1=O |r| Show InChI InChI=1S/C21H24ClN3O5S/c22-16-3-1-15-12-18(4-2-14(15)11-16)31(29,30)13-19(26)20(27)24-8-5-17(6-9-24)25-10-7-23-21(25)28/h1-4,11-12,17,19,26H,5-10,13H2,(H,23,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317106
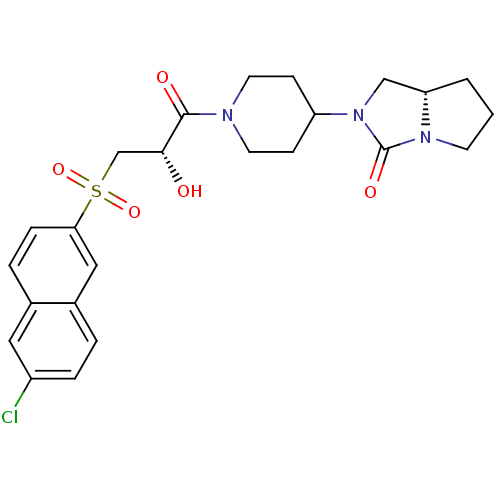
((7aS)-2-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfo...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1C[C@@H]2CCCN2C1=O |r| Show InChI InChI=1S/C24H28ClN3O5S/c25-18-5-3-17-13-21(6-4-16(17)12-18)34(32,33)15-22(29)23(30)26-10-7-19(8-11-26)28-14-20-2-1-9-27(20)24(28)31/h3-6,12-13,19-20,22,29H,1-2,7-11,14-15H2/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317093
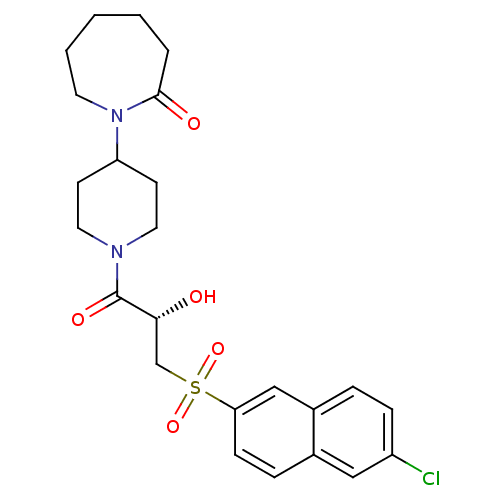
(1-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCCCC1=O |r| Show InChI InChI=1S/C24H29ClN2O5S/c25-19-7-5-18-15-21(8-6-17(18)14-19)33(31,32)16-22(28)24(30)26-12-9-20(10-13-26)27-11-3-1-2-4-23(27)29/h5-8,14-15,20,22,28H,1-4,9-13,16H2/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299081
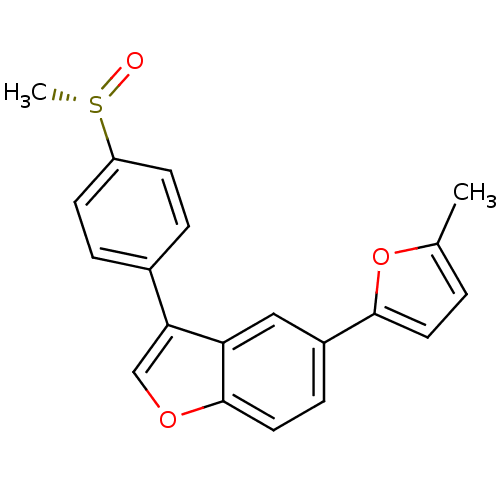
((S)-5-(5-methylfuran-2-yl)-3-(4-(methylsulfinyl)ph...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)[S@](C)=O)c2c1 |r| Show InChI InChI=1S/C20H16O3S/c1-13-3-9-19(23-13)15-6-10-20-17(11-15)18(12-22-20)14-4-7-16(8-5-14)24(2)21/h3-12H,1-2H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299080
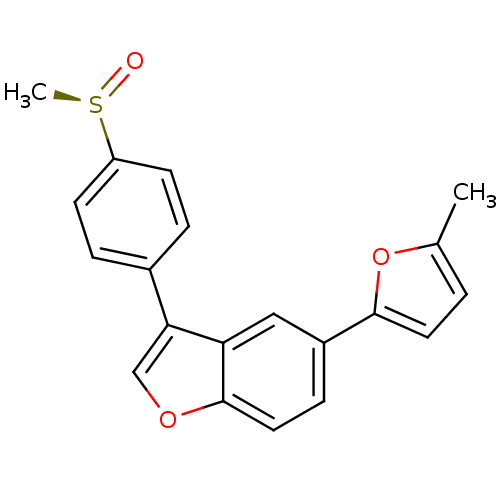
((R)-5-(5-methylfuran-2-yl)-3-(4-(methylsulfinyl)ph...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)[S@@](C)=O)c2c1 |r| Show InChI InChI=1S/C20H16O3S/c1-13-3-9-19(23-13)15-6-10-20-17(11-15)18(12-22-20)14-4-7-16(8-5-14)24(2)21/h3-12H,1-2H3/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299082
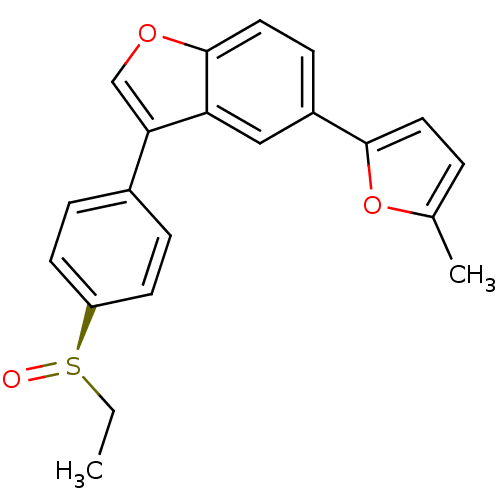
((R)-3-(4-(ethylsulfinyl)phenyl)-5-(5-methylfuran-2...)Show SMILES CC[S@@](=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 |r| Show InChI InChI=1S/C21H18O3S/c1-3-25(22)17-8-5-15(6-9-17)19-13-23-21-11-7-16(12-18(19)21)20-10-4-14(2)24-20/h4-13H,3H2,1-2H3/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299084
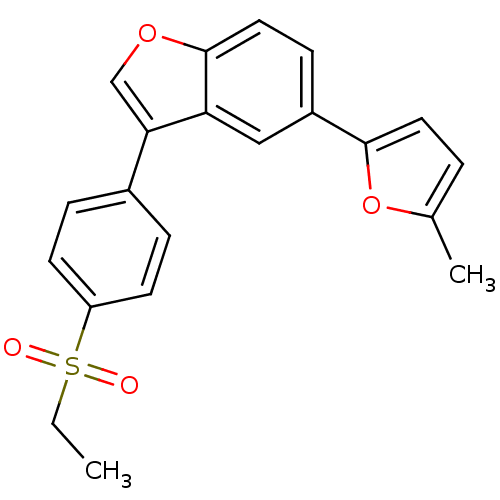
(2-{3-[4-(Ethylsulfonyl)phenyl]-1-benzofuran-5-yl}-...)Show SMILES CCS(=O)(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 Show InChI InChI=1S/C21H18O4S/c1-3-26(22,23)17-8-5-15(6-9-17)19-13-24-21-11-7-16(12-18(19)21)20-10-4-14(2)25-20/h4-13H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299085
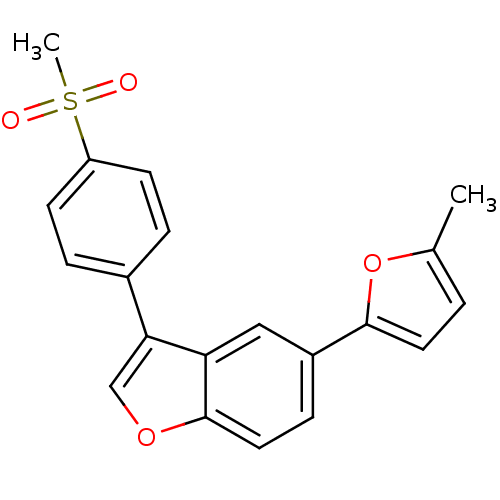
(2-Methyl-5-{3-[4-(methylsulfonyl)phenyl]-1-benzofu...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)S(C)(=O)=O)c2c1 Show InChI InChI=1S/C20H16O4S/c1-13-3-9-19(24-13)15-6-10-20-17(11-15)18(12-23-20)14-4-7-16(8-5-14)25(2,21)22/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317103
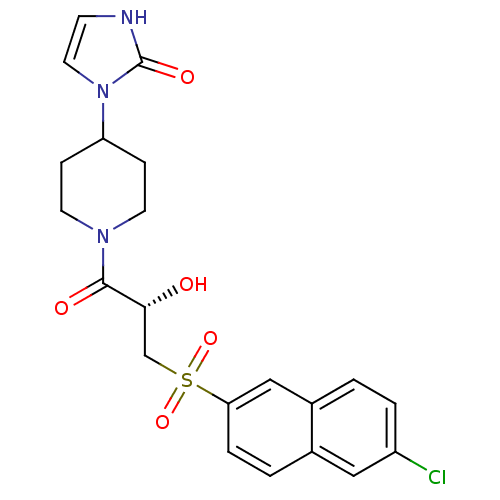
(1-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)n1cc[nH]c1=O |r| Show InChI InChI=1S/C21H22ClN3O5S/c22-16-3-1-15-12-18(4-2-14(15)11-16)31(29,30)13-19(26)20(27)24-8-5-17(6-9-24)25-10-7-23-21(25)28/h1-4,7,10-12,17,19,26H,5-6,8-9,13H2,(H,23,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317094
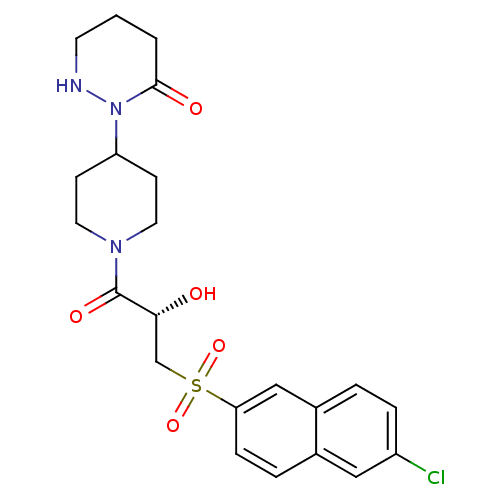
(2-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1NCCCC1=O |r| Show InChI InChI=1S/C22H26ClN3O5S/c23-17-5-3-16-13-19(6-4-15(16)12-17)32(30,31)14-20(27)22(29)25-10-7-18(8-11-25)26-21(28)2-1-9-24-26/h3-6,12-13,18,20,24,27H,1-2,7-11,14H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299086

(4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofuran-...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)C(O)=O)c2c1 Show InChI InChI=1S/C20H14O4/c1-12-2-8-18(24-12)15-7-9-19-16(10-15)17(11-23-19)13-3-5-14(6-4-13)20(21)22/h2-11H,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317105
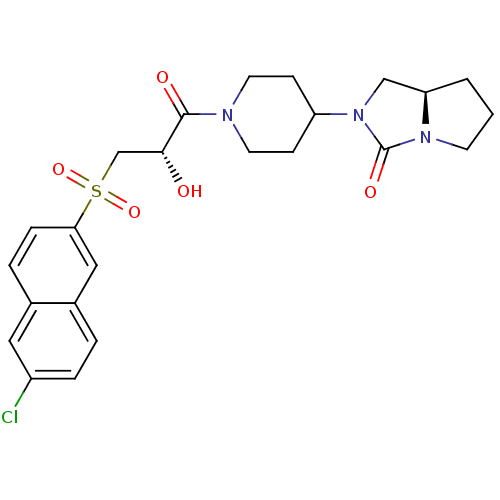
((7aR)-2-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfo...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1C[C@H]2CCCN2C1=O |r| Show InChI InChI=1S/C24H28ClN3O5S/c25-18-5-3-17-13-21(6-4-16(17)12-18)34(32,33)15-22(29)23(30)26-10-7-19(8-11-26)28-14-20-2-1-9-27(20)24(28)31/h3-6,12-13,19-20,22,29H,1-2,7-11,14-15H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299087
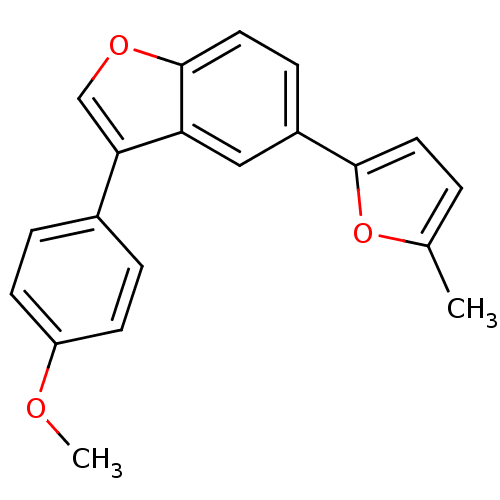
(2-[3-(4-Methoxyphenyl)-1-benzofuran-5-yl]-5-methyl...)Show InChI InChI=1S/C20H16O3/c1-13-3-9-19(23-13)15-6-10-20-17(11-15)18(12-22-20)14-4-7-16(21-2)8-5-14/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317102
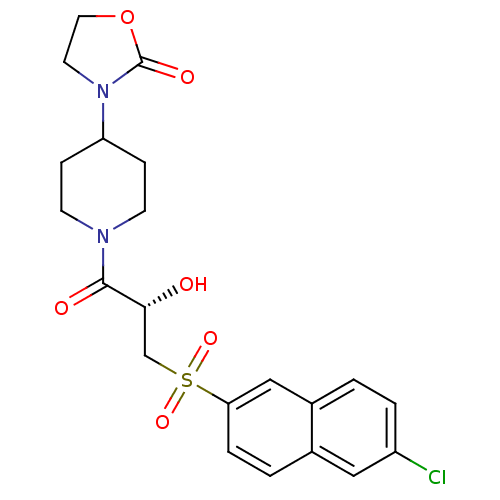
(3-(1-{(2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-2...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCOC1=O |r| Show InChI InChI=1S/C21H23ClN2O6S/c22-16-3-1-15-12-18(4-2-14(15)11-16)31(28,29)13-19(25)20(26)23-7-5-17(6-8-23)24-9-10-30-21(24)27/h1-4,11-12,17,19,25H,5-10,13H2/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299088
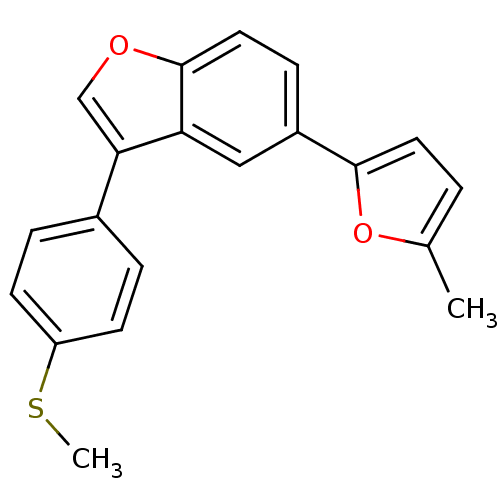
(2-Methyl-5-{3-[4-(methylsulfanyl)phenyl]-1-benzofu...)Show InChI InChI=1S/C20H16O2S/c1-13-3-9-19(22-13)15-6-10-20-17(11-15)18(12-21-20)14-4-7-16(23-2)8-5-14/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299089
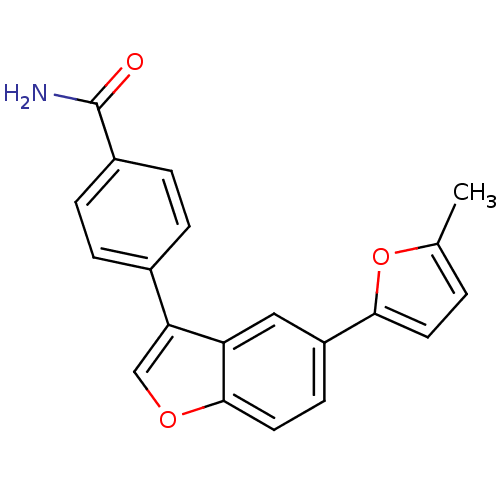
(4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofuran-...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)C(N)=O)c2c1 Show InChI InChI=1S/C20H15NO3/c1-12-2-8-18(24-12)15-7-9-19-16(10-15)17(11-23-19)13-3-5-14(6-4-13)20(21)22/h2-11H,1H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299072
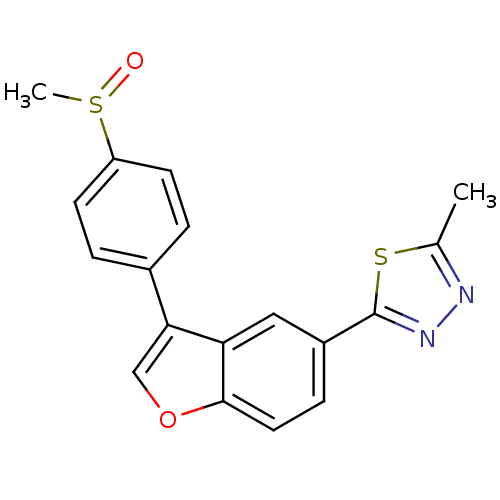
(2-Methyl-5-{3-[4-(methylsulfinyl)phenyl]-1-benzofu...)Show SMILES Cc1nnc(s1)-c1ccc2occ(-c3ccc(cc3)S(C)=O)c2c1 Show InChI InChI=1S/C18H14N2O2S2/c1-11-19-20-18(23-11)13-5-8-17-15(9-13)16(10-22-17)12-3-6-14(7-4-12)24(2)21/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299090

(2-[3-(3-Methoxyphenyl)-1-benzofuran-5-yl]-5-methyl...)Show InChI InChI=1S/C20H16O3/c1-13-6-8-19(23-13)15-7-9-20-17(11-15)18(12-22-20)14-4-3-5-16(10-14)21-2/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299091
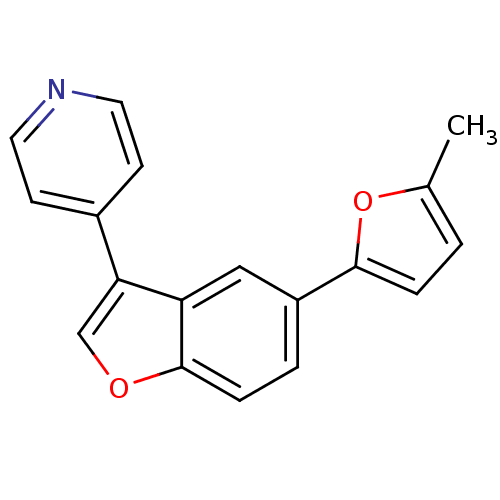
(4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofuran-...)Show InChI InChI=1S/C18H13NO2/c1-12-2-4-17(21-12)14-3-5-18-15(10-14)16(11-20-18)13-6-8-19-9-7-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299073

(3-Methyl-5-{3-[4-(methylsulfinyl)phenyl]-1-benzofu...)Show SMILES Cc1nnc([nH]1)-c1ccc2occ(-c3ccc(cc3)S(C)=O)c2c1 Show InChI InChI=1S/C18H15N3O2S/c1-11-19-18(21-20-11)13-5-8-17-15(9-13)16(10-23-17)12-3-6-14(7-4-12)24(2)22/h3-10H,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299077

(5-{3-[4-(Methylsulfinyl)phenyl]-1-benzofuran-5-yl}...)Show SMILES CS(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1nnc(S)o1 Show InChI InChI=1S/C17H12N2O3S2/c1-24(20)12-5-2-10(3-6-12)14-9-21-15-7-4-11(8-13(14)15)16-18-19-17(23)22-16/h2-9H,1H3,(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299092
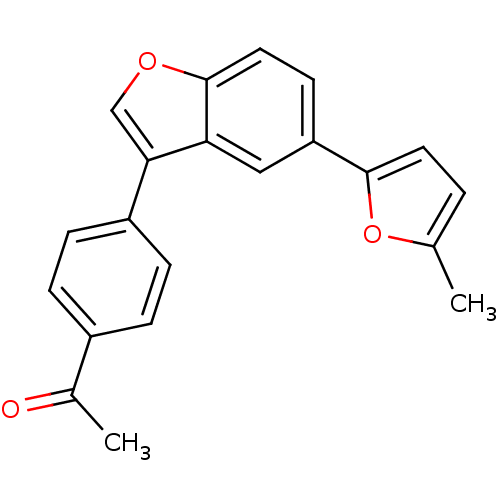
(1-{4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofur...)Show SMILES CC(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 Show InChI InChI=1S/C21H16O3/c1-13-3-9-20(24-13)17-8-10-21-18(11-17)19(12-23-21)16-6-4-15(5-7-16)14(2)22/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299093
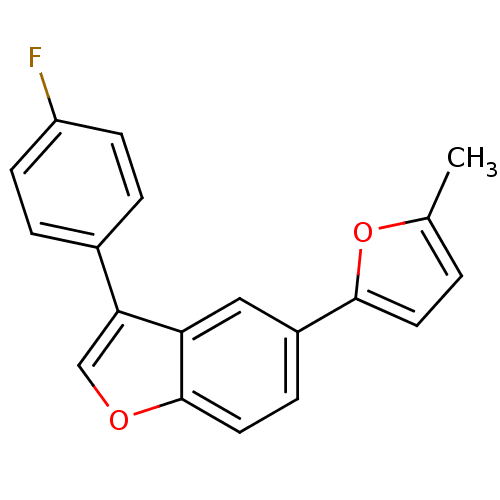
