 Found 497 hits with Last Name = 'theroff' and Initial = 'jp'
Found 497 hits with Last Name = 'theroff' and Initial = 'jp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693
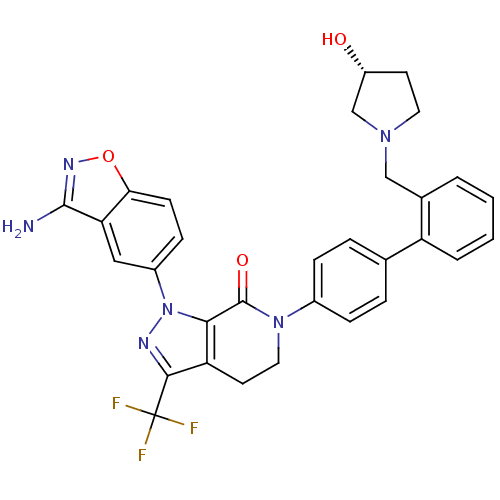
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228913
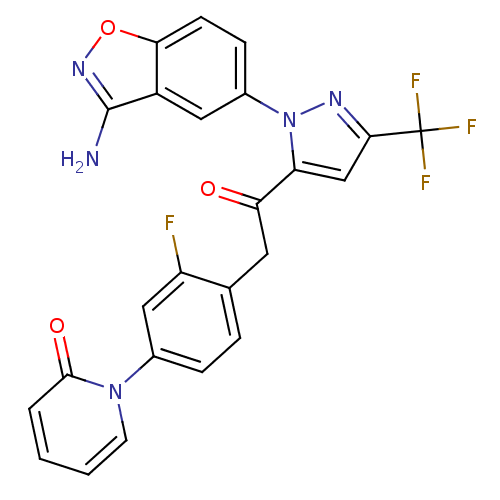
(1-(4-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifl...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H15F4N5O3/c25-17-11-14(32-8-2-1-3-22(32)35)5-4-13(17)9-19(34)18-12-21(24(26,27)28)30-33(18)15-6-7-20-16(10-15)23(29)31-36-20/h1-8,10-12H,9H2,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228907

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H18F4N4O4S/c1-39(36,37)23-5-3-2-4-17(23)14-6-7-15(19(27)10-14)11-21(35)20-13-24(26(28,29)30)32-34(20)16-8-9-22-18(12-16)25(31)33-38-22/h2-10,12-13H,11H2,1H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228926

