 Found 1032 hits with Last Name = 'thomson' and Initial = 'sa'
Found 1032 hits with Last Name = 'thomson' and Initial = 'sa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8143
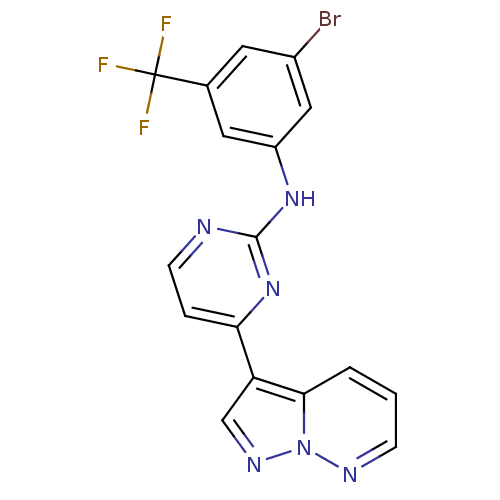
(N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...)Show SMILES FC(F)(F)c1cc(Br)cc(Nc2nccc(n2)-c2cnn3ncccc23)c1 Show InChI InChI=1S/C17H10BrF3N6/c18-11-6-10(17(19,20)21)7-12(8-11)25-16-22-5-3-14(26-16)13-9-24-27-15(13)2-1-4-23-27/h1-9H,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8145

(N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10Cl2N6/c17-10-6-11(18)8-12(7-10)22-16-19-5-3-14(23-16)13-9-21-24-15(13)2-1-4-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8138
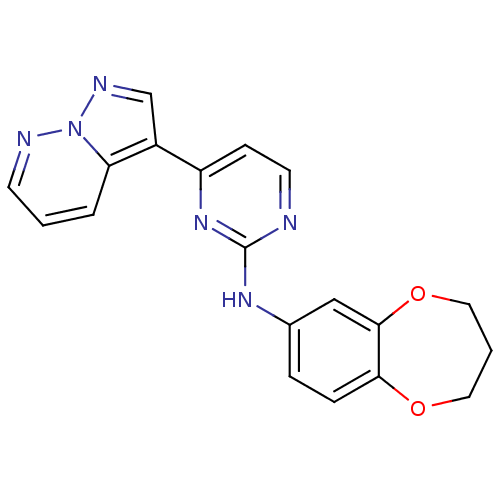
(N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...)Show InChI InChI=1S/C19H16N6O2/c1-3-16-14(12-22-25(16)21-7-1)15-6-8-20-19(24-15)23-13-4-5-17-18(11-13)27-10-2-9-26-17/h1,3-8,11-12H,2,9-10H2,(H,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8136

(N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...)Show InChI InChI=1S/C18H14N6O2/c1-2-15-13(11-21-24(15)20-6-1)14-5-7-19-18(23-14)22-12-3-4-16-17(10-12)26-9-8-25-16/h1-7,10-11H,8-9H2,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8146

(N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C18H16N6/c1-12-8-13(2)10-14(9-12)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8144
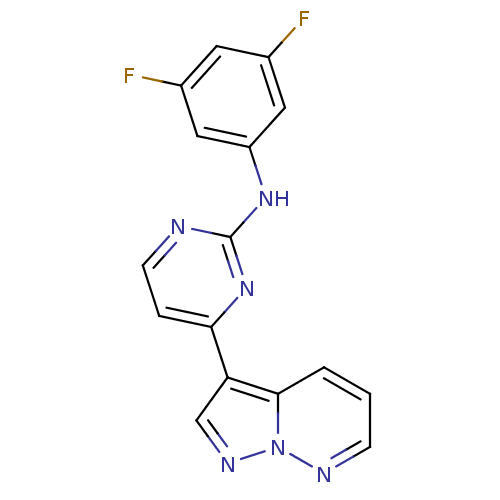
(N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10F2N6/c17-10-6-11(18)8-12(7-10)22-16-19-5-3-14(23-16)13-9-21-24-15(13)2-1-4-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8142

