

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575780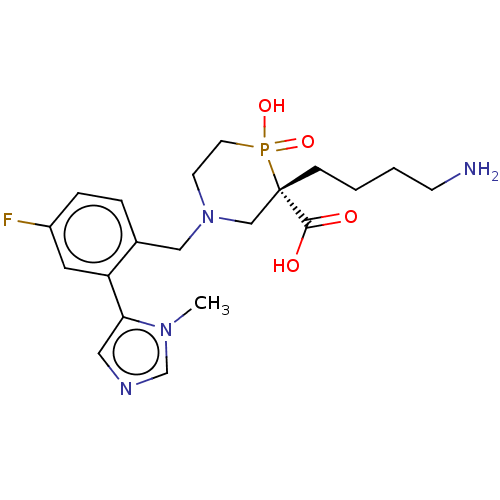 (CHEMBL4878039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50575780 (CHEMBL4878039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human pancreatic CPB incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase N catalytic chain (Homo sapiens (Human)) | BDBM50575780 (CHEMBL4878039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human plasma CPN incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575776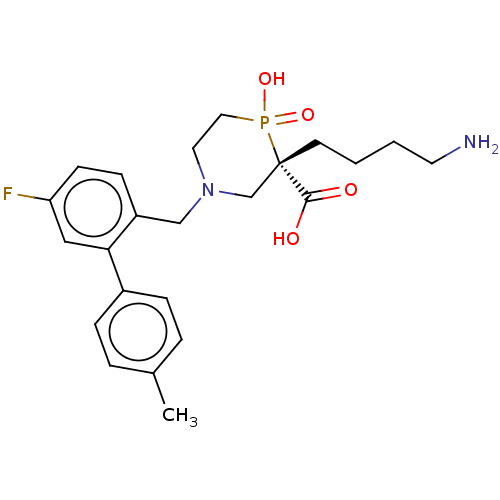 (CHEMBL4868605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575768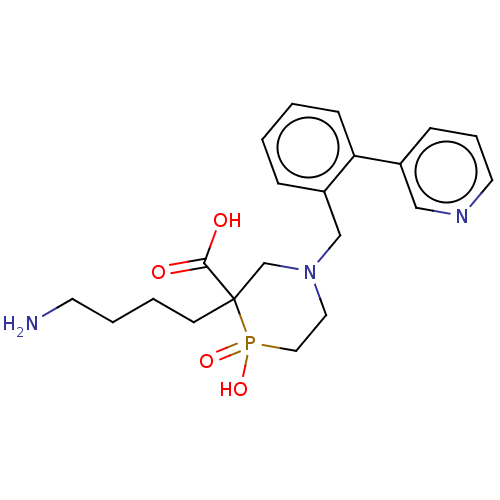 (CHEMBL4846664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575775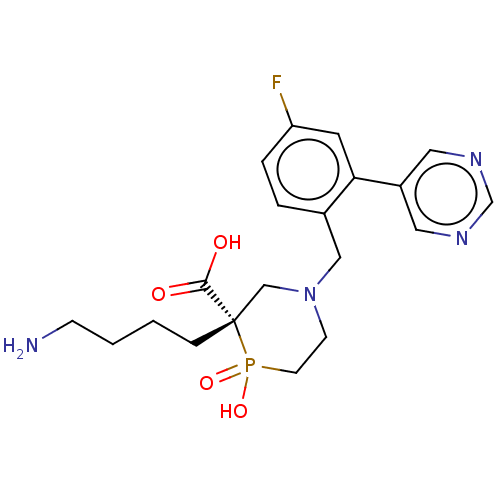 (CHEMBL4861532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575770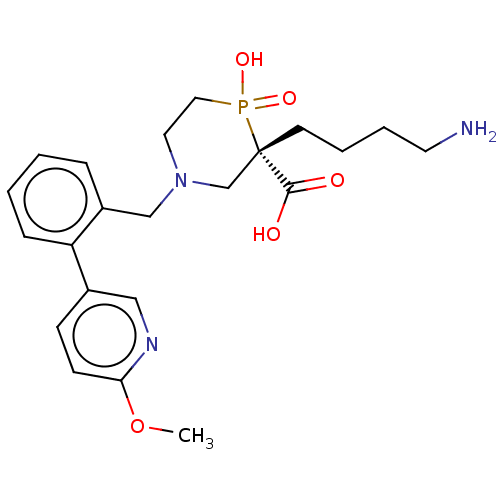 (CHEMBL4858095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575780 (CHEMBL4878039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575769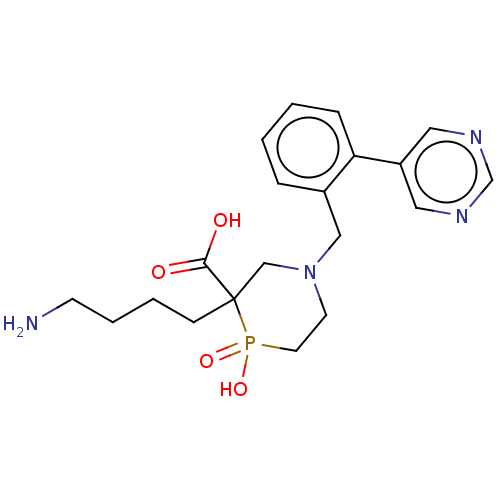 (CHEMBL4853133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575779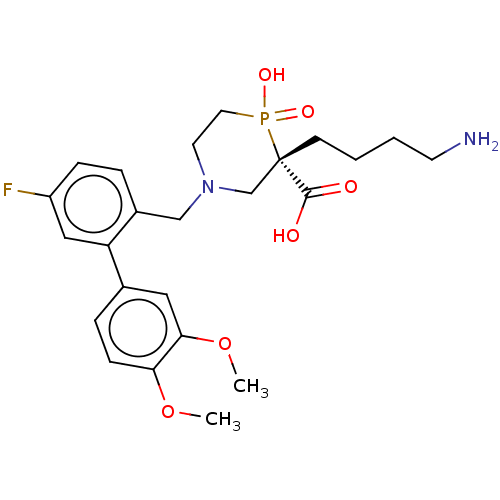 (CHEMBL4858909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575777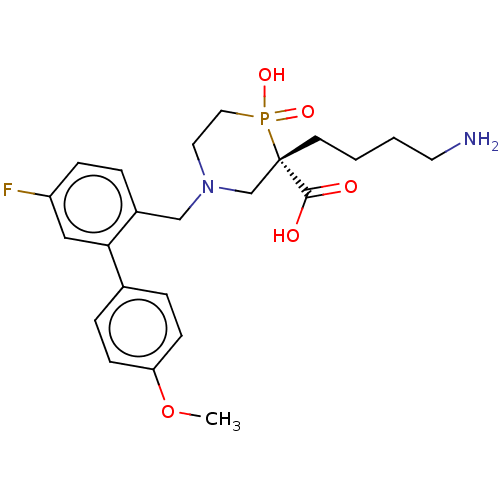 (CHEMBL4852722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575772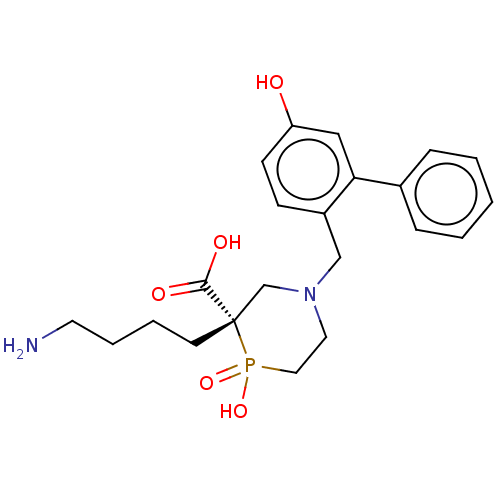 (CHEMBL4875723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575771 (CHEMBL4852607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575773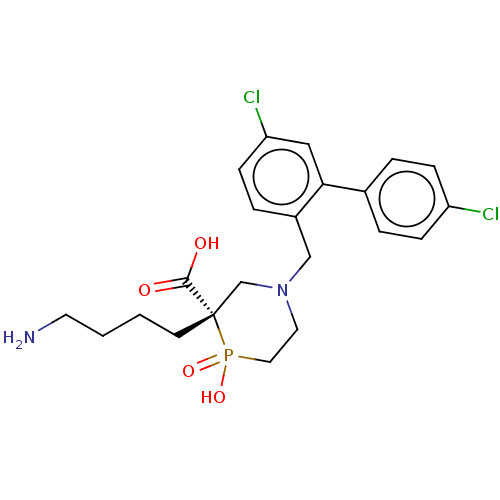 (CHEMBL4861840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575774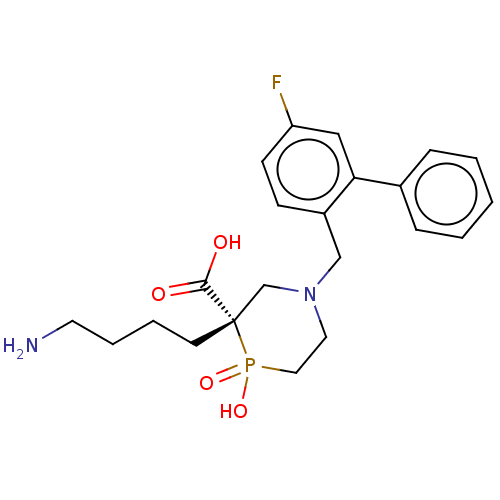 (CHEMBL4854445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575764 (CHEMBL4866725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575778 (CHEMBL4868496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127745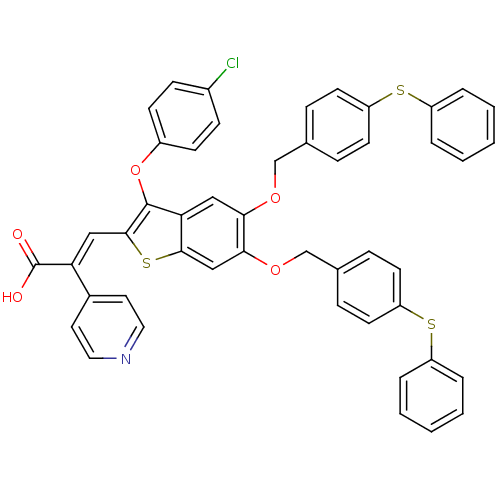 ((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4-phenylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575763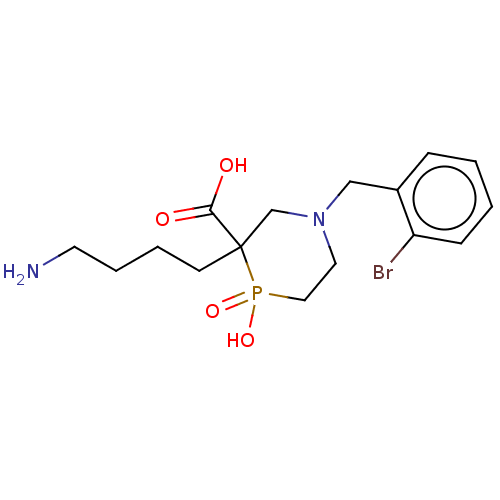 (CHEMBL4858525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127733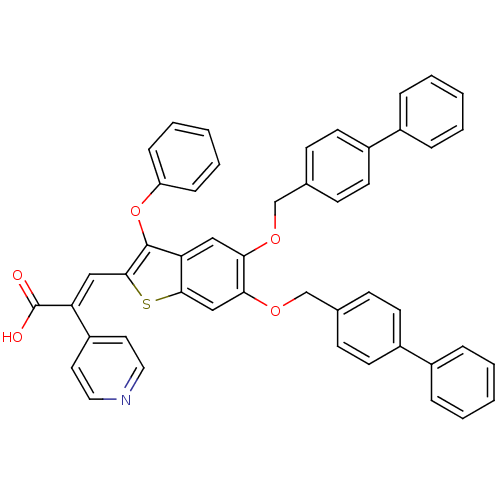 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-phenoxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127735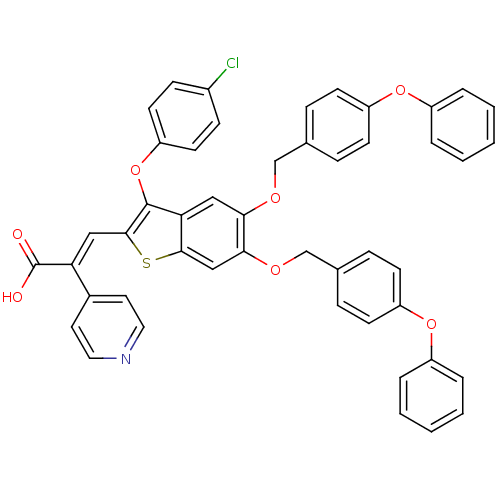 ((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4-phenoxy-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575741 (CHEMBL4856598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575757 (CHEMBL4861694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575761 (CHEMBL4846016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127743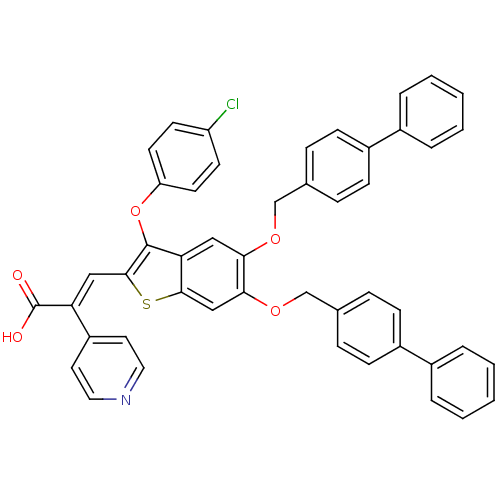 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127732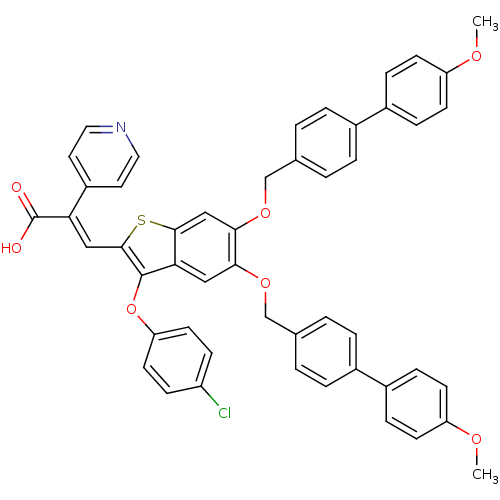 ((E)-3-[3-(4-Chloro-phenoxy)-5,6-bis-(4'-methoxy-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575760 (CHEMBL4855391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575758 (CHEMBL4847492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575739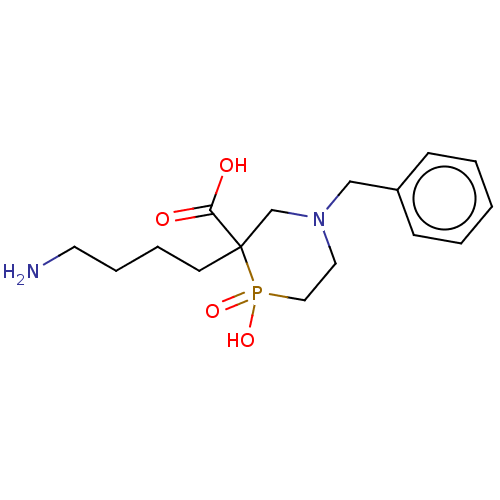 (CHEMBL4862631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575755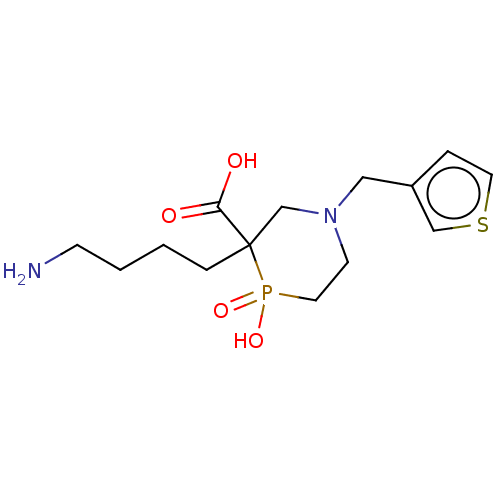 (CHEMBL4857164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575740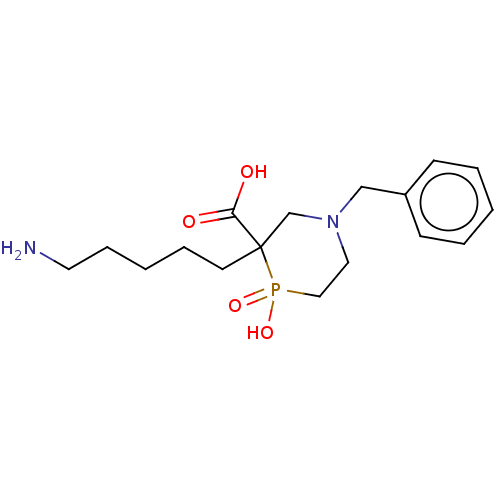 (CHEMBL4869435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127742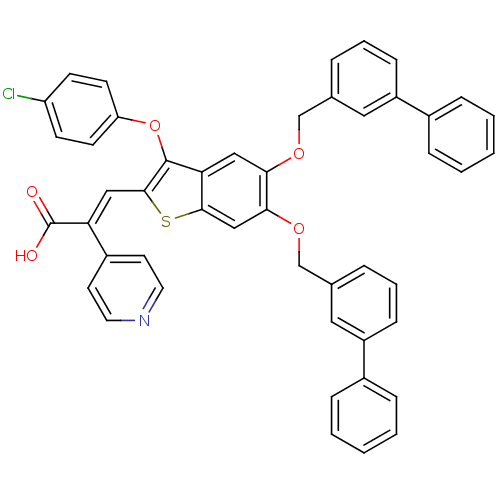 ((E)-3-[5,6-Bis-(biphenyl-3-ylmethoxy)-3-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127726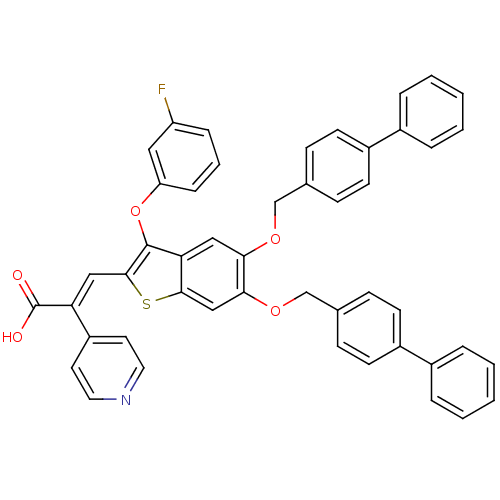 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(3-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127730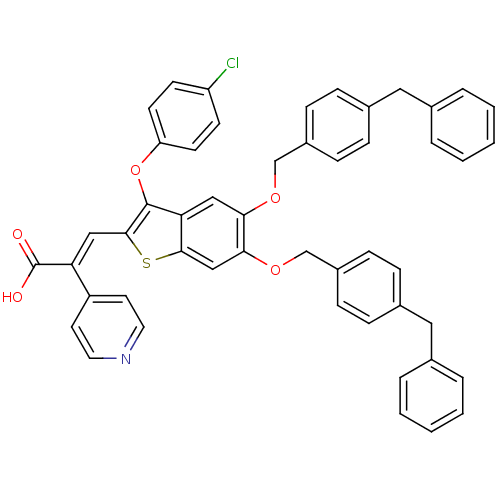 ((E)-3-[5,6-Bis-(4-benzyl-benzyloxy)-3-(4-chloro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127741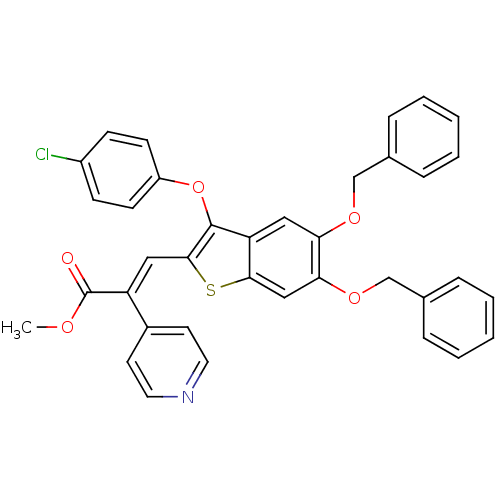 ((E)-3-[5,6-Bis-benzyloxy-3-(4-chloro-phenoxy)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127727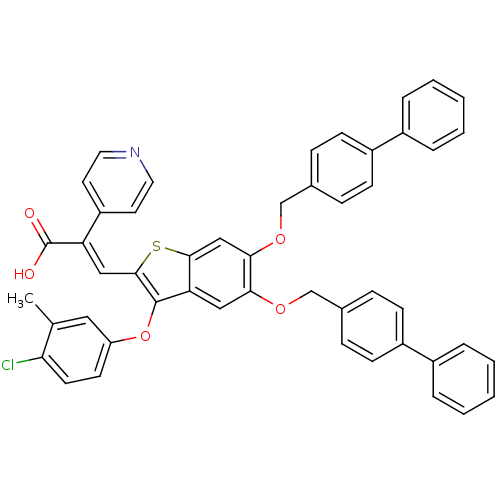 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575743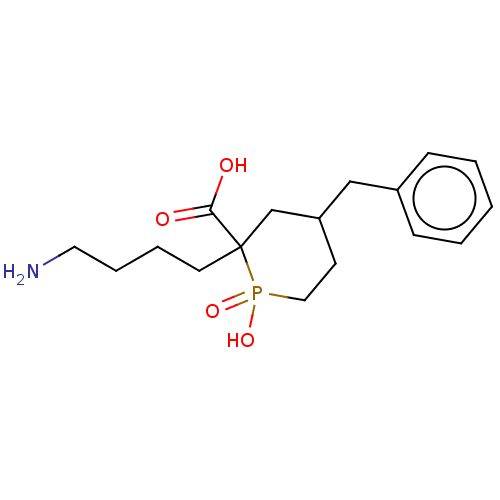 (CHEMBL4861443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575752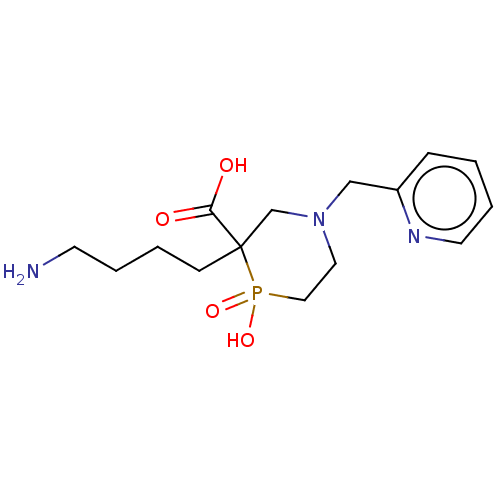 (CHEMBL4855720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575762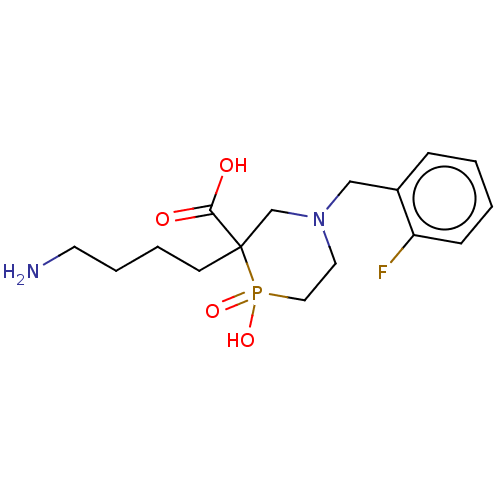 (CHEMBL4848798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127739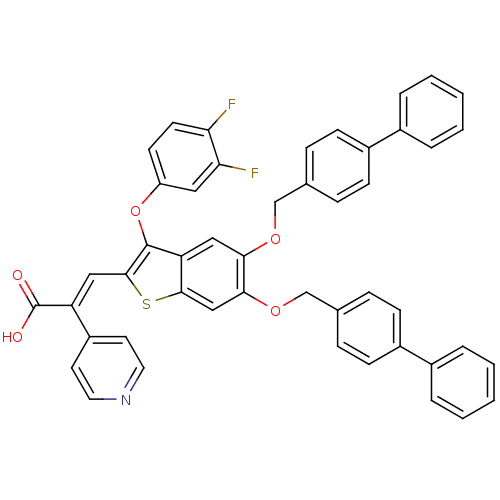 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(3,4-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575759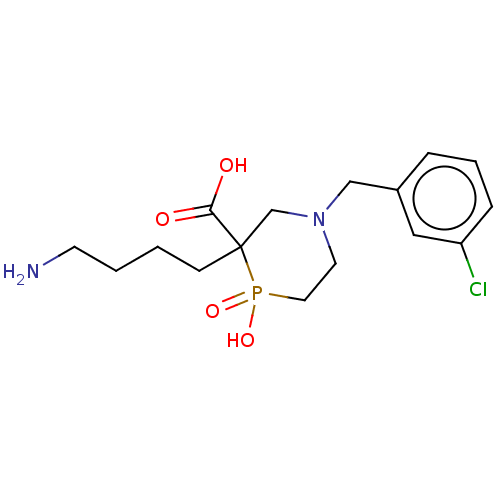 (CHEMBL4864051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127747 ((E)-3-[5,6-Bis-(4-benzenesulfonyl-benzyloxy)-3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127749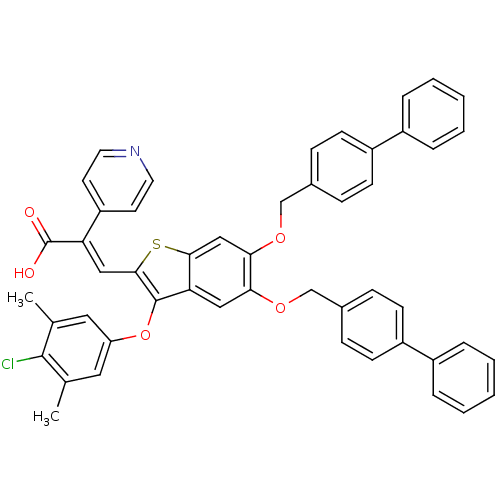 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575754 (CHEMBL4851707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575736 (CHEMBL4858857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575753 (CHEMBL4879366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575737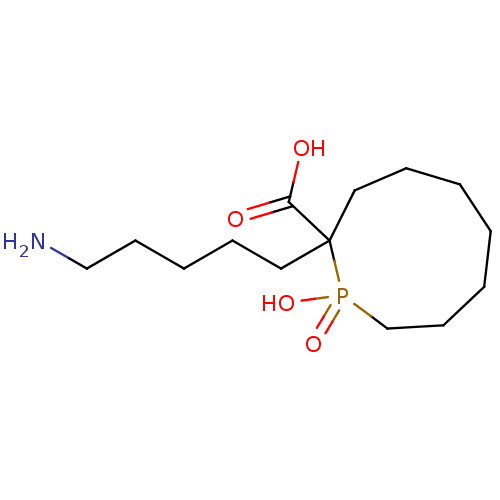 (CHEMBL4861262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575734 (CHEMBL4877745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127746 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50127736 ((E)-3-[5,6-Bis-(biphenyl-4-ylmethoxy)-3-(4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier Curated by ChEMBL | Assay Description Inhibitory activity against plasminogen activator inhibitor 1 (PAI-1) was evaluated by inhibition of tissue plasminogen activator/PAI-1 complex forma... | Bioorg Med Chem Lett 13: 1705-8 (2003) BindingDB Entry DOI: 10.7270/Q2J965SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |