

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358044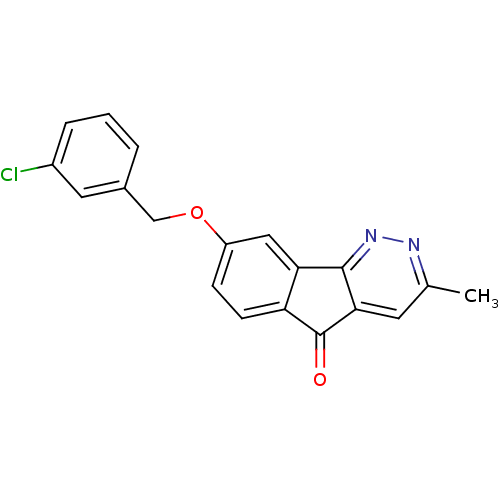 (CHEMBL1917940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358044 (CHEMBL1917940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50121688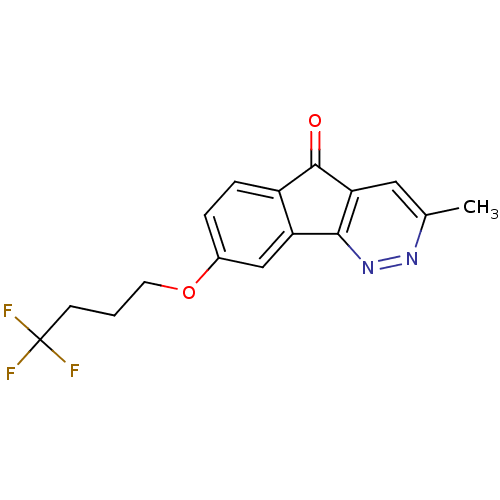 (3-Methyl-8-(4,4,4-trifluoro-butoxy)-indeno[1,2-c]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358045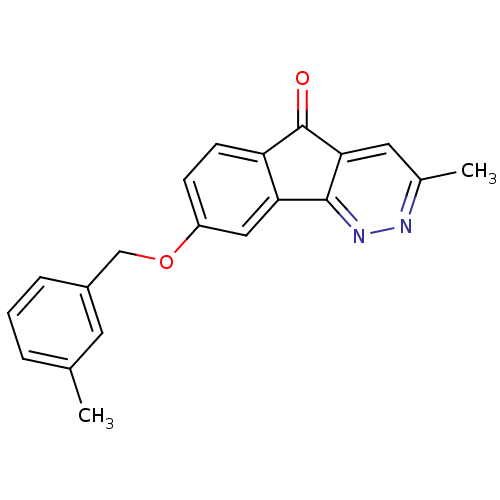 (CHEMBL1914652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3604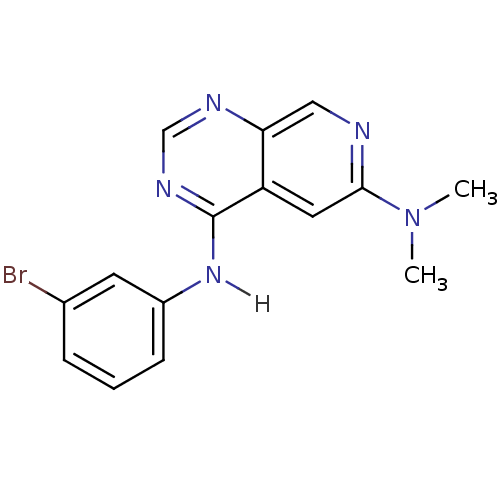 (4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3603 (4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554469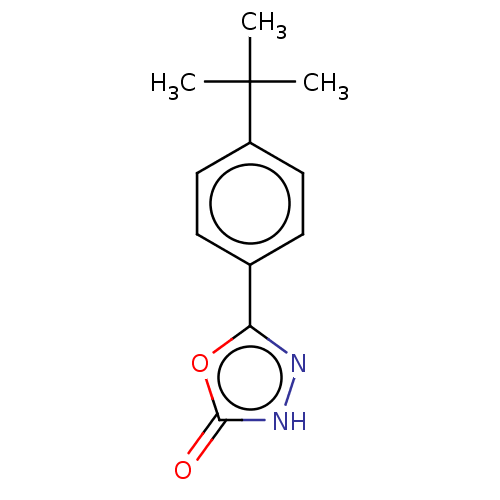 (CHEMBL4796694) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554470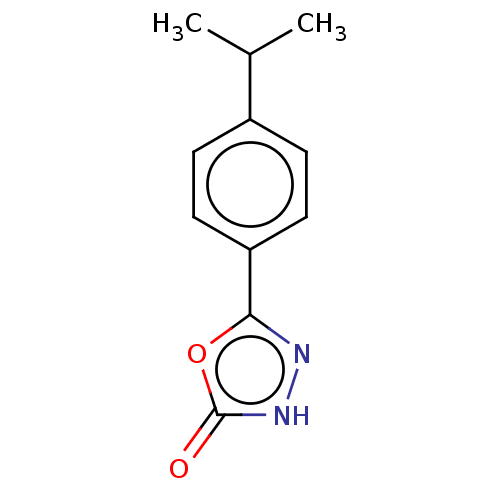 (CHEMBL4752559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3595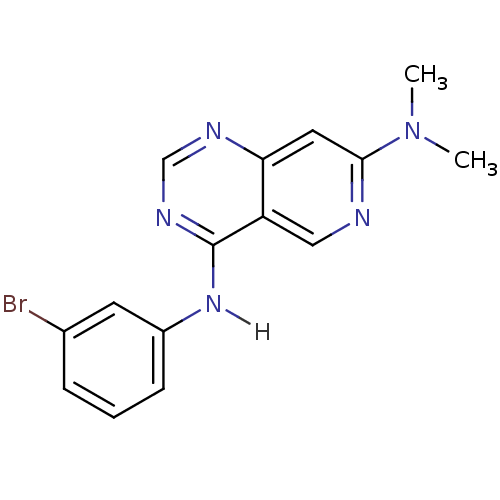 (4-N-(3-bromophenyl)-7-N,7-N-dimethylpyrido[4,3-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3594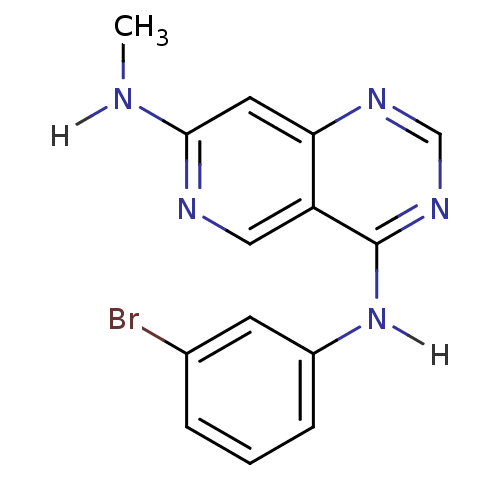 (4-N-(3-bromophenyl)-7-N-methylpyrido[4,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3600 (4-N-(3-bromophenyl)pyrido[3,4-d]pyrimidine-4,6-dia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50087082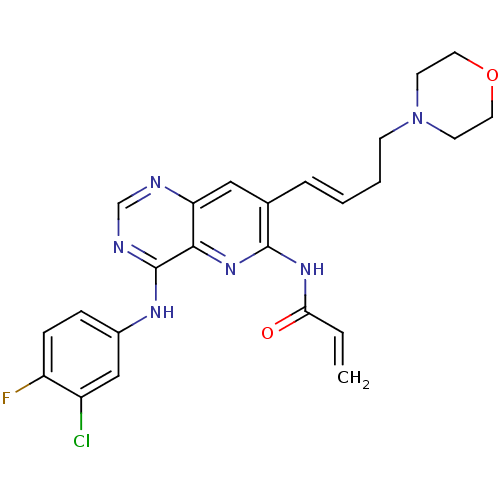 (CHEMBL417478 | N-[4-(3-Chloro-4-fluoro-phenylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of a polyglutamic acid/tyrosine random copolymer by EGFR enzyme prepared from human A431 carcinoma cell vesicles by imm... | J Med Chem 43: 1380-97 (2001) BindingDB