

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297592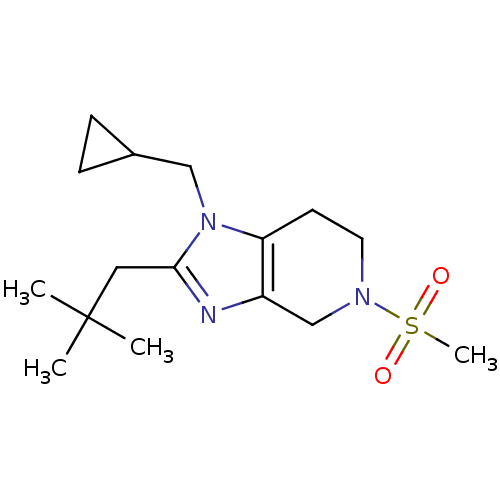 (1-(cyclopropylmethyl)-5-(methylsulfonyl)-2-neopent...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 439 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354863 (CHEMBL1834544) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.97 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297594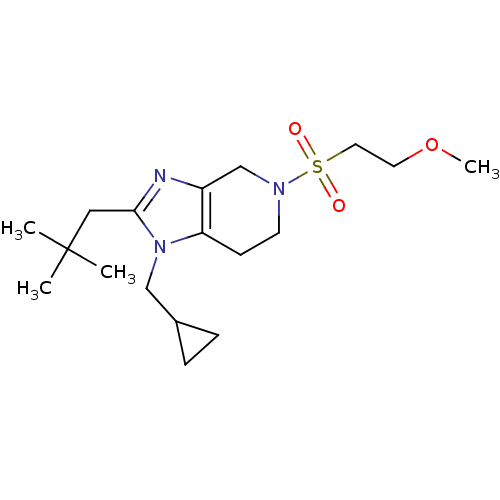 (1-(cyclopropylmethyl)-5-(2-methoxyethylsulfonyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297595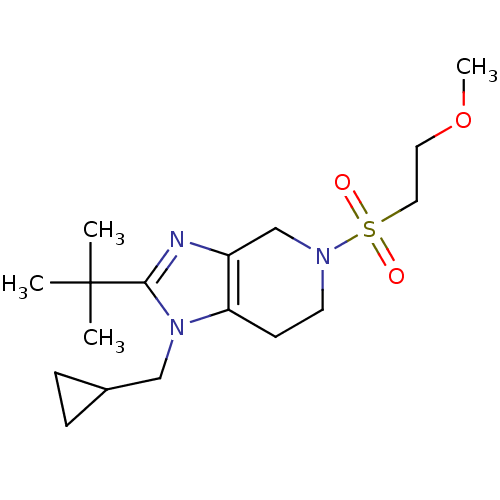 (2-tert-butyl-1-(cyclopropylmethyl)-5-(2-methoxyeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26.7 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297596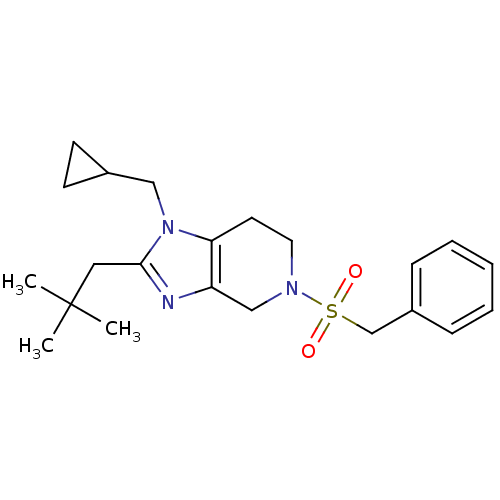 (5-(benzylsulfonyl)-1-(cyclopropylmethyl)-2-neopent...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297597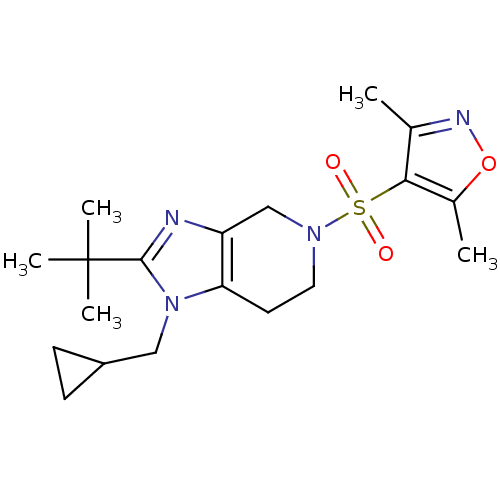 (4-(2-tert-butyl-1-(cyclopropylmethyl)-6,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297598 (1-(1-(cyclopropylmethyl)-2-neopentyl-6,7-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297599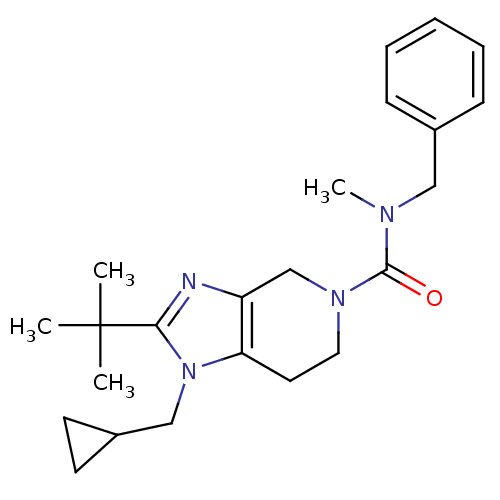 (CHEMBL562149 | N-benzyl-2-tert-butyl-1-(cyclopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 637 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297600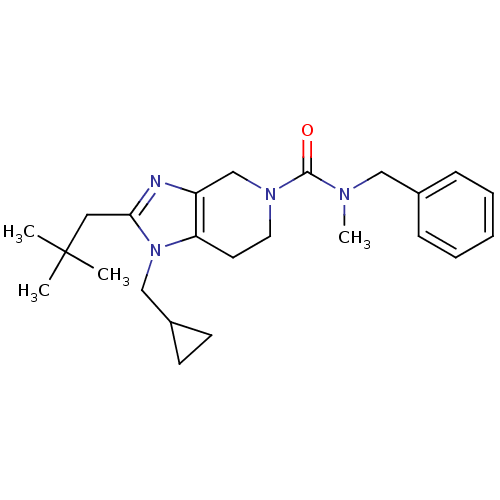 (CHEMBL564065 | N-benzyl-1-(cyclopropylmethyl)-N-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 523 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297601 (1-(2-tert-butyl-1-(cyclopropylmethyl)-6,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297602 (5-(benzylsulfonyl)-2-tert-butyl-1-(cyclopropylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21.8 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297603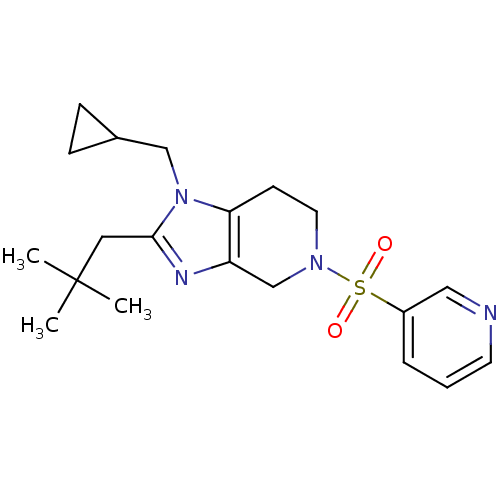 (1-(cyclopropylmethyl)-2-neopentyl-5-(pyridin-3-yls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297604 (2-tert-butyl-1-(cyclopropylmethyl)-5-(pyridin-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14.2 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297605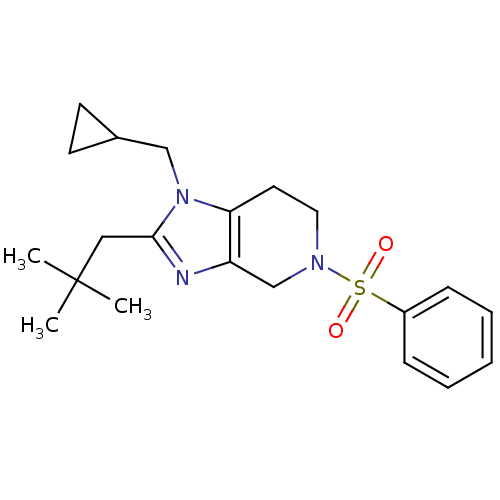 (1-(cyclopropylmethyl)-2-neopentyl-5-(phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297606 (2-tert-butyl-1-(cyclopropylmethyl)-5-(phenylsulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297607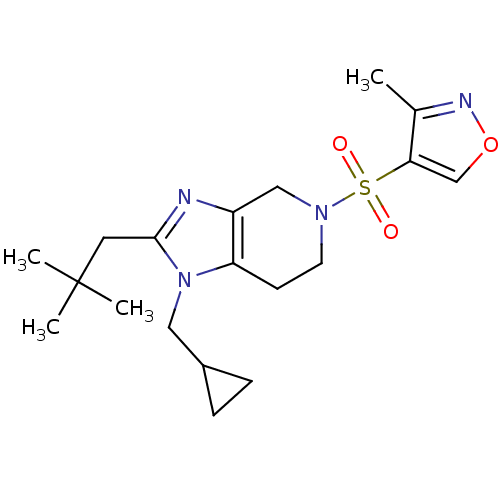 (4-(1-(cyclopropylmethyl)-2-neopentyl-6,7-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297608 (4-(2-tert-butyl-1-(cyclopropylmethyl)-6,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55.4 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297609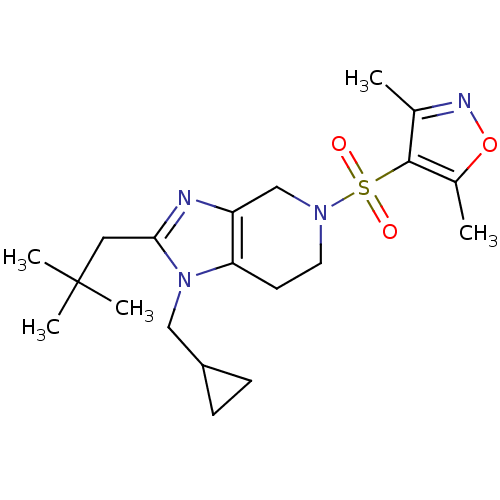 (4-(1-(cyclopropylmethyl)-2-neopentyl-6,7-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297610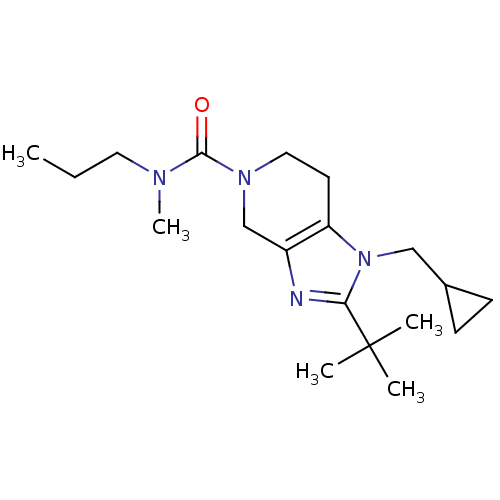 (2-tert-butyl-1-(cyclopropylmethyl)-N-methyl-N-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297611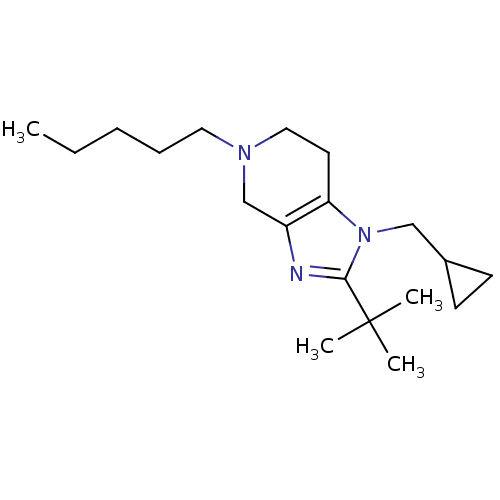 (2-tert-butyl-1-(cyclopropylmethyl)-5-pentyl-4,5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 