

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50295693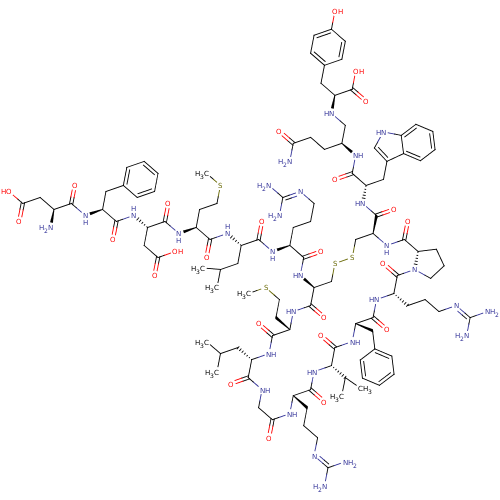 (CHEMBL557629) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Mus musculus) | BDBM50295693 (CHEMBL557629) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50295690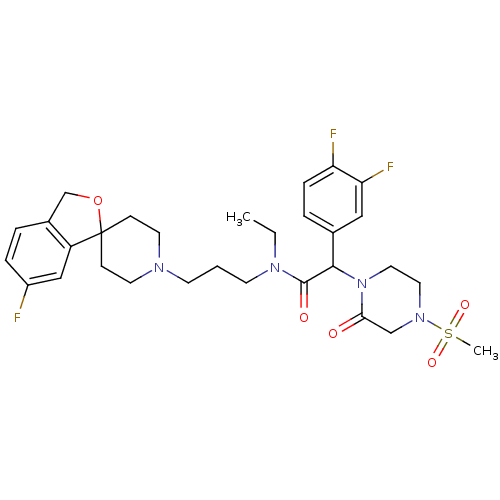 ((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human MCH1R expressed in CHO cells by scintillation counting per mg of protein | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237869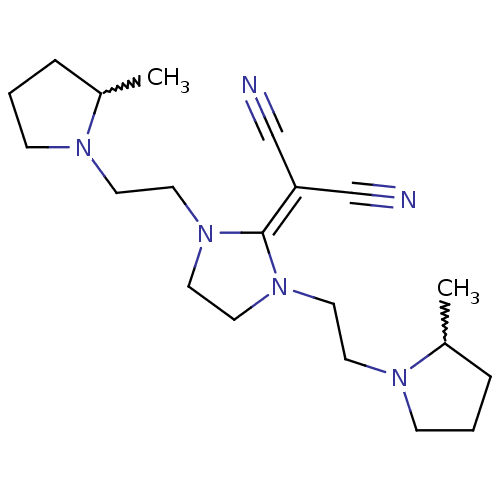 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237874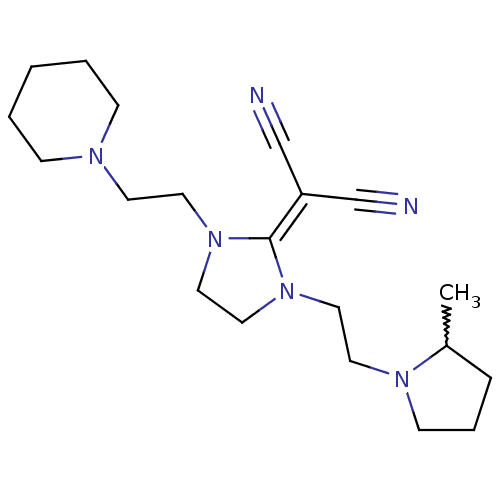 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50295690 ((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237872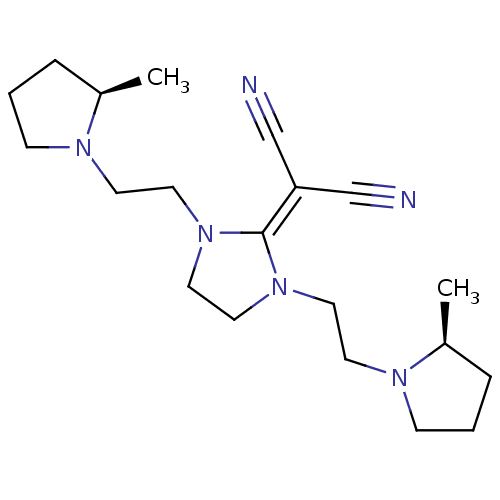 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Mus musculus) | BDBM50295690 ((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110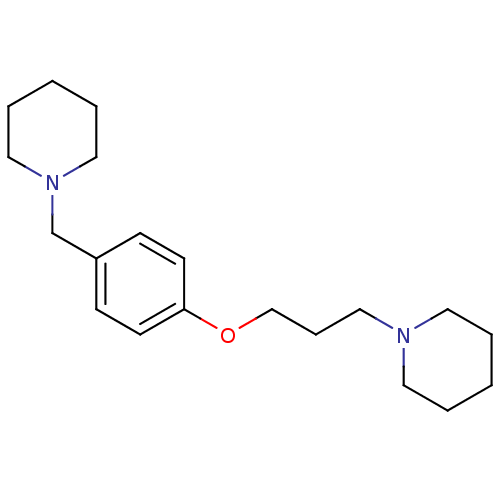 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237869 