Entry DOI: 10.7270/Q2H13179 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4780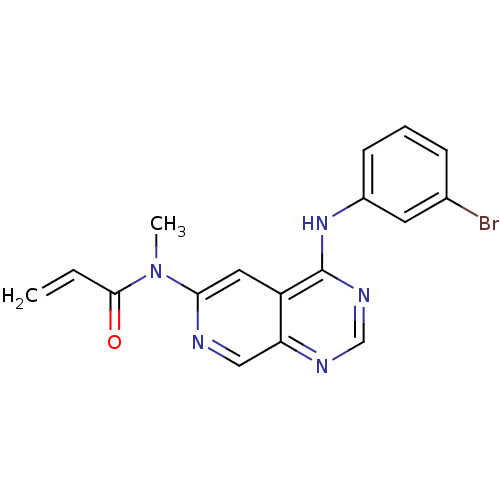 (4-Anilinopyrido[3,4-d]pyrimidine 7 | N-[4-(3-Bromo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3702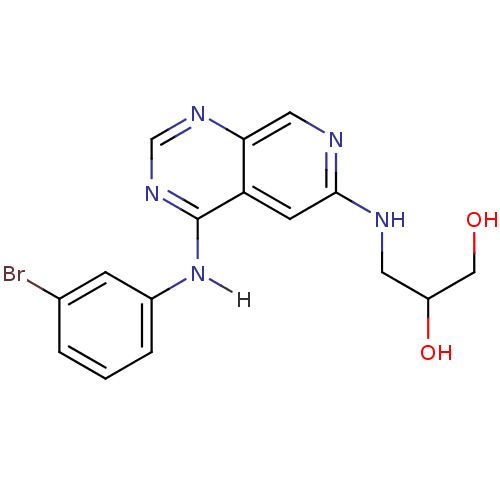 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554462 (CHEMBL4784029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3724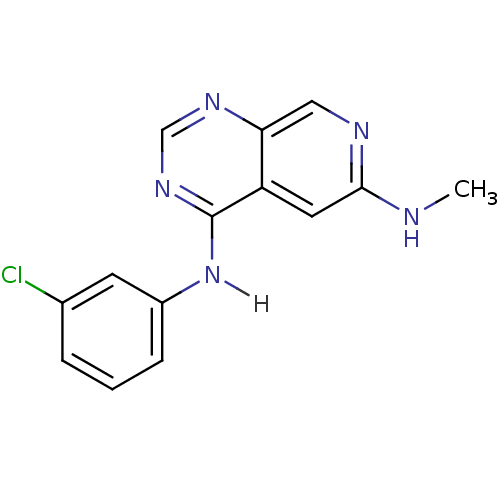 (4-N-(3-chlorophenyl)-6-N-methylpyrido[3,4-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554475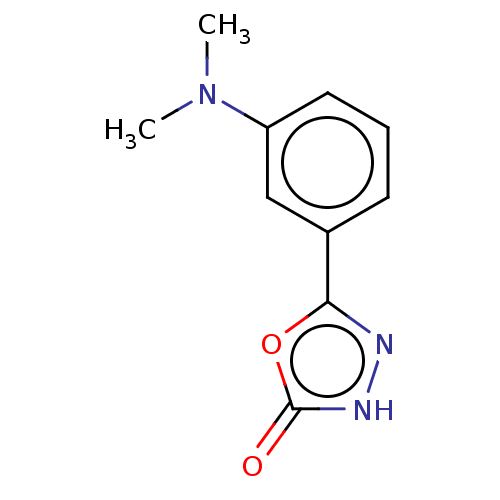 (CHEMBL4757064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3700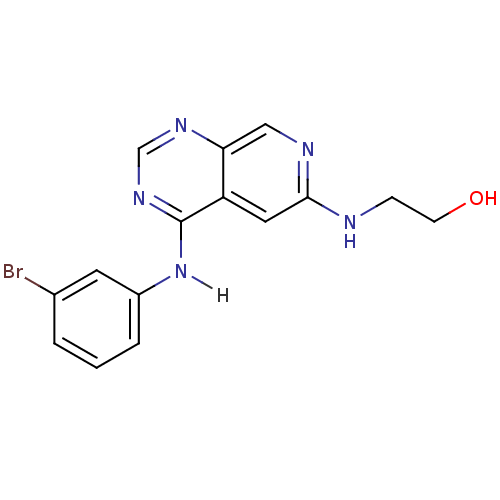 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3701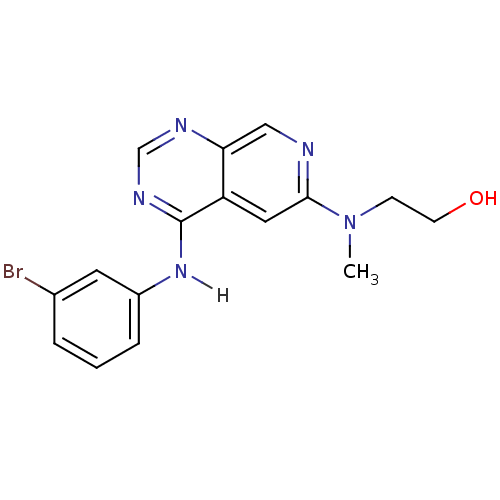 