 Found 309 hits with Last Name = 'warner' and Initial = 'r'
Found 309 hits with Last Name = 'warner' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50003154
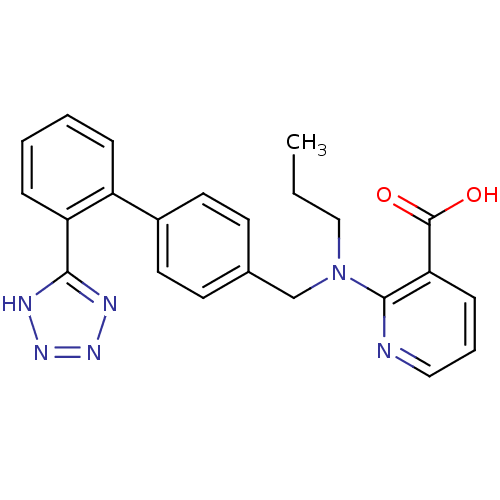
(2-{Propyl-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncccc1C(O)=O Show InChI InChI=1S/C23H22N6O2/c1-2-14-29(22-20(23(30)31)8-5-13-24-22)15-16-9-11-17(12-10-16)18-6-3-4-7-19(18)21-25-27-28-26-21/h3-13H,2,14-15H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050
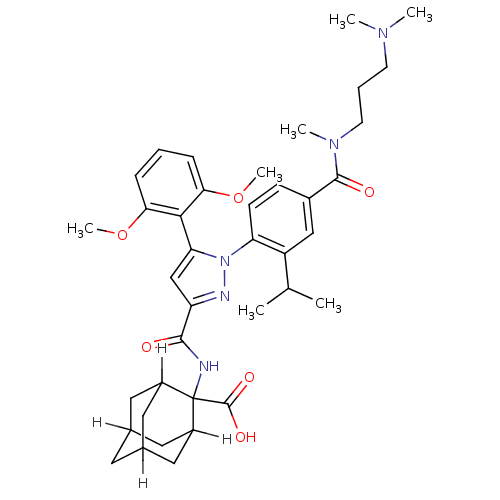
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
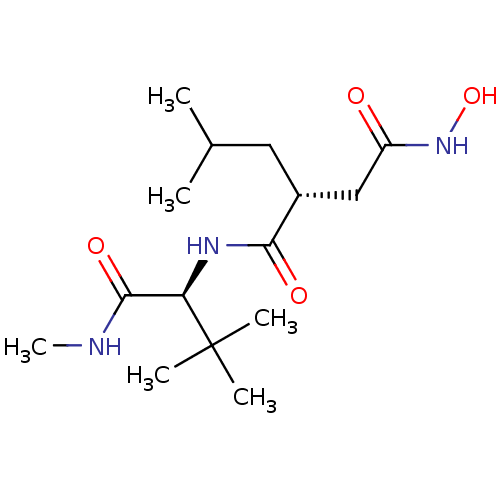
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063920

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003158

(2-Methyl-4-{propyl-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1nc(C)ncc1C(O)=O Show InChI InChI=1S/C23H23N7O2/c1-3-12-30(22-20(23(31)32)13-24-15(2)25-22)14-16-8-10-17(11-9-16)18-6-4-5-7-19(18)21-26-28-29-27-21/h4-11,13H,3,12,14H2,1-2H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003155

(4-{Butyl-[2''-(2H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1ncncc1C(O)=O Show InChI InChI=1S/C23H23N7O2/c1-2-3-12-30(22-20(23(31)32)13-24-15-25-22)14-16-8-10-17(11-9-16)18-6-4-5-7-19(18)21-26-28-29-27-21/h4-11,13,15H,2-3,12,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS2 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 2 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103099
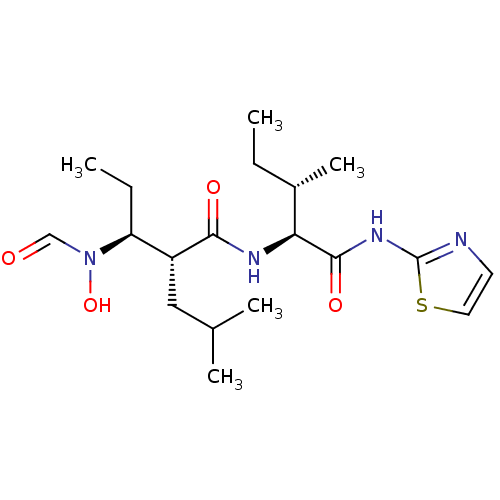
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003156
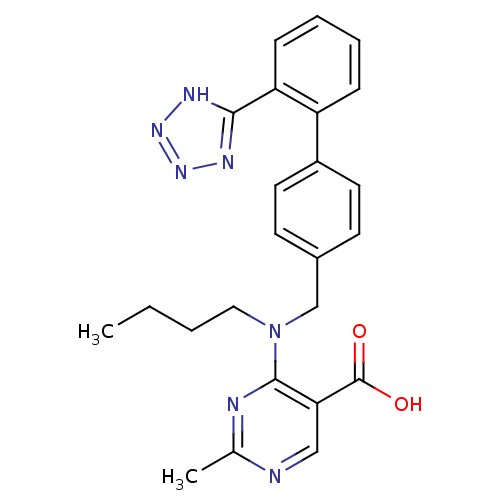
(4-{Butyl-[2''-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)c1nc(C)ncc1C(O)=O Show InChI InChI=1S/C24H25N7O2/c1-3-4-13-31(23-21(24(32)33)14-25-16(2)26-23)15-17-9-11-18(12-10-17)19-7-5-6-8-20(19)22-27-29-30-28-22/h5-12,14H,3-4,13,15H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against angiotensin II receptor, type 1 in rat liver |
J Med Chem 35: 3714-7 (1992)
BindingDB Entry DOI: 10.7270/Q2H41QCN |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103097
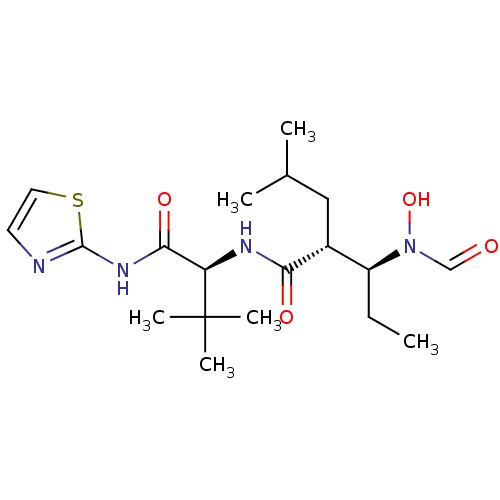
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103092
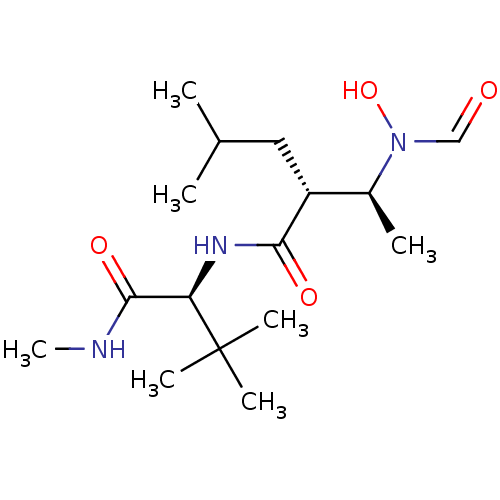
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103096
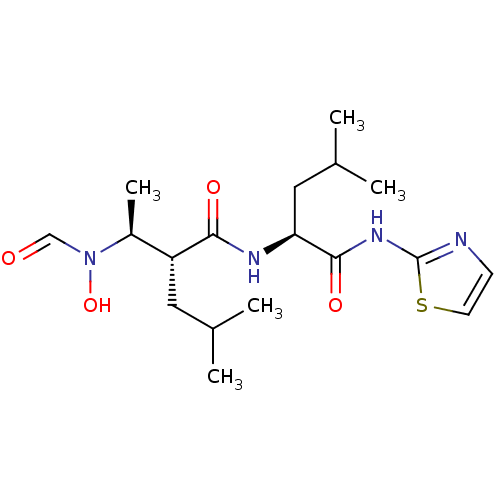
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103102
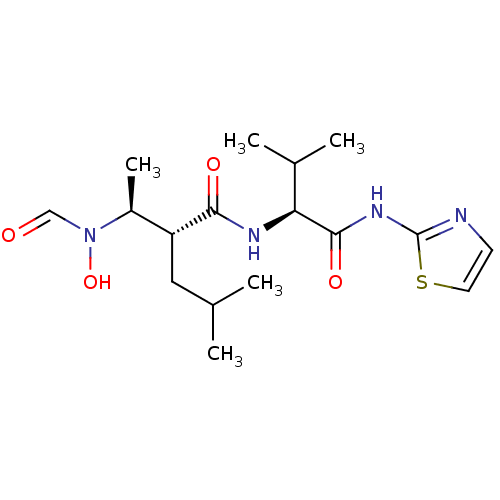
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103098
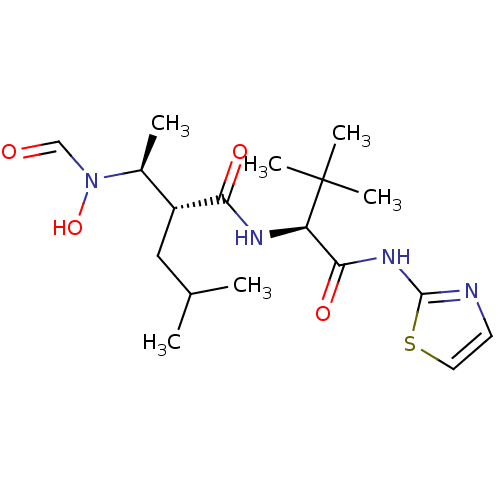
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103093
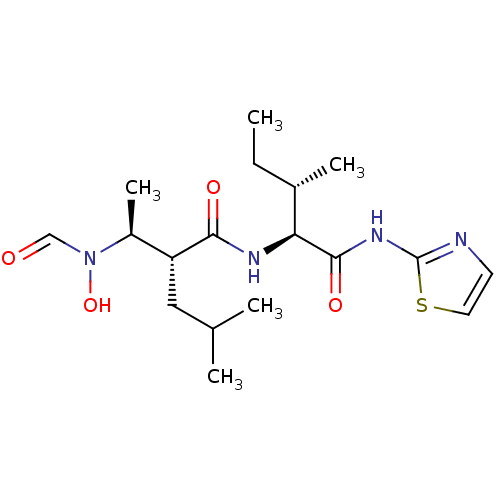
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 2 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103092