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237874 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237872 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171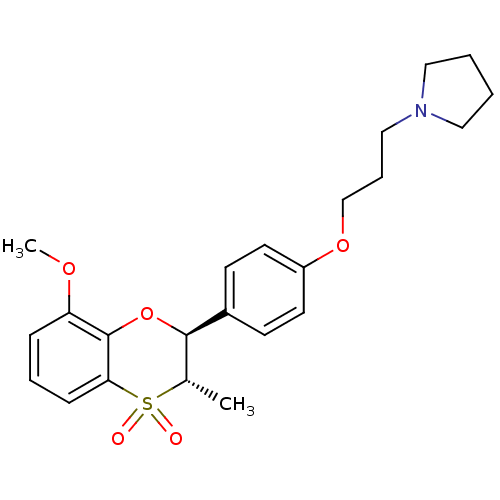 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM50295693 (CHEMBL557629) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274200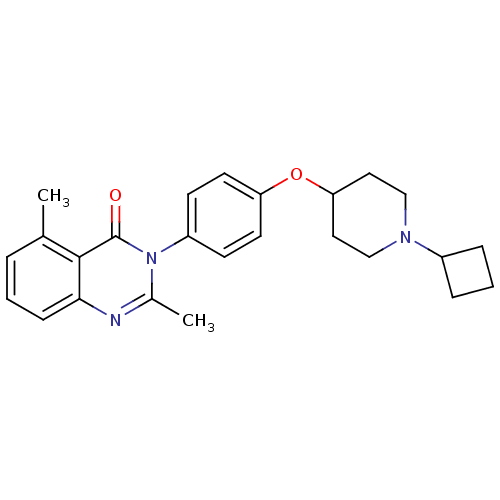 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22911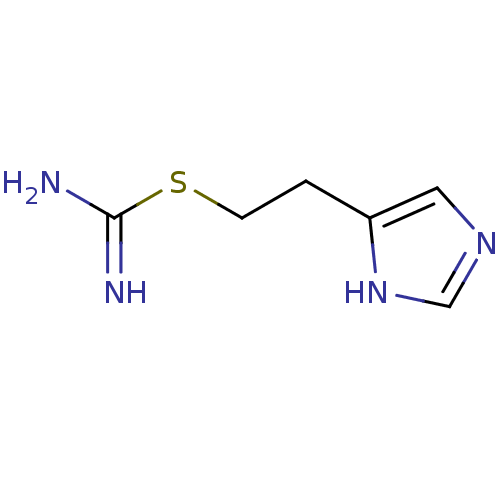 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274235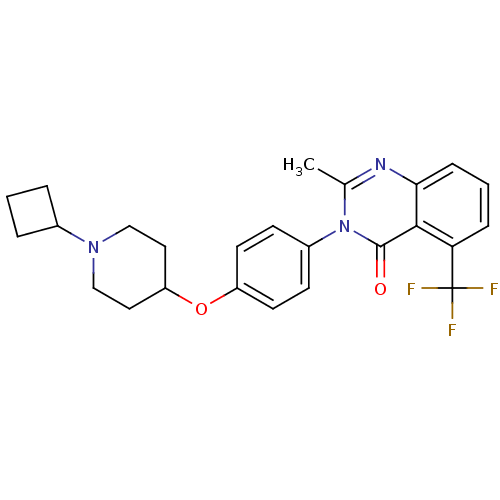 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50246289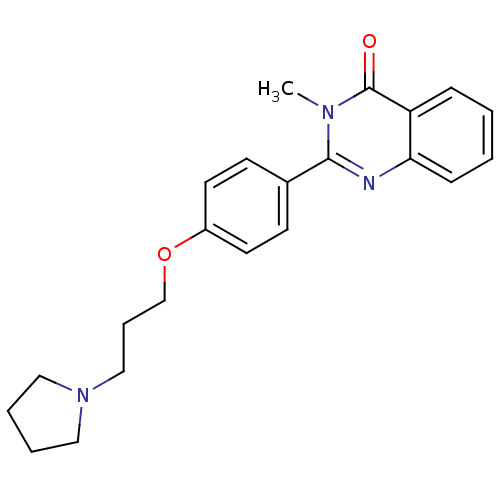 (3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237878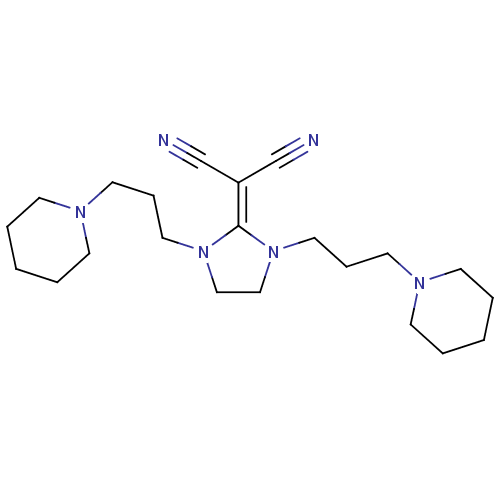 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237878 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246289 (3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor by competitive binding assay | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237871 