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3646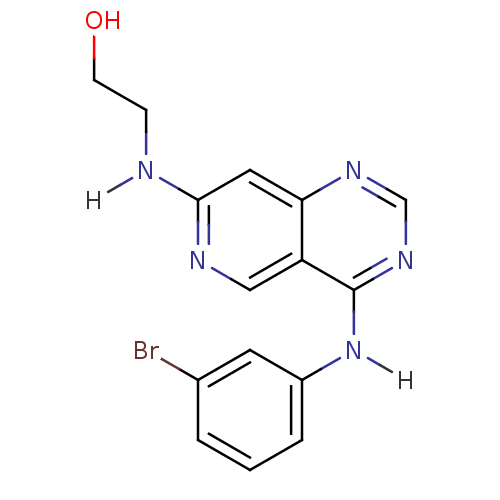 (2-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077239 (CHEMBL52913 | N-[4-(3-Chloro-phenylamino)-quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554471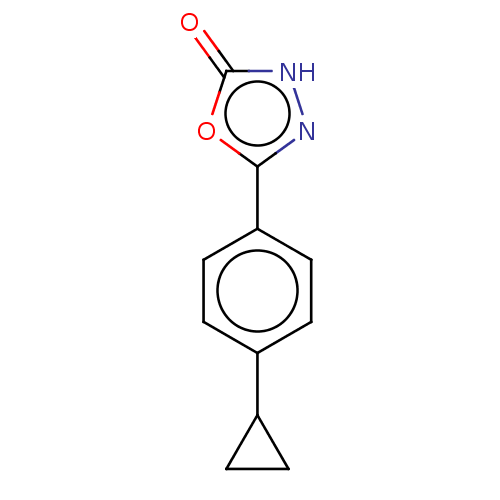 (CHEMBL4761454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554479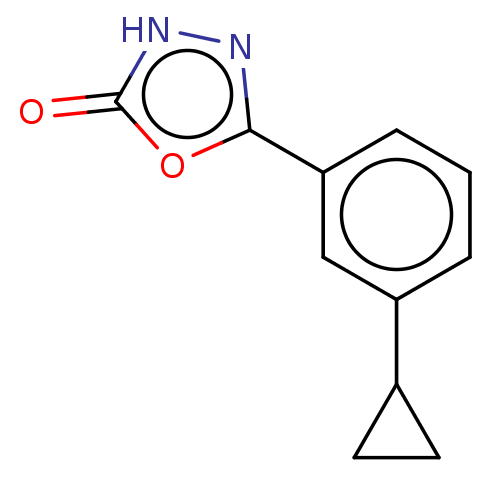 (CHEMBL4741570) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554472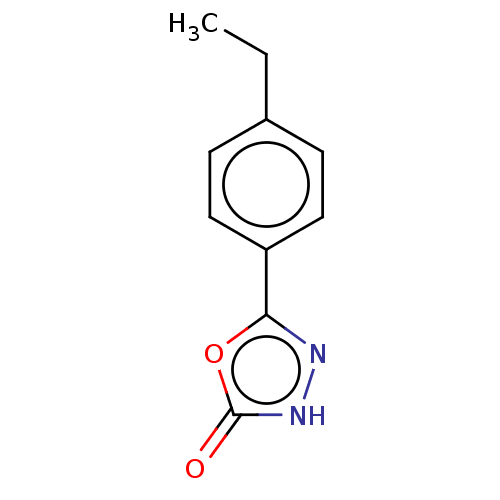 (CHEMBL4787488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3722 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3671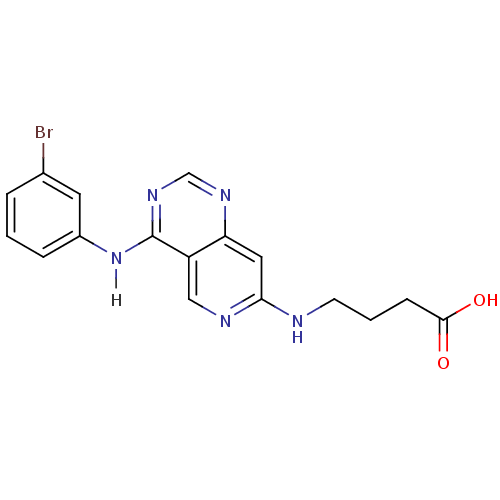 (4-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3720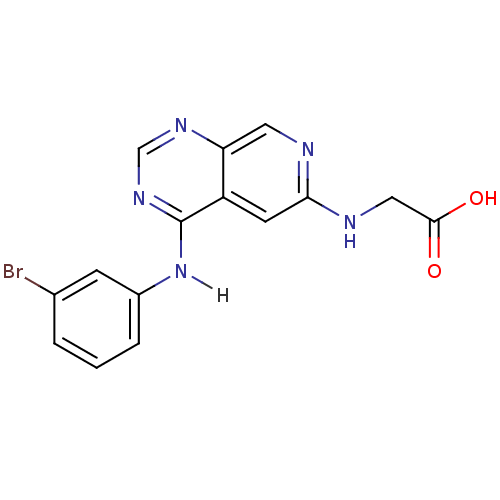 