(1-(3-fluoro-4-(2-(1-(4-methoxyphenyl)-3-(trifluoro...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H17F4N3O3/c1-34-18-9-7-16(8-10-18)31-20(14-22(29-31)24(26,27)28)21(32)12-15-5-6-17(13-19(15)25)30-11-3-2-4-23(30)33/h2-11,13-14H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228914
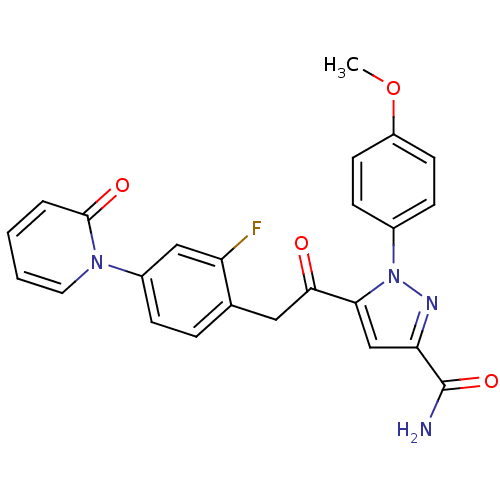
(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228909
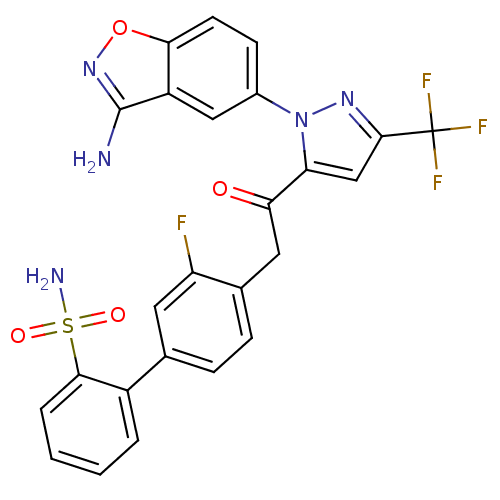
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H17F4N5O4S/c26-18-9-13(16-3-1-2-4-22(16)39(31,36)37)5-6-14(18)10-20(35)19-12-23(25(27,28)29)32-34(19)15-7-8-21-17(11-15)24(30)33-38-21/h1-9,11-12H,10H2,(H2,30,33)(H2,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228912
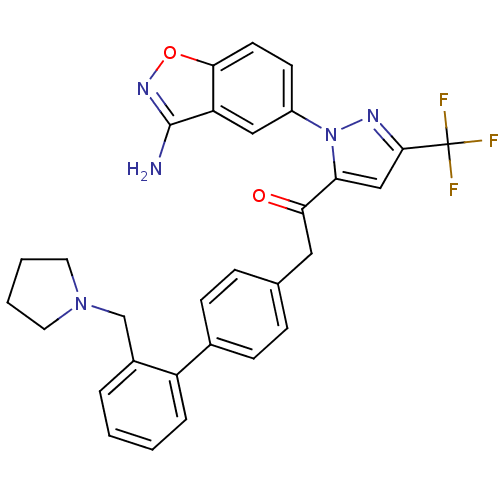
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CCCC1)C(F)(F)F Show InChI InChI=1S/C30H26F3N5O2/c31-30(32,33)28-17-25(38(35-28)22-11-12-27-24(16-22)29(34)36-40-27)26(39)15-19-7-9-20(10-8-19)23-6-2-1-5-21(23)18-37-13-3-4-14-37/h1-2,5-12,16-17H,3-4,13-15,18H2,(H2,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228922

(1-(4-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifl...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C24H20F3N5O3/c25-24(26,27)21-13-18(32(29-21)16-8-9-20-17(12-16)23(28)30-35-20)19(33)11-14-4-6-15(7-5-14)31-10-2-1-3-22(31)34/h4-9,12-13H,1-3,10-11H2,(H2,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228919
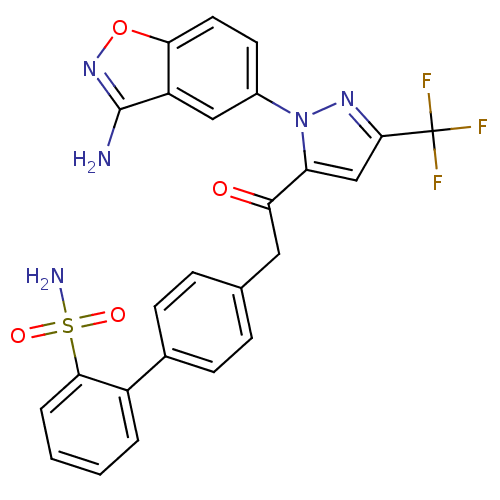
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H18F3N5O4S/c26-25(27,28)23-13-19(33(31-23)16-9-10-21-18(12-16)24(29)32-37-21)20(34)11-14-5-7-15(8-6-14)17-3-1-2-4-22(17)38(30,35)36/h1-10,12-13H,11H2,(H2,29,32)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228917
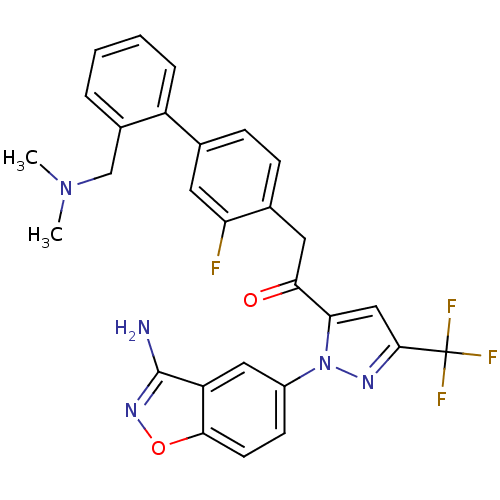
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CN(C)Cc1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H23F4N5O2/c1-36(2)15-18-5-3-4-6-20(18)16-7-8-17(22(29)11-16)12-24(38)23-14-26(28(30,31)32)34-37(23)19-9-10-25-21(13-19)27(33)35-39-25/h3-11,13-14H,12,15H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228924

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)cc1 Show InChI InChI=1S/C26H19F3N4O4S/c1-38(35,36)23-5-3-2-4-18(23)16-8-6-15(7-9-16)12-21(34)20-14-24(26(27,28)29)31-33(20)17-10-11-22-19(13-17)25(30)32-37-22/h2-11,13-14H,12H2,1H3,(H2,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228915
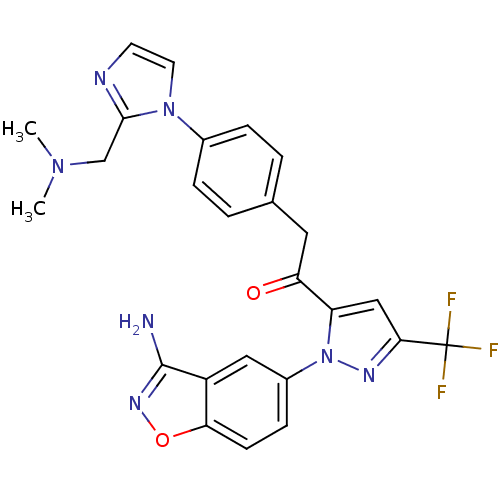
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CN(C)Cc1nccn1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)cc1 Show InChI InChI=1S/C25H22F3N7O2/c1-33(2)14-23-30-9-10-34(23)16-5-3-15(4-6-16)11-20(36)19-13-22(25(26,27)28)31-35(19)17-7-8-21-18(12-17)24(29)32-37-21/h3-10,12-13H,11,14H2,1-2H3,(H2,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228906