(N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{pyrazol...)Show SMILES COc1cc(Nc2nccc(n2)-c2cnn3ncccc23)cc(c1)C(F)(F)F Show InChI InChI=1S/C18H13F3N6O/c1-28-13-8-11(18(19,20)21)7-12(9-13)25-17-22-6-4-15(26-17)14-10-24-27-16(14)3-2-5-23-27/h2-10H,1H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8140
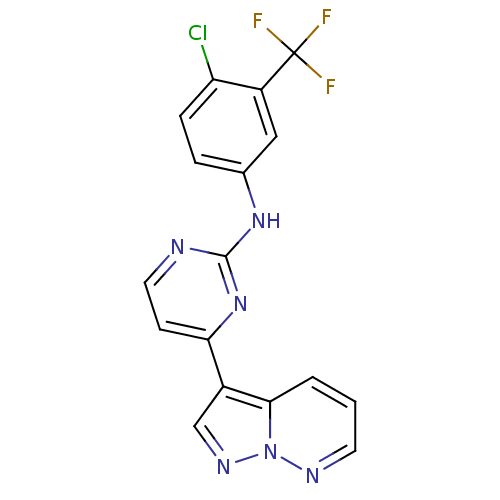
(N-[4-chloro-3-(trifluoromethyl)phenyl]-4-{pyrazolo...)Show SMILES FC(F)(F)c1cc(Nc2nccc(n2)-c2cnn3ncccc23)ccc1Cl Show InChI InChI=1S/C17H10ClF3N6/c18-13-4-3-10(8-12(13)17(19,20)21)25-16-22-7-5-14(26-16)11-9-24-27-15(11)2-1-6-23-27/h1-9H,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8135
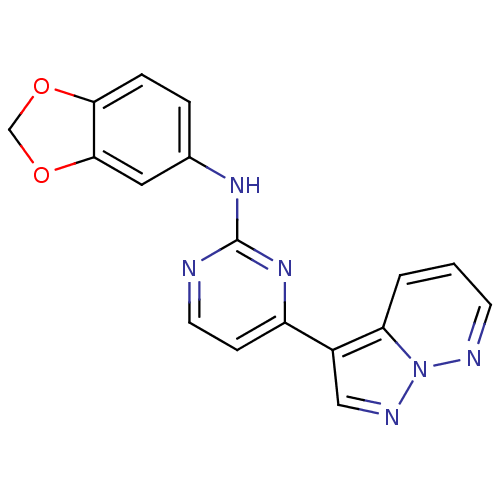
(N-(2H-1,3-benzodioxol-5-yl)-4-{pyrazolo[1,5-a]pyri...)Show InChI InChI=1S/C17H12N6O2/c1-2-14-12(9-20-23(14)19-6-1)13-5-7-18-17(22-13)21-11-3-4-15-16(8-11)25-10-24-15/h1-9H,10H2,(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8129

(4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...)Show InChI InChI=1S/C17H11N7/c18-10-12-3-5-13(6-4-12)22-17-19-9-7-15(23-17)14-11-21-24-16(14)2-1-8-20-24/h1-9,11H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8130
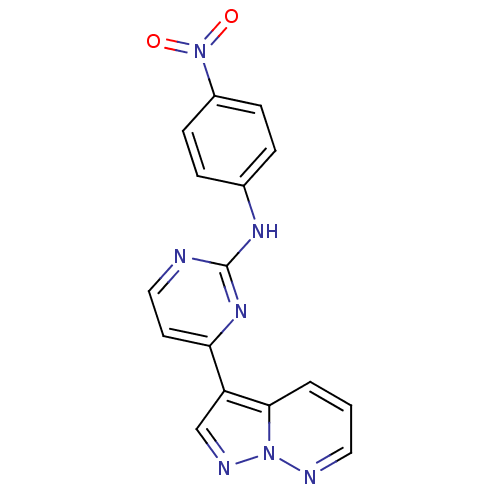
(N-(4-nitrophenyl)-4-{pyrazolo[1,5-a]pyridazin-3-yl...)Show InChI InChI=1S/C16H11N7O2/c24-23(25)12-5-3-11(4-6-12)20-16-17-9-7-14(21-16)13-10-19-22-15(13)2-1-8-18-22/h1-10H,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8171
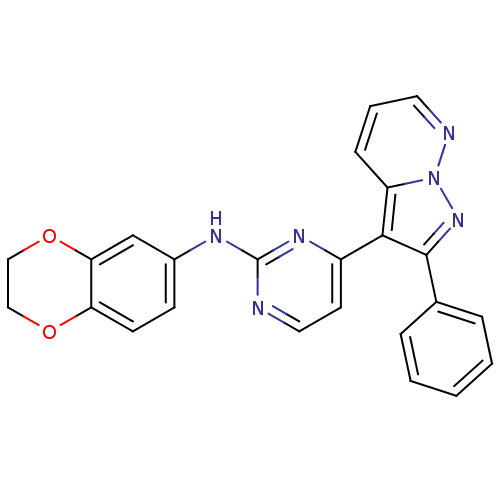
(N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-phenylpy...)Show SMILES C1COc2cc(Nc3nccc(n3)-c3c(nn4ncccc34)-c3ccccc3)ccc2O1 Show InChI InChI=1S/C24H18N6O2/c1-2-5-16(6-3-1)23-22(19-7-4-11-26-30(19)29-23)18-10-12-25-24(28-18)27-17-8-9-20-21(15-17)32-14-13-31-20/h1-12,15H,13-14H2,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50276413