878 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297612 (1-(cyclopropylmethyl)-2-neopentyl-5-pentyl-4,5,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297613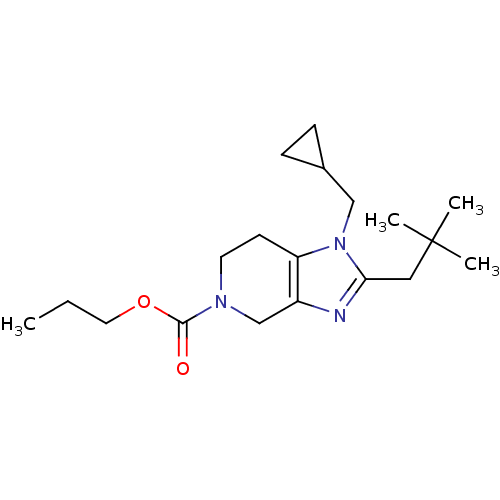 (CHEMBL561890 | propyl 1-(cyclopropylmethyl)-2-neop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297614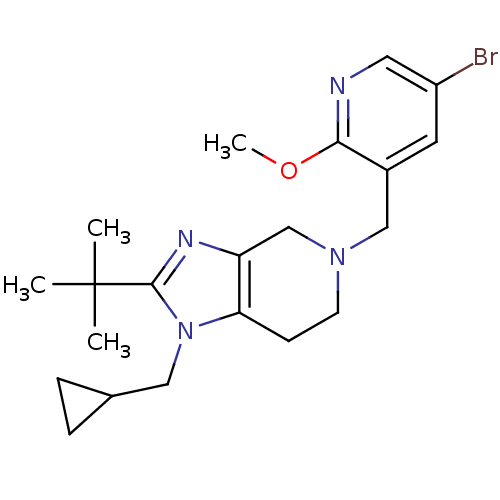 (5-((5-bromo-2-methoxypyridin-3-yl)methyl)-2-tert-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50297597 (4-(2-tert-butyl-1-(cyclopropylmethyl)-6,7-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHO cells by FLIPR assay | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50297608 (4-(2-tert-butyl-1-(cyclopropylmethyl)-6,7-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHO cells by FLIPR assay | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50297594 (1-(cyclopropylmethyl)-5-(2-methoxyethylsulfonyl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHO cells by FLIPR assay | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849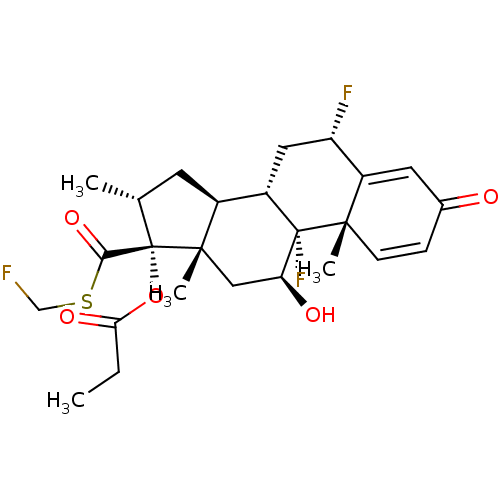 