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3714 (2-{[3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554464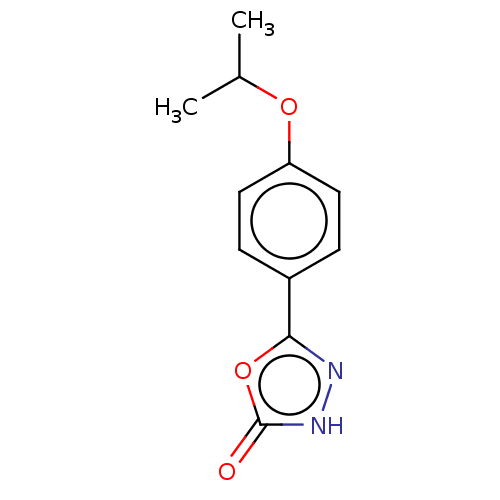 (CHEMBL4750152) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4791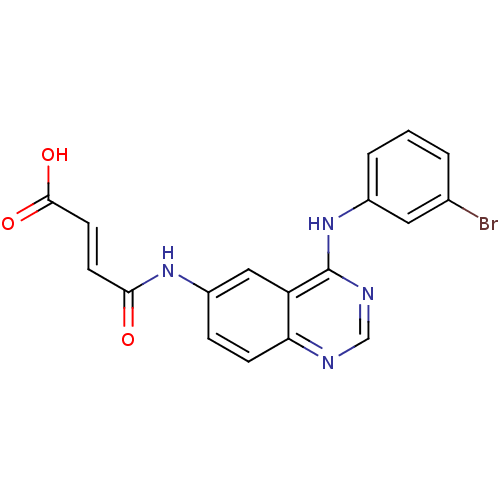 ((2E)-3-({4-[(3-bromophenyl)amino]quinazolin-6-yl}c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50144578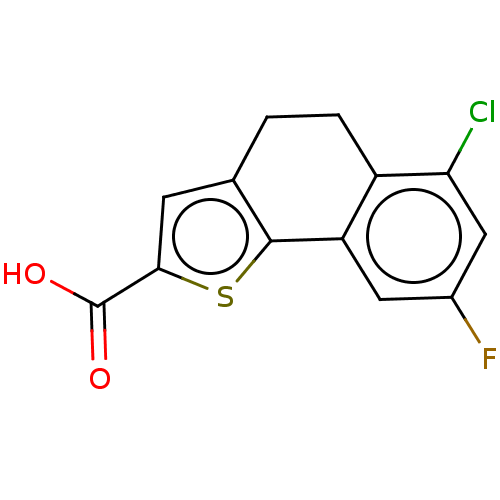 (CHEMBL3760062) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01974 BindingDB Entry DOI: 10.7270/Q2NP28F9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077247 (CHEMBL51741 | N-[4-(6-Bromo-2,3-dihydro-indol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077244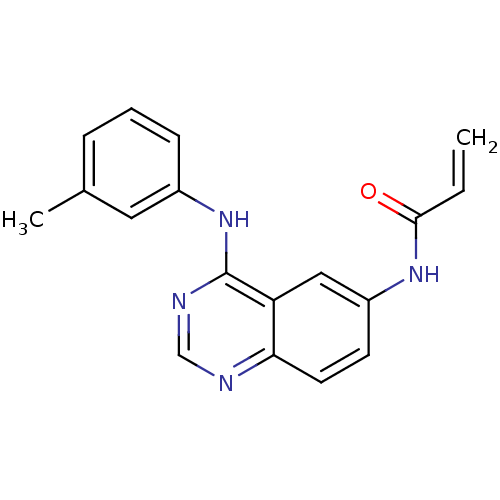 (CHEMBL31815 | N-(4-m-Tolylamino-quinazolin-6-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077244 (CHEMBL31815 | N-(4-m-Tolylamino-quinazolin-6-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of a polyglutamic acid/tyrosine random copolymer by EGFR enzyme prepared from human A431 carcinoma cell vesicles by imm... | J Med Chem 43: 1380-97 (2001) BindingDB Entry DOI: 10.7270/Q2H13179 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4808 (4-Anilinopyrido[3,4-d]pyrimidine 35 | N-(3-Bromoph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554484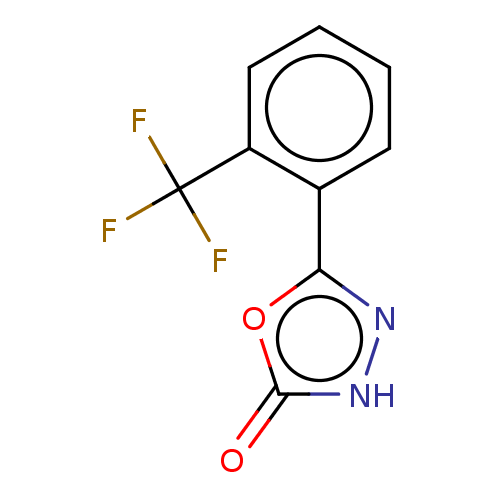 (CHEMBL4747825) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3721 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4798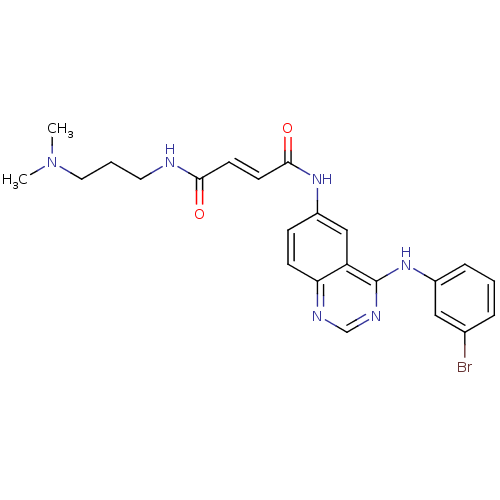 ((2E)-N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3727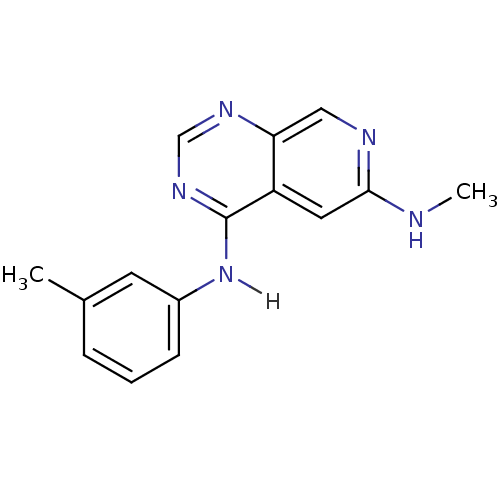 (6-(Methylamino)-4-[(3-methylphenyl)amino]pyrido[3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554480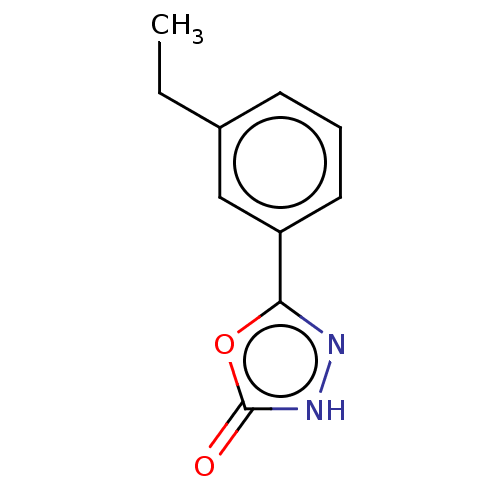 (CHEMBL4757561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077233 (CHEMBL443523 | N-(4-(3-bromophenylamino) quinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM50554467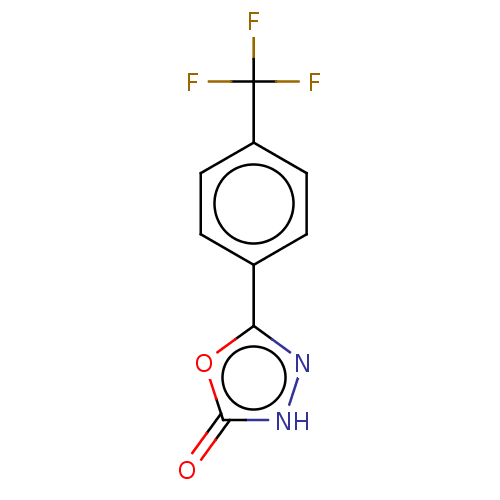 (CHEMBL4759088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Notum (S81-T451 residues) C330S mutant expressed in HEK293S cells using OPTS substrate incubated for 40 mins by fluorescence base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01391 BindingDB Entry DOI: 10.7270/Q2988BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077236 (CHEMBL54088 | N-(4-m-Tolylamino-pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4783 ((2E)-N-[4-(3-Bromoanilino)pyrido[3,4-d]pyrimidin-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3667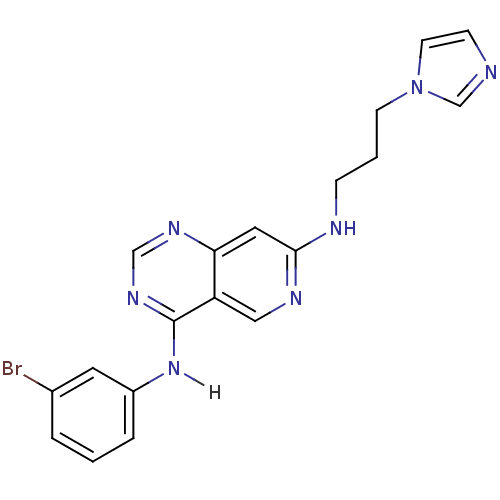 (4-N-(3-bromophenyl)-7-N-[3-(1H-imidazol-1-yl)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077246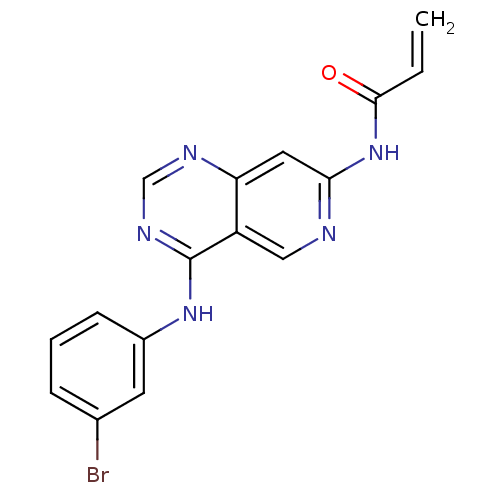 (CHEMBL49986 | N-[4-(3-Bromo-phenylamino)-pyrido[4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4596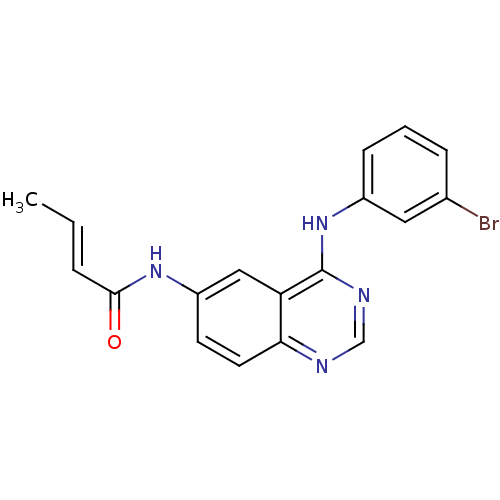 ((2E)-N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4802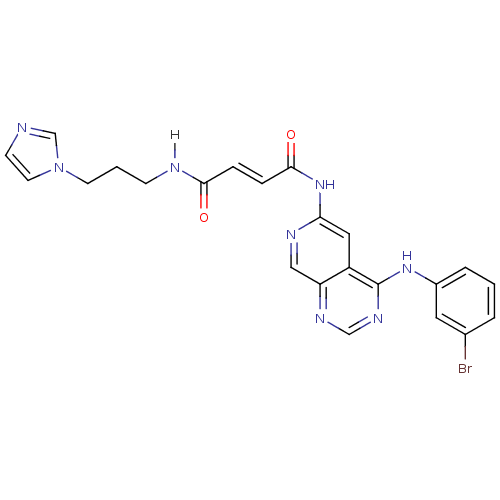 ((2E)-N-{4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3703 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3670 (3-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 957 total ) | Next | Last >> |