(7-isothiocyanatotetrahydroisoquinoline | CHEMBL459...)Show InChI InChI=1S/C10H10N2S/c13-7-12-10-2-1-8-3-4-11-6-9(8)5-10/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of phenylethanolamine from human PNMT by competitive inhibition assay |
Bioorg Med Chem Lett 19: 1071-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.014
BindingDB Entry DOI: 10.7270/Q2ZS2WCP |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50276413

(7-isothiocyanatotetrahydroisoquinoline | CHEMBL459...)Show InChI InChI=1S/C10H10N2S/c13-7-12-10-2-1-8-3-4-11-6-9(8)5-10/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to wild type human PNMT by liquid scintillation spectrometry in presence of [3H]AdoMet |
Bioorg Med Chem Lett 19: 1071-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.014
BindingDB Entry DOI: 10.7270/Q2ZS2WCP |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13017

(1,2,3,4-tetrahydroisoquinoline-7-sulfonamide | CHE...)Show InChI InChI=1S/C9H12N2O2S/c10-14(12,13)9-2-1-7-3-4-11-6-8(7)5-9/h1-2,5,11H,3-4,6H2,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Binding affinity to wild type human PNMT by liquid scintillation spectrometry in presence of [3H]AdoMet |
Bioorg Med Chem Lett 19: 1071-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.014
BindingDB Entry DOI: 10.7270/Q2ZS2WCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8129

(4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...)Show InChI InChI=1S/C17H11N7/c18-10-12-3-5-13(6-4-12)22-17-19-9-7-15(23-17)14-11-21-24-16(14)2-1-8-20-24/h1-9,11H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8144

(N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10F2N6/c17-10-6-11(18)8-12(7-10)22-16-19-5-3-14(23-16)13-9-21-24-15(13)2-1-4-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8139

(N-(3,4-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10Cl2N6/c17-12-4-3-10(8-13(12)18)22-16-19-7-5-14(23-16)11-9-21-24-15(11)2-1-6-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50219326

(PYRAZOLOPYRIMIDINE 1)Show SMILES CN(C)CCNC(=O)c1ccc(\C=N\Nc2ncnc3n(ncc23)-c2cccc3[nH]cnc23)cc1 Show InChI InChI=1S/C24H24N10O/c1-33(2)11-10-25-24(35)17-8-6-16(7-9-17)12-30-32-22-18-13-31-34(23(18)29-15-28-22)20-5-3-4-19-21(20)27-14-26-19/h3-9,12-15H,10-11H2,1-2H3,(H,25,35)(H,26,27)(H,28,29,32)/b30-12+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of glycogen synthase kinase-3 (GSK3-beta) |
Bioorg Med Chem Lett 14: 2127-30 (2004)
BindingDB Entry DOI: 10.7270/Q2Q242DJ |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8137

(N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C16H10F2N6/c17-12-4-3-10(8-13(12)18)22-16-19-7-5-14(23-16)11-9-21-24-15(11)2-1-6-20-24/h1-9H,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 3
(Homo sapiens (Human)) | BDBM50185446
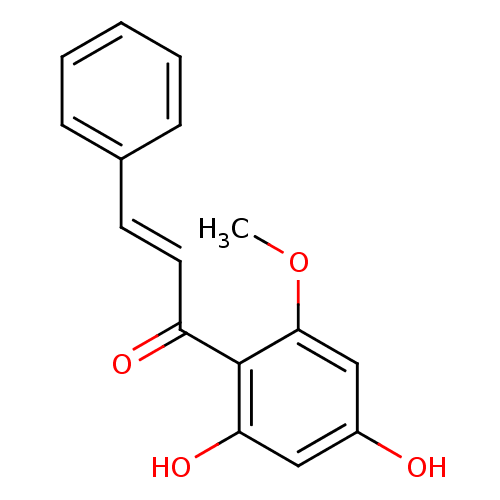
((E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop...)Show InChI InChI=1S/C16H14O4/c1-20-15-10-12(17)9-14(19)16(15)13(18)8-7-11-5-3-2-4-6-11/h2-10,17,19H,1H3/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human voltage-gated potassium channel Kv1.3 expressed in L929 cells by whole-cell patch clamp assay |
Bioorg Med Chem Lett 20: 6983-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.132
BindingDB Entry DOI: 10.7270/Q2N58MMS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8128

(N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...)Show InChI InChI=1S/C17H14N6O/c1-24-13-5-2-4-12(10-13)21-17-18-9-7-15(22-17)14-11-20-23-16(14)6-3-8-19-23/h2-11H,1H3,(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615811