(1-(4-(2-(1-(4-methoxyphenyl)-3-(trifluoromethyl)-1...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C24H22F3N3O3/c1-33-19-11-9-18(10-12-19)30-20(15-22(28-30)24(25,26)27)21(31)14-16-5-7-17(8-6-16)29-13-3-2-4-23(29)32/h5-12,15H,2-4,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228921
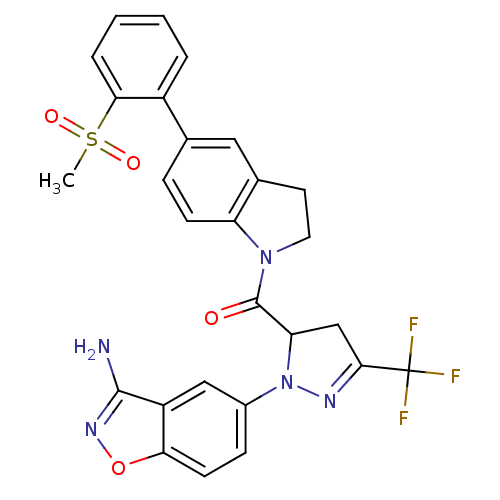
((1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluorometh...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc2N(CCc2c1)C(=O)C1CC(=NN1c1ccc2onc(N)c2c1)C(F)(F)F |c:26| Show InChI InChI=1S/C27H22F3N5O4S/c1-40(37,38)23-5-3-2-4-18(23)15-6-8-20-16(12-15)10-11-34(20)26(36)21-14-24(27(28,29)30)32-35(21)17-7-9-22-19(13-17)25(31)33-39-22/h2-9,12-13,21H,10-11,14H2,1H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228925
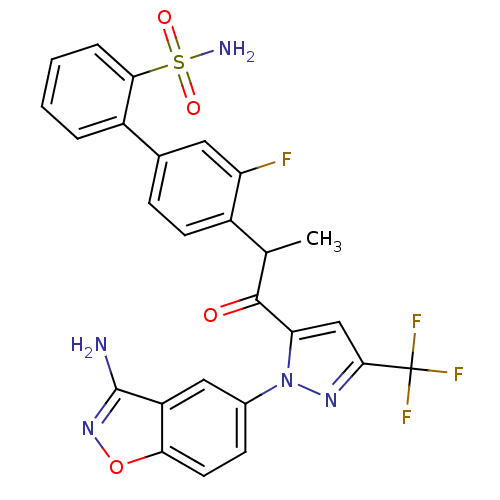
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1F)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C26H19F4N5O4S/c1-13(16-8-6-14(10-19(16)27)17-4-2-3-5-22(17)40(32,37)38)24(36)20-12-23(26(28,29)30)33-35(20)15-7-9-21-18(11-15)25(31)34-39-21/h2-13H,1H3,(H2,31,34)(H2,32,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228911
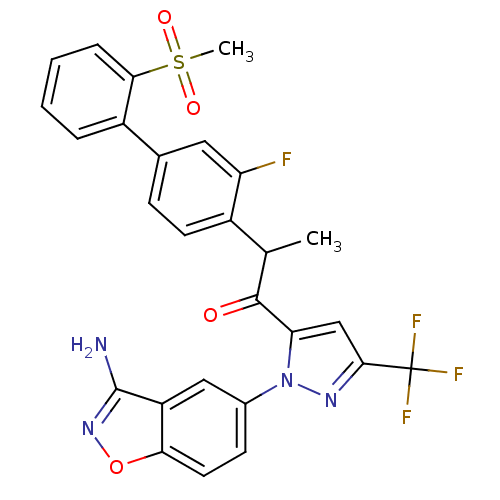
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1F)-c1ccccc1S(C)(=O)=O Show InChI InChI=1S/C27H20F4N4O4S/c1-14(17-9-7-15(11-20(17)28)18-5-3-4-6-23(18)40(2,37)38)25(36)21-13-24(27(29,30)31)33-35(21)16-8-10-22-19(12-16)26(32)34-39-22/h3-14H,1-2H3,(H2,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228910

(2-[2'-((S)-3-hydroxy-pyrrolidin-1-ylmethyl)-biphen...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CC[C@H](O)C1)C(F)(F)F Show InChI InChI=1S/C30H28F3N3O3/c1-39-25-12-10-23(11-13-25)36-27(17-29(34-36)30(31,32)33)28(38)16-20-6-8-21(9-7-20)26-5-3-2-4-22(26)18-35-15-14-24(37)19-35/h2-13,17,24,37H,14-16,18-19H2,1H3/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228920