(2-[3-(4-Fluorophenyl)-1-benzofuran-5-yl]-5-methyl-...)Show InChI InChI=1S/C19H13FO2/c1-12-2-8-18(22-12)14-5-9-19-16(10-14)17(11-21-19)13-3-6-15(20)7-4-13/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299078

(5-{3-[4-(Methylsulfinyl)phenyl]-1-benzofuran-5-yl}...)Show SMILES CS(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1n[nH]c(=O)o1 Show InChI InChI=1S/C17H12N2O4S/c1-24(21)12-5-2-10(3-6-12)14-9-22-15-7-4-11(8-13(14)15)16-18-19-17(20)23-16/h2-9H,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299080

((R)-5-(5-methylfuran-2-yl)-3-(4-(methylsulfinyl)ph...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)[S@@](C)=O)c2c1 |r| Show InChI InChI=1S/C20H16O3S/c1-13-3-9-19(23-13)15-6-10-20-17(11-15)18(12-22-20)14-4-7-16(8-5-14)24(2)21/h3-12H,1-2H3/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317099
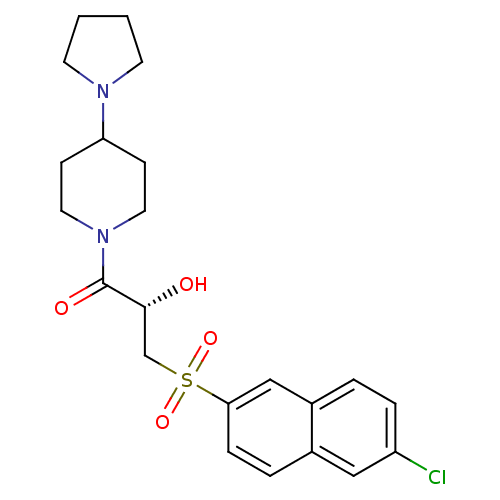
((2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-1-oxo-1...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCC1 |r| Show InChI InChI=1S/C22H27ClN2O4S/c23-18-5-3-17-14-20(6-4-16(17)13-18)30(28,29)15-21(26)22(27)25-11-7-19(8-12-25)24-9-1-2-10-24/h3-6,13-14,19,21,26H,1-2,7-12,15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299094

(1-{4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofur...)Show InChI InChI=1S/C21H18O3/c1-13-3-9-20(24-13)17-8-10-21-18(11-17)19(12-23-21)16-6-4-15(5-7-16)14(2)22/h3-12,14,22H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299095