(CHEMBL5272869)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(N3CCC3)c(Cl)cc2c1 |r,wU:7.10,wD:4.3,(8.38,4.12,;7.6,2.78,;6.83,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-7.05,-2.61,;-7.45,-4.12,;-8.94,-3.72,;-8.53,-2.21,;-5.71,-.31,;-7.05,.46,;-4.4,.46,;-3.06,-.3,;-1.74,.48,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50219327
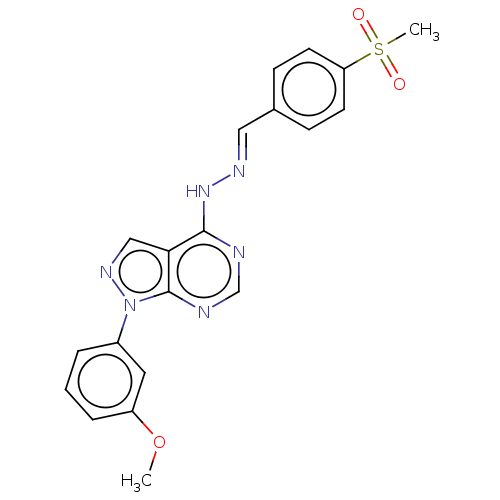
(GW811168X | PYRAZOLOPYRIMIDINE 2)Show SMILES COc1cccc(c1)-n1ncc2c(N\N=C\c3ccc(cc3)S(C)(=O)=O)ncnc12 Show InChI InChI=1S/C20H18N6O3S/c1-29-16-5-3-4-15(10-16)26-20-18(12-24-26)19(21-13-22-20)25-23-11-14-6-8-17(9-7-14)30(2,27)28/h3-13H,1-2H3,(H,21,22,25)/b23-11+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of glycogen synthase kinase-3 (GSK3-beta) |
Bioorg Med Chem Lett 14: 2127-30 (2004)
BindingDB Entry DOI: 10.7270/Q2Q242DJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8688
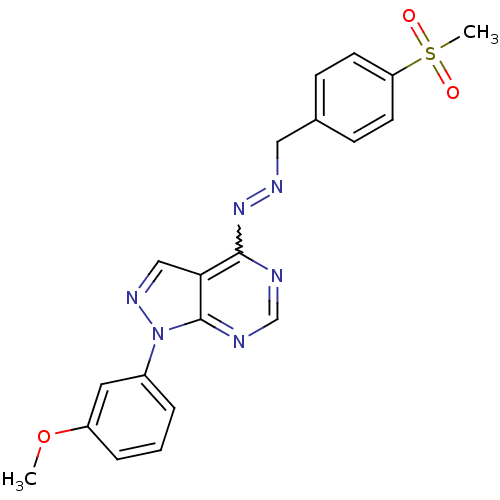
((1E)-1-[(4-methanesulfonylphenyl)methylidene]-2-[1...)Show SMILES COc1cccc(c1)-n1ncc2c(N=NCc3ccc(cc3)S(C)(=O)=O)ncnc12 |w:13.13| Show InChI InChI=1S/C20H18N6O3S/c1-29-16-5-3-4-15(10-16)26-20-18(12-24-26)19(21-13-22-20)25-23-11-14-6-8-17(9-7-14)30(2,27)28/h3-10,12-13H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... |
Bioorg Med Chem Lett 14: 2121-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.036
BindingDB Entry DOI: 10.7270/Q2NV9GF5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8686
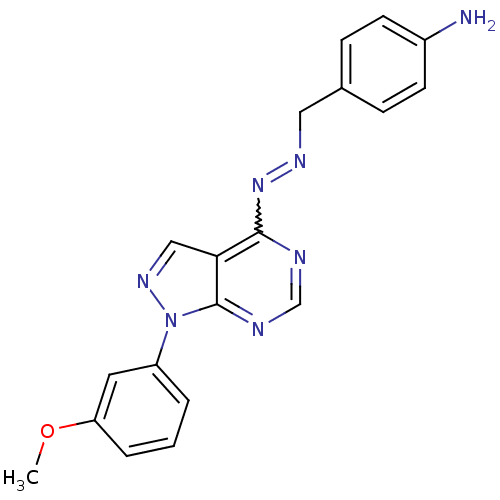
(4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]...)Show SMILES COc1cccc(c1)-n1ncc2c(N=NCc3ccc(N)cc3)ncnc12 |w:13.13| Show InChI InChI=1S/C19H17N7O/c1-27-16-4-2-3-15(9-16)26-19-17(11-24-26)18(21-12-22-19)25-23-10-13-5-7-14(20)8-6-13/h2-9,11-12H,10,20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... |
Bioorg Med Chem Lett 14: 2121-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.036
BindingDB Entry DOI: 10.7270/Q2NV9GF5 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8146

(N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...)Show InChI InChI=1S/C18H16N6/c1-12-8-13(2)10-14(9-12)22-18-19-7-5-16(23-18)15-11-21-24-17(15)4-3-6-20-24/h3-11H,1-2H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... |
J Med Chem 47: 4716-30 (2004)
Article DOI: 10.1021/jm040063i
BindingDB Entry DOI: 10.7270/Q2VM49HJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27747