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1)-c1ccccc1S(C)(=O)=O Show InChI InChI=1S/C27H21F3N4O4S/c1-15(16-7-9-17(10-8-16)19-5-3-4-6-23(19)39(2,36)37)25(35)21-14-24(27(28,29)30)32-34(21)18-11-12-22-20(13-18)26(31)33-38-22/h3-15H,1-2H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228908
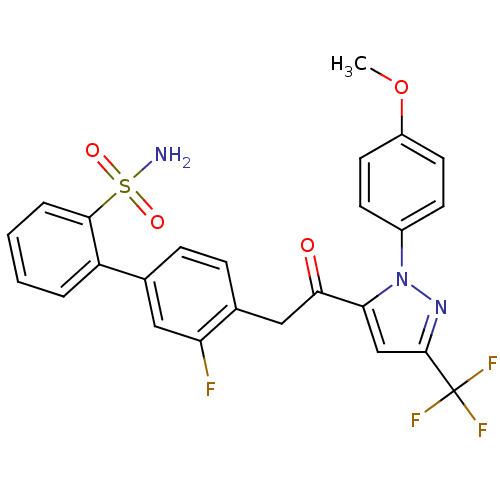
(3'-fluoro-4'-{2-[2-(4-methoxy-phenyl)-5-trifluorom...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H19F4N3O4S/c1-36-18-10-8-17(9-11-18)32-21(14-24(31-32)25(27,28)29)22(33)13-16-7-6-15(12-20(16)26)19-4-2-3-5-23(19)37(30,34)35/h2-12,14H,13H2,1H3,(H2,30,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228923
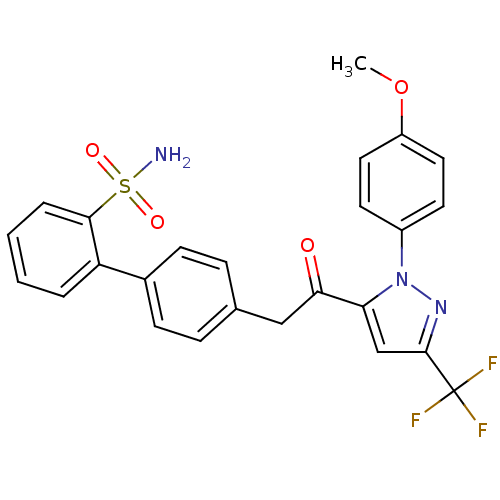
(4'-{2-[2-(4-methoxy-phenyl)-5-trifluoromethyl-2H-p...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3N3O4S/c1-35-19-12-10-18(11-13-19)31-21(15-24(30-31)25(26,27)28)22(32)14-16-6-8-17(9-7-16)20-4-2-3-5-23(20)36(29,33)34/h2-13,15H,14H2,1H3,(H2,29,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228918
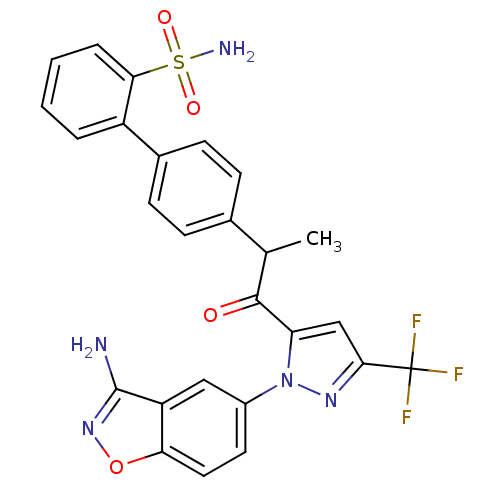
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C26H20F3N5O4S/c1-14(15-6-8-16(9-7-15)18-4-2-3-5-22(18)39(31,36)37)24(35)20-13-23(26(27,28)29)32-34(20)17-10-11-21-19(12-17)25(30)33-38-21/h2-14H,1H3,(H2,30,33)(H2,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM12693

(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228912

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CCCC1)C(F)(F)F Show InChI InChI=1S/C30H26F3N5O2/c31-30(32,33)28-17-25(38(35-28)22-11-12-27-24(16-22)29(34)36-40-27)26(39)15-19-7-9-20(10-8-19)23-6-2-1-5-21(23)18-37-13-3-4-14-37/h1-2,5-12,16-17H,3-4,13-15,18H2,(H2,34,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228913

(1-(4-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifl...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H15F4N5O3/c25-17-11-14(32-8-2-1-3-22(32)35)5-4-13(17)9-19(34)18-12-21(24(26,27)28)30-33(18)15-6-7-20-16(10-15)23(29)31-36-20/h1-8,10-12H,9H2,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 349 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228915

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CN(C)Cc1nccn1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)cc1 Show InChI InChI=1S/C25H22F3N7O2/c1-33(2)14-23-30-9-10-34(23)16-5-3-15(4-6-16)11-20(36)19-13-22(25(26,27)28)31-35(19)17-7-8-21-18(12-17)24(29)32-37-21/h3-10,12-13H,11,14H2,1-2H3,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228910