(4-[5-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-benzofuran-...)Show InChI InChI=1S/C19H14O3/c1-12-2-8-18(22-12)14-5-9-19-16(10-14)17(11-21-19)13-3-6-15(20)7-4-13/h2-11,20H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299082

((R)-3-(4-(ethylsulfinyl)phenyl)-5-(5-methylfuran-2...)Show SMILES CC[S@@](=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 |r| Show InChI InChI=1S/C21H18O3S/c1-3-25(22)17-8-5-15(6-9-17)19-13-23-21-11-7-16(12-18(19)21)20-10-4-14(2)24-20/h4-13H,3H2,1-2H3/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50317100
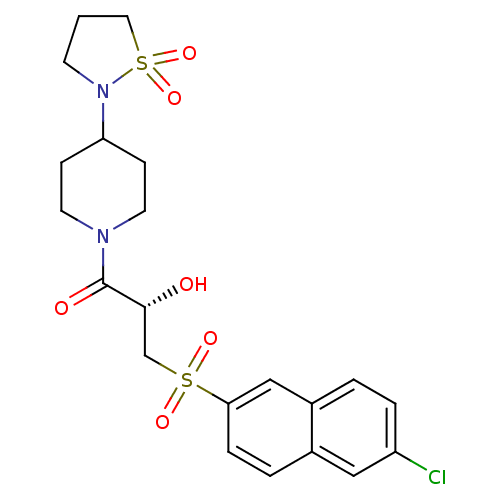
((2S)-3-[(6-Chloronaphthalen-2-yl)sulfonyl]-1-[4-(1...)Show SMILES O[C@H](CS(=O)(=O)c1ccc2cc(Cl)ccc2c1)C(=O)N1CCC(CC1)N1CCCS1(=O)=O |r| Show InChI InChI=1S/C21H25ClN2O6S2/c22-17-4-2-16-13-19(5-3-15(16)12-17)31(27,28)14-20(25)21(26)23-9-6-18(7-10-23)24-8-1-11-32(24,29)30/h2-5,12-13,18,20,25H,1,6-11,14H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assay |
J Med Chem 53: 3517-31 (2010)
Article DOI: 10.1021/jm901699j
BindingDB Entry DOI: 10.7270/Q2PR7W52 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299096
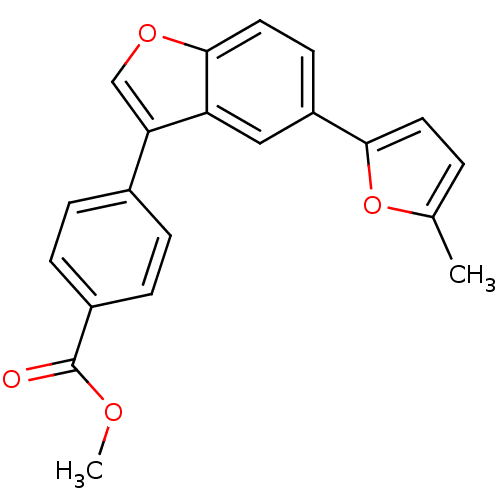
(CHEMBL573480 | Methyl 4-[5-(5-Methyl-1,3,4-oxadiaz...)Show SMILES COC(=O)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 Show InChI InChI=1S/C21H16O4/c1-13-3-9-19(25-13)16-8-10-20-17(11-16)18(12-24-20)14-4-6-15(7-5-14)21(22)23-2/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299099