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)-c1cccc(F)c1)C(O)=O |r| Show InChI InChI=1S/C34H40FN3O5/c1-19-14-23(16-22-10-11-22)15-20(2)29(19)38-33(42)36-28-18-25(24-8-7-9-26(35)17-24)12-13-27(28)31(39)37-30(32(40)41)21(3)43-34(4,5)6/h7-9,12-15,17-18,21-22,30H,10-11,16H2,1-6H3,(H,37,39)(H,40,41)(H2,36,38,42)/t21-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27730
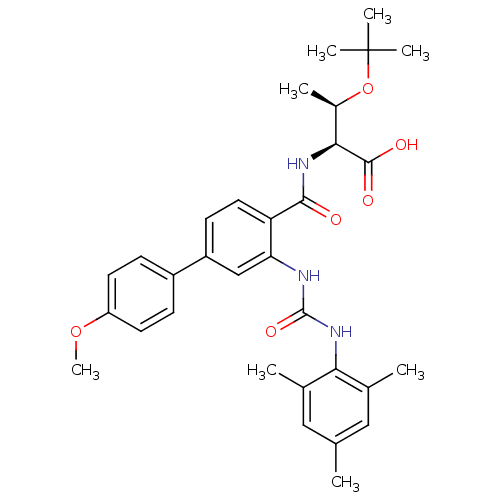
((2S,3R)-3-(tert-butoxy)-2-{[4-(4-methoxyphenyl)-2-...)Show SMILES COc1ccc(cc1)-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-15-19(2)27(20(3)16-18)35-31(39)33-26-17-23(22-9-12-24(40-8)13-10-22)11-14-25(26)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615823

(CHEMBL5289520)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@@H](C1)C(C)(C)O |r,wU:19.21,wD:21.26,1.0,(-8.73,-.66,;-7.96,-1.99,;-8.37,-3.51,;-6.88,-3.91,;-6.48,-2.39,;-5.14,-1.62,;-3.81,-2.39,;-2.49,-1.62,;-1.16,-2.39,;.17,-1.62,;.17,-.08,;-1.17,.69,;-2.49,-.09,;-3.83,.68,;-5.14,-.1,;-6.48,.67,;1.51,.69,;1.51,2.23,;2.84,-.08,;4.17,.69,;4.58,2.2,;6.07,1.8,;5.66,.29,;7.4,2.57,;8.17,3.91,;6.63,3.91,;8.73,1.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8674
(4-{4-[(2E)-2-(pyridin-4-ylmethylidene)hydrazin-1-y...)Show SMILES C(N=Nc1ncnc2n(ncc12)-c1ccncc1)c1ccncc1 |w:2.2| Show InChI InChI=1S/C16H12N8/c1-5-17-6-2-12(1)9-21-23-15-14-10-22-24(16(14)20-11-19-15)13-3-7-18-8-4-13/h1-8,10-11H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... |
Bioorg Med Chem Lett 14: 2121-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.036
BindingDB Entry DOI: 10.7270/Q2NV9GF5 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615812

(CHEMBL5289730)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:19.21,wD:1.0,22.28,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-4.2,-2.6,;-2.88,-1.83,;-1.55,-2.6,;-.21,-1.84,;-.21,-.3,;-1.55,.48,;-2.88,-.3,;-4.22,.46,;-5.53,-.31,;-6.86,.46,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;8.56,4.12,;7.02,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615775

(CHEMBL5279860)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(ncc2c1)N1CCC1 |r,wU:7.10,wD:4.3,(6.84,4.12,;7.61,2.78,;8.38,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-5.71,-.31,;-4.4,.46,;-3.06,-.3,;-1.74,.48,;-7.05,-2.61,;-8.53,-2.21,;-8.94,-3.72,;-7.45,-4.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615791

(CHEMBL5275536)Show SMILES CC(C)(O)[C@H]1CC2(C[C@@H](C2)NC(=O)c2cnc3nc(ccc3c2)C2CC2)C1 |r,wU:8.10,wD:4.3,(8.76,4.37,;7.99,3.04,;7.22,4.37,;9.32,2.27,;6.66,2.27,;5.17,2.67,;4.76,1.15,;3.27,1.55,;2.87,.04,;4.36,-.36,;1.54,-.73,;.2,.04,;.2,1.58,;-1.13,-.73,;-1.13,-2.27,;-2.46,-3.04,;-3.8,-2.27,;-5.12,-3.03,;-6.45,-2.27,;-6.45,-.75,;-5.13,.03,;-3.8,-.74,;-2.47,.04,;-7.78,-3.04,;-8.55,-4.37,;-9.32,-3.04,;6.25,.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615797