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237871 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296870 ((1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296879 ((4,4-diphenylpiperidin-1-yl)((1R,4R)-4-((R)-1-(4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237873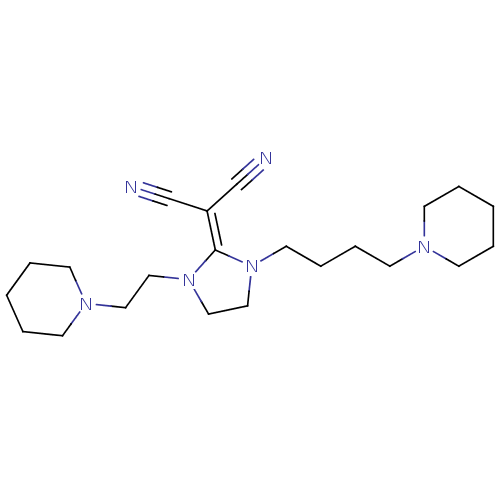 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296884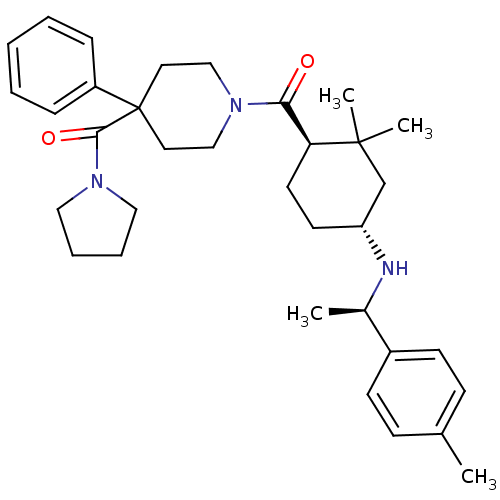 ((1-((1R,4R)-2,2-dimethyl-4-((R)-1-p-tolylethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50246289 (3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to rat histamine H3 receptor by competitive binding assay | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296885 (1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237873 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296873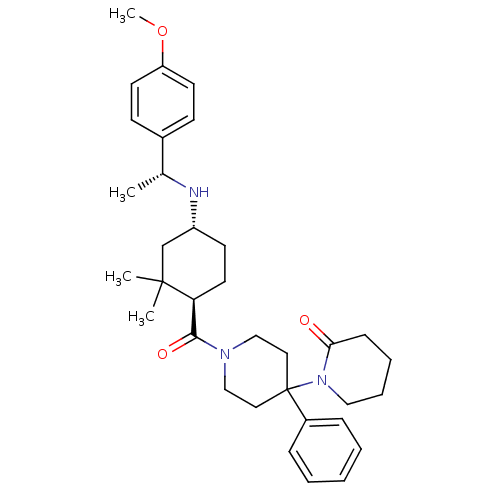 (1'-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22530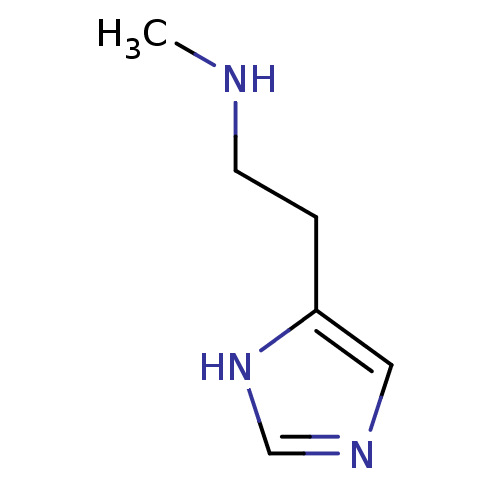 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 955 total ) | Next | Last >> |