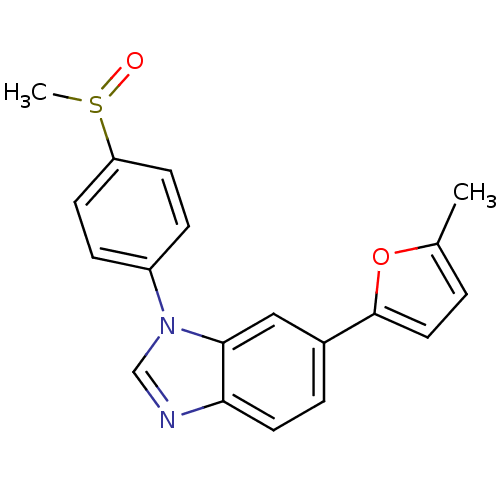
(6-(5-Methyl-1,3,4-oxadiazol-2-yl)-1-[4-(methylsulf...)Show SMILES Cc1ccc(o1)-c1ccc2ncn(-c3ccc(cc3)S(C)=O)c2c1 Show InChI InChI=1S/C19H16N2O2S/c1-13-3-10-19(23-13)14-4-9-17-18(11-14)21(12-20-17)15-5-7-16(8-6-15)24(2)22/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299097
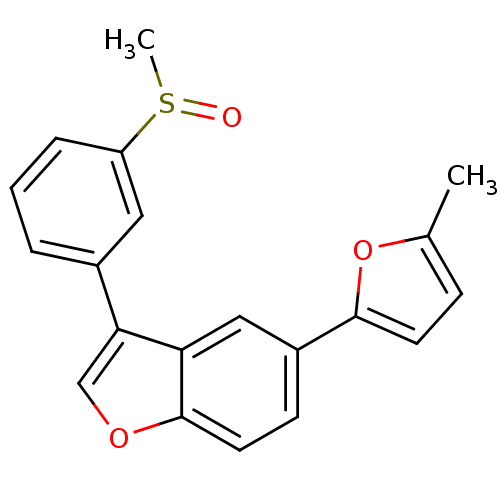
(2-Methyl-5-{3-[3-(methylsulfinyl)phenyl]-1-benzofu...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3cccc(c3)S(C)=O)c2c1 Show InChI InChI=1S/C20H16O3S/c1-13-6-8-19(23-13)15-7-9-20-17(11-15)18(12-22-20)14-4-3-5-16(10-14)24(2)21/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299102

(5-(5-Methyl-1,3,4-oxadiazol-2-yl)-3-[4-(methylsulf...)Show SMILES Cc1ccc(o1)-c1ccc2occ(-c3ccc(cc3)S(C)=O)c2n1 Show InChI InChI=1S/C19H15NO3S/c1-12-3-9-17(23-12)16-8-10-18-19(20-16)15(11-22-18)13-4-6-14(7-5-13)24(2)21/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50299098
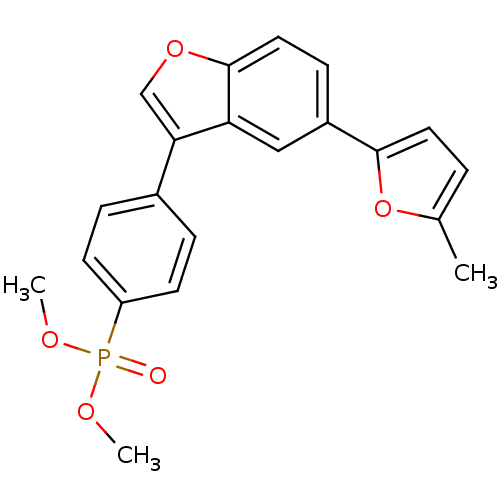
(CHEMBL578861 | Dimethyl {4-[5-(5-Methyl-1,3,4-oxad...)Show SMILES COP(=O)(OC)c1ccc(cc1)-c1coc2ccc(cc12)-c1ccc(C)o1 Show InChI InChI=1S/C21H19O5P/c1-14-4-10-20(26-14)16-7-11-21-18(12-16)19(13-25-21)15-5-8-17(9-6-15)27(22,23-2)24-3/h4-13H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta |
J Med Chem 52: 6270-86 (2009)
Article DOI: 10.1021/jm900647e
BindingDB Entry DOI: 10.7270/Q2CZ377V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data