(CHEMBL5282861)Show SMILES CC(C)(O)[C@H]1C[C@@H](C1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:6.8,wD:4.3,(7.82,3.82,;7.05,2.48,;6.28,3.82,;8.39,1.71,;5.72,1.71,;4.23,2.11,;3.83,.6,;5.31,.2,;2.49,-.18,;1.16,.59,;1.16,2.13,;-.18,-.18,;-.18,-1.71,;-1.51,-2.48,;-2.84,-1.71,;-4.18,-2.48,;-5.51,-1.71,;-6.84,-2.48,;-7.62,-3.82,;-8.39,-2.48,;-5.51,-.18,;-6.84,.59,;-4.18,.59,;-2.84,-.18,;-1.51,.59,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8695
(ethyl({4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo...)Show SMILES CCNCc1ccc(CN=Nc2ncnc3n(ncc23)-c2cccc(OC)c2)cc1 |w:10.10| Show InChI InChI=1S/C22H23N7O/c1-3-23-12-16-7-9-17(10-8-16)13-26-28-21-20-14-27-29(22(20)25-15-24-21)18-5-4-6-19(11-18)30-2/h4-11,14-15,23H,3,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... |
Bioorg Med Chem Lett 14: 2121-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.036
BindingDB Entry DOI: 10.7270/Q2NV9GF5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8687

((1E)-1-[(3-methanesulfonylphenyl)methylidene]-2-[1...)Show SMILES COc1cccc(c1)-n1ncc2c(N=NCc3cccc(c3)S(C)(=O)=O)ncnc12 |w:13.13| Show InChI InChI=1S/C20H18N6O3S/c1-29-16-7-4-6-15(10-16)26-20-18(12-24-26)19(21-13-22-20)25-23-11-14-5-3-8-17(9-14)30(2,27)28/h3-10,12-13H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... |
Bioorg Med Chem Lett 14: 2121-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.036
BindingDB Entry DOI: 10.7270/Q2NV9GF5 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27743

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(F)cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H36FN3O5/c1-15-11-19(13-18-7-8-18)12-16(2)23(15)32-27(36)30-22-14-20(29)9-10-21(22)25(33)31-24(26(34)35)17(3)37-28(4,5)6/h9-12,14,17-18,24H,7-8,13H2,1-6H3,(H,31,33)(H,34,35)(H2,30,32,36)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27745
((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(OC)cc2)c(C)c1 |r| Show InChI InChI=1S/C34H43N3O6/c1-9-10-23-17-20(2)29(21(3)18-23)37-33(41)35-28-19-25(24-11-14-26(42-8)15-12-24)13-16-27(28)31(38)36-30(32(39)40)22(4)43-34(5,6)7/h11-19,22,30H,9-10H2,1-8H3,(H,36,38)(H,39,40)(H2,35,37,41)/t22-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27746

((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2cccc(F)c2)c(C)c1 |r| Show InChI InChI=1S/C33H40FN3O5/c1-8-10-22-15-19(2)28(20(3)16-22)37-32(41)35-27-18-24(23-11-9-12-25(34)17-23)13-14-26(27)30(38)36-29(31(39)40)21(4)42-33(5,6)7/h9,11-18,21,29H,8,10H2,1-7H3,(H,36,38)(H,39,40)(H2,35,37,41)/t21-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50219340
(CHEMBL65944)Show SMILES N(\N=C\c1ccncc1)c1ncnc2n(ncc12)-c1cccc2[nH]cnc12 Show InChI InChI=1S/C18H13N9/c1-2-14-16(21-10-20-14)15(3-1)27-18-13(9-25-27)17(22-11-23-18)26-24-8-12-4-6-19-7-5-12/h1-11H,(H,20,21)(H,22,23,26)/b24-8+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of glycogen synthase kinase-3 (GSK3-beta) |
Bioorg Med Chem Lett 14: 2127-30 (2004)
BindingDB Entry DOI: 10.7270/Q2Q242DJ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615779
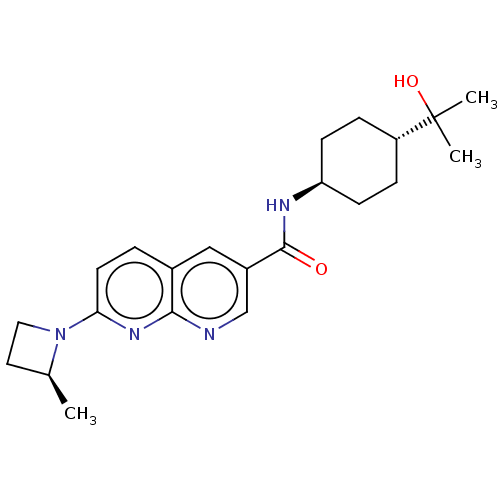
(CHEMBL5285125)Show SMILES C[C@H]1CCN1c1ccc2cc(cnc2n1)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:18.20,wD:1.0,21.27,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-5.53,-.31,;-4.22,.46,;-2.88,-.3,;-1.55,.48,;-.21,-.3,;-.21,-1.84,;-1.54,-2.6,;-2.88,-1.83,;-4.2,-2.6,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;7.02,4.12,;8.56,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615800
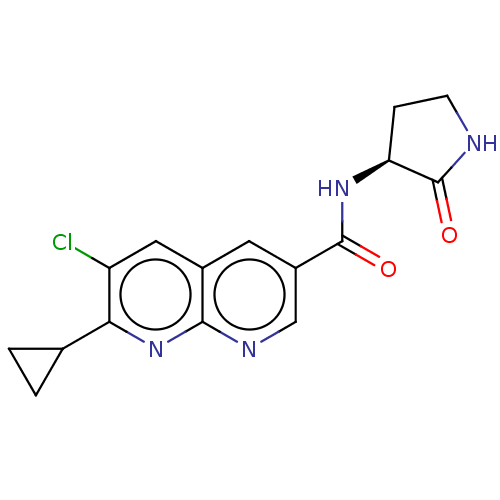
(CHEMBL5266142)Show SMILES Clc1cc2cc(cnc2nc1C1CC1)C(=O)N[C@H]1CCNC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615795