(2-[2'-((S)-3-hydroxy-pyrrolidin-1-ylmethyl)-biphen...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CC[C@H](O)C1)C(F)(F)F Show InChI InChI=1S/C30H28F3N3O3/c1-39-25-12-10-23(11-13-25)36-27(17-29(34-36)30(31,32)33)28(38)16-20-6-8-21(9-7-20)26-5-3-2-4-22(26)18-35-15-14-24(37)19-35/h2-13,17,24,37H,14-16,18-19H2,1H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228926

(1-(3-fluoro-4-(2-(1-(4-methoxyphenyl)-3-(trifluoro...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H17F4N3O3/c1-34-18-9-7-16(8-10-18)31-20(14-22(29-31)24(26,27)28)21(32)12-15-5-6-17(13-19(15)25)30-11-3-2-4-23(30)33/h2-11,13-14H,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228906

(1-(4-(2-(1-(4-methoxyphenyl)-3-(trifluoromethyl)-1...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C24H22F3N3O3/c1-33-19-11-9-18(10-12-19)30-20(15-22(28-30)24(25,26)27)21(31)14-16-5-7-17(8-6-16)29-13-3-2-4-23(29)32/h5-12,15H,2-4,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228908

(3'-fluoro-4'-{2-[2-(4-methoxy-phenyl)-5-trifluorom...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H19F4N3O4S/c1-36-18-10-8-17(9-11-18)32-21(14-24(31-32)25(27,28)29)22(33)13-16-7-6-15(12-20(16)26)19-4-2-3-5-23(19)37(30,34)35/h2-12,14H,13H2,1H3,(H2,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228923

(4'-{2-[2-(4-methoxy-phenyl)-5-trifluoromethyl-2H-p...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3N3O4S/c1-35-19-12-10-18(11-13-19)31-21(15-24(30-31)25(26,27)28)22(32)14-16-6-8-17(9-7-16)20-4-2-3-5-23(20)36(29,33)34/h2-13,15H,14H2,1H3,(H2,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50228914