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354850 (BUDESONIDE | US10869929, Compound Budesonide | US1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354851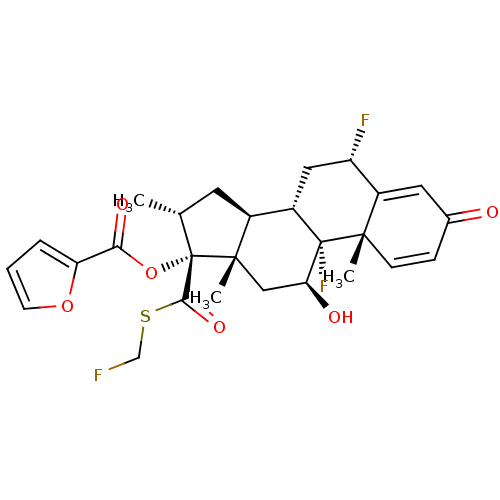 (FLUTICASONE FUROATE | Veramyst) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354852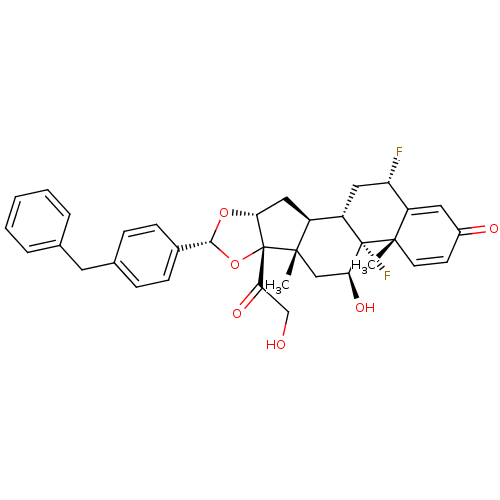 (CHEMBL1834533) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354853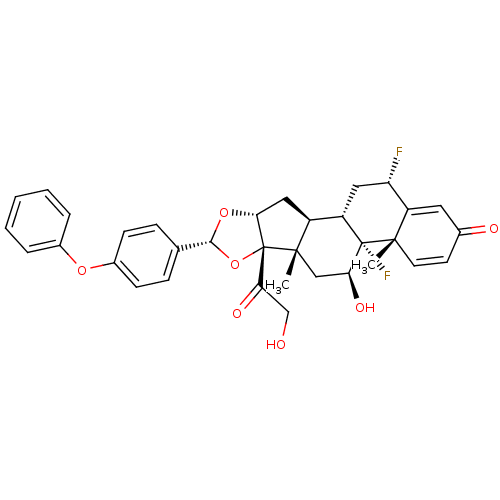 (CHEMBL1834534) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354854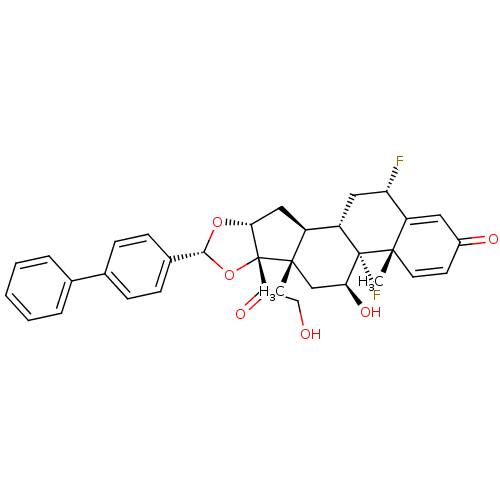 (CHEMBL1834535) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.78 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354855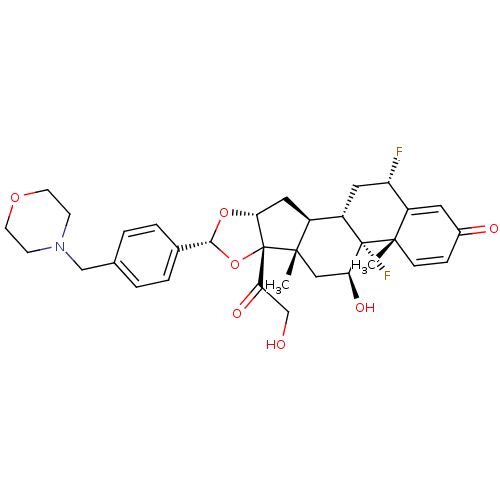 (CHEMBL1834536) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354856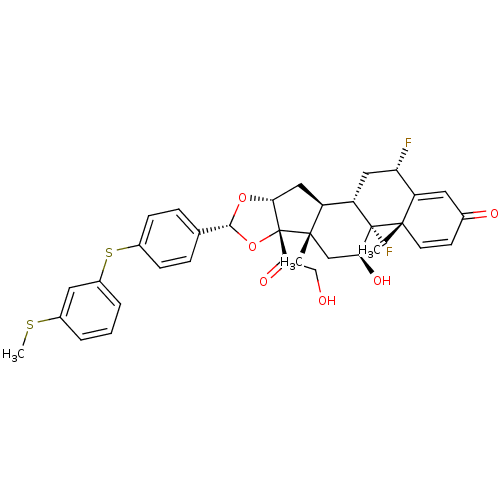 (CHEMBL1834537) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.93 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354857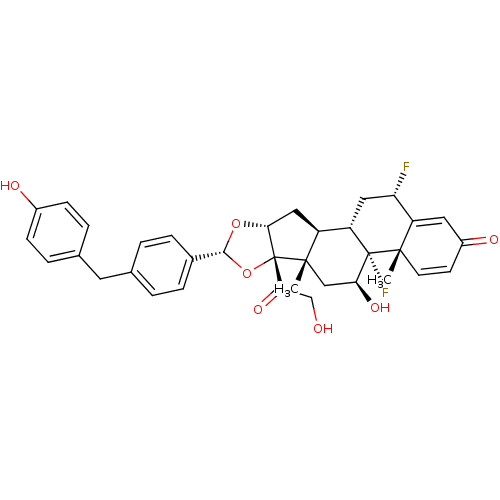 (CHEMBL1834538) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354858 (CHEMBL1834539) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354859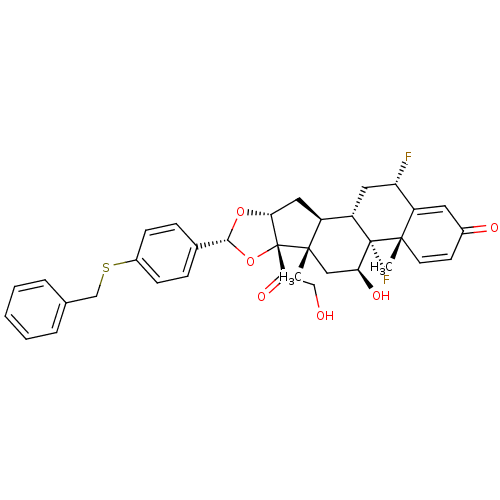 (CHEMBL1834540) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.35 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354860 (CHEMBL1834541) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354861 (CHEMBL1834542) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.87 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354862 (CHEMBL1834543) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50297593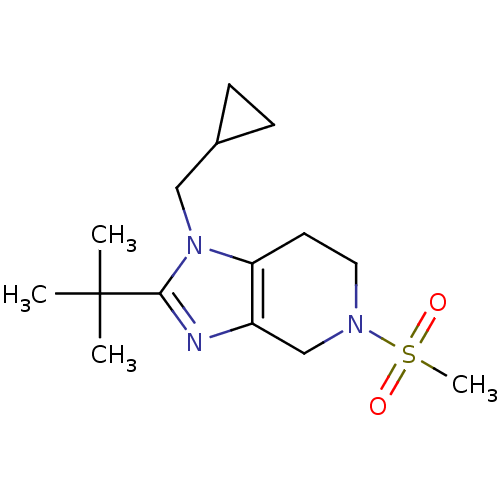 (2-tert-butyl-1-(cyclopropylmethyl)-5-(methylsulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 403 | n/a | n/a | n/a | n/a |
Pfizer Discovery Chemistry Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release | Bioorg Med Chem Lett 19: 4406-9 (2009) Article DOI: 10.1016/j.bmcl.2009.05.062 BindingDB Entry DOI: 10.7270/Q28G8KRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||