(CHEMBL5279371)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CC[C@](C)(O)CC1 |r,wU:19.21,wD:22.26,1.0,(-8.57,-.49,;-7.8,-1.82,;-8.2,-3.33,;-6.72,-3.73,;-6.31,-2.22,;-4.98,-1.45,;-3.64,-2.22,;-2.31,-1.45,;-.98,-2.22,;.36,-1.45,;.36,.09,;-.98,.86,;-2.31,.09,;-3.64,.86,;-4.98,.09,;-6.31,.86,;1.69,.86,;1.69,2.4,;3.02,.09,;4.36,.86,;4.36,2.4,;5.69,3.17,;7.03,2.4,;8.57,2.4,;7.8,3.73,;7.03,.86,;5.69,.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615813
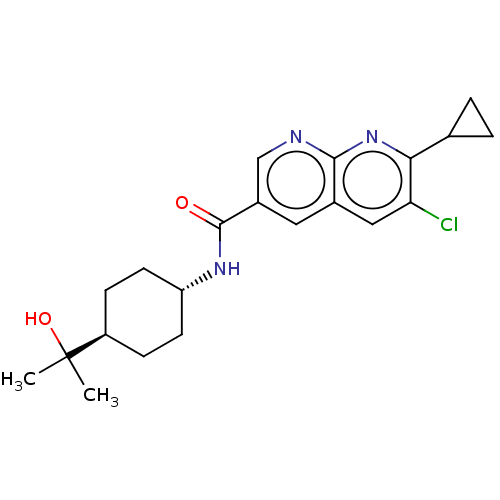
(CHEMBL5269778)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:7.10,wD:4.3,(8.2,4.03,;7.43,2.69,;6.66,4.03,;8.76,1.92,;6.1,1.92,;4.76,2.69,;3.43,1.92,;3.43,.38,;4.76,-.39,;6.1,.38,;2.1,-.39,;.76,.38,;.76,1.92,;-.57,-.39,;-.57,-1.93,;-1.9,-2.69,;-3.24,-1.92,;-4.56,-2.69,;-5.89,-1.93,;-7.22,-2.7,;-7.99,-4.03,;-8.76,-2.7,;-5.89,-.4,;-7.22,.37,;-4.57,.37,;-3.24,-.39,;-1.91,.39,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615802