(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human tPA |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361211
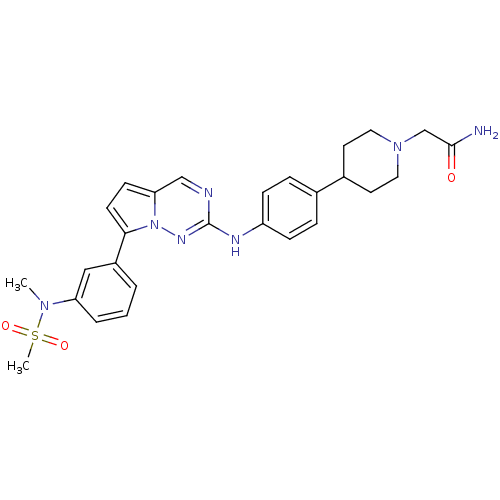
(CHEMBL1934340)Show SMILES CN(c1cccc(c1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12)S(C)(=O)=O Show InChI InChI=1S/C27H31N7O3S/c1-32(38(2,36)37)23-5-3-4-21(16-23)25-11-10-24-17-29-27(31-34(24)25)30-22-8-6-19(7-9-22)20-12-14-33(15-13-20)18-26(28)35/h3-11,16-17,20H,12-15,18H2,1-2H3,(H2,28,35)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50353049
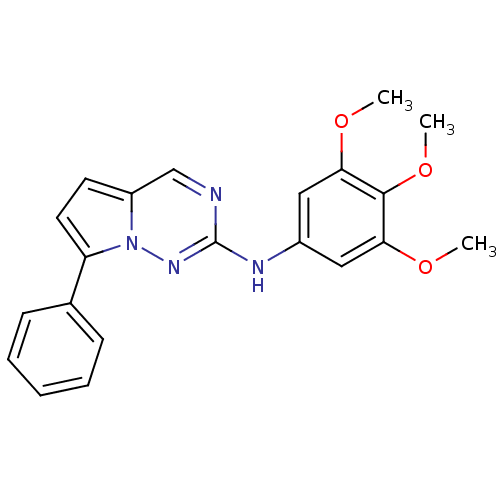
(CHEMBL1822511)Show SMILES COc1cc(Nc2ncc3ccc(-c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20N4O3/c1-26-18-11-15(12-19(27-2)20(18)28-3)23-21-22-13-16-9-10-17(25(16)24-21)14-7-5-4-6-8-14/h4-13H,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361213
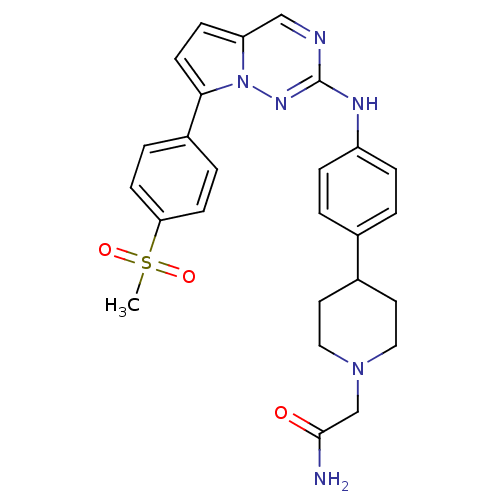
(CHEMBL1934342)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12 Show InChI InChI=1S/C26H28N6O3S/c1-36(34,35)23-9-4-20(5-10-23)24-11-8-22-16-28-26(30-32(22)24)29-21-6-2-18(3-7-21)19-12-14-31(15-13-19)17-25(27)33/h2-11,16,19H,12-15,17H2,1H3,(H2,27,33)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361210
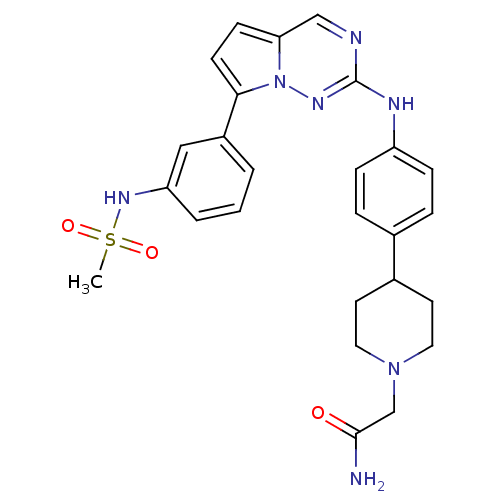
(CHEMBL1934339)Show SMILES CS(=O)(=O)Nc1cccc(c1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12 Show InChI InChI=1S/C26H29N7O3S/c1-37(35,36)31-22-4-2-3-20(15-22)24-10-9-23-16-28-26(30-33(23)24)29-21-7-5-18(6-8-21)19-11-13-32(14-12-19)17-25(27)34/h2-10,15-16,19,31H,11-14,17H2,1H3,(H2,27,34)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361212
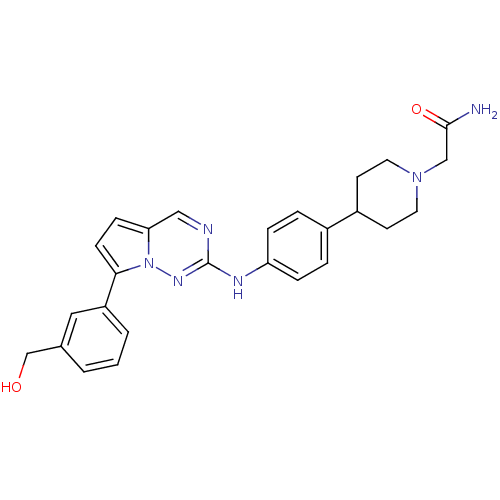
(CHEMBL1934341)Show SMILES NC(=O)CN1CCC(CC1)c1ccc(Nc2ncc3ccc(-c4cccc(CO)c4)n3n2)cc1 Show InChI InChI=1S/C26H28N6O2/c27-25(34)16-31-12-10-20(11-13-31)19-4-6-22(7-5-19)29-26-28-15-23-8-9-24(32(23)30-26)21-3-1-2-18(14-21)17-33/h1-9,14-15,20,33H,10-13,16-17H2,(H2,27,34)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data