(CHEMBL5269001)Show SMILES C[C@H]1CCN1c1cc2ncc(cc2cn1)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:18.20,wD:1.0,21.27,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-4.2,-2.6,;-2.88,-1.83,;-1.55,-2.6,;-.21,-1.84,;-.21,-.3,;-1.55,.48,;-2.88,-.3,;-4.22,.46,;-5.53,-.31,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;8.56,4.12,;7.02,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615792
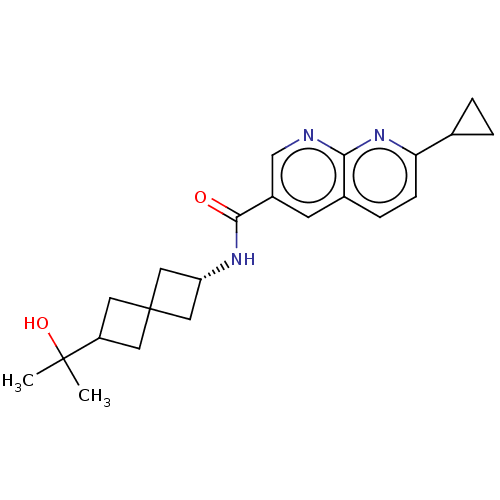
(CHEMBL5279720)Show SMILES CC(C)(O)C1CC2(C[C@@H](C2)NC(=O)c2cnc3nc(ccc3c2)C2CC2)C1 |r,wU:8.10,(7.22,4.37,;7.99,3.04,;8.76,4.37,;9.32,2.27,;6.66,2.27,;5.17,2.67,;4.76,1.15,;3.27,1.55,;2.87,.04,;4.36,-.36,;1.54,-.73,;.2,.04,;.2,1.58,;-1.13,-.73,;-1.13,-2.27,;-2.46,-3.04,;-3.8,-2.27,;-5.12,-3.03,;-6.45,-2.27,;-6.45,-.75,;-5.13,.03,;-3.8,-.74,;-2.47,.04,;-7.78,-3.04,;-8.55,-4.37,;-9.32,-3.04,;6.25,.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50219001
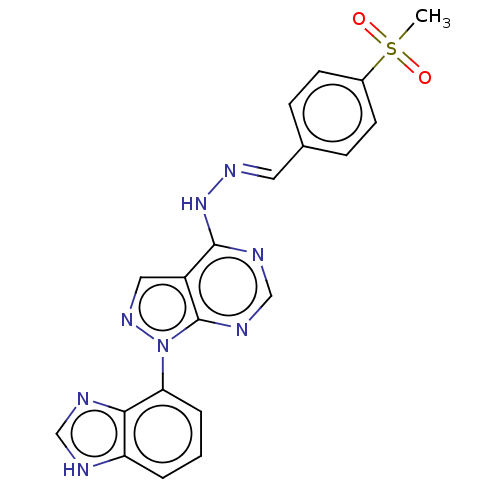
(CHEMBL69687)Show SMILES CS(=O)(=O)c1ccc(\C=N\Nc2ncnc3n(ncc23)-c2cccc3[nH]cnc23)cc1 Show InChI InChI=1S/C20H16N8O2S/c1-31(29,30)14-7-5-13(6-8-14)9-25-27-19-15-10-26-28(20(15)24-12-23-19)17-4-2-3-16-18(17)22-11-21-16/h2-12H,1H3,(H,21,22)(H,23,24,27)/b25-9+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of glycogen synthase kinase-3 (GSK3-beta) |
Bioorg Med Chem Lett 14: 2127-30 (2004)
BindingDB Entry DOI: 10.7270/Q2Q242DJ |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615825

(CHEMBL5266618)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:19.21,21.25,wD:1.0,(-8.17,-.27,;-7.4,-1.61,;-7.81,-3.12,;-6.32,-3.52,;-5.91,-2.01,;-4.58,-1.24,;-3.25,-2,;-1.93,-1.23,;-.6,-2,;.74,-1.24,;.74,.3,;-.6,1.08,;-1.93,.3,;-3.27,1.06,;-4.58,.29,;-5.91,1.06,;2.07,1.07,;2.07,2.61,;3.4,.3,;4.74,1.07,;5.14,2.58,;6.63,2.19,;7.4,3.52,;8.17,2.19,;6.22,.67,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615824
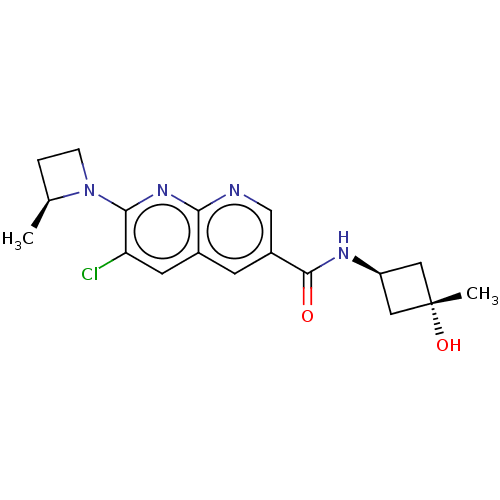
(CHEMBL5278680)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@](C)(O)C1 |r,wU:19.21,wD:21.25,1.0,(-8.17,-.27,;-7.4,-1.61,;-7.81,-3.12,;-6.32,-3.52,;-5.91,-2.01,;-4.58,-1.24,;-3.25,-2,;-1.93,-1.23,;-.6,-2,;.74,-1.24,;.74,.3,;-.6,1.08,;-1.93,.3,;-3.27,1.06,;-4.58,.29,;-5.91,1.06,;2.07,1.07,;2.07,2.61,;3.4,.3,;4.74,1.07,;5.14,2.58,;6.63,2.19,;8.17,2.19,;7.4,3.52,;6.22,.67,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data