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041427
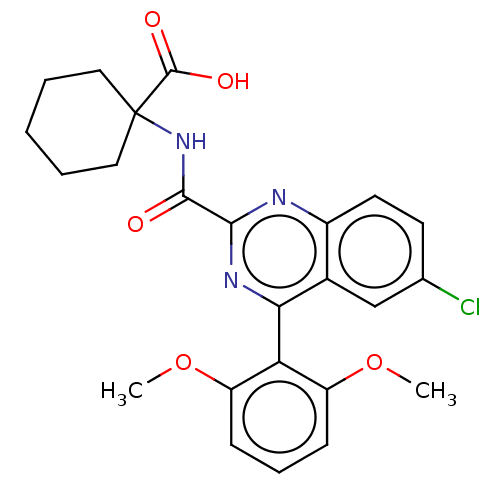
(CHEMBL3356853)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)NC1(CCCCC1)C(O)=O |(41.35,-22.47,;42.69,-23.24,;42.69,-24.79,;41.36,-25.56,;41.36,-27.11,;42.7,-27.88,;44.04,-27.1,;45.37,-27.87,;45.38,-29.42,;44.03,-25.55,;45.36,-24.78,;46.7,-25.55,;48.03,-24.77,;48.03,-23.23,;46.67,-22.47,;46.66,-20.94,;45.33,-20.18,;44.01,-20.96,;42.66,-20.21,;44.02,-22.47,;45.35,-23.24,;49.37,-25.54,;49.38,-27.08,;50.71,-24.76,;52.04,-25.53,;53.38,-26.3,;54.7,-25.53,;54.71,-23.99,;53.38,-23.22,;52.04,-23.99,;52.05,-27.08,;50.72,-27.85,;53.39,-27.84,)| Show InChI InChI=1S/C24H24ClN3O5/c1-32-17-7-6-8-18(33-2)19(17)20-15-13-14(25)9-10-16(15)26-21(27-20)22(29)28-24(23(30)31)11-4-3-5-12-24/h6-10,13H,3-5,11-12H2,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041428

(CHEMBL3356855)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r,wU:24.27,(22.57,-23.73,;23.91,-24.49,;23.91,-26.03,;22.58,-26.8,;22.58,-28.35,;23.92,-29.12,;25.25,-28.35,;26.59,-29.11,;26.59,-30.65,;25.25,-26.8,;26.57,-26.02,;27.91,-26.79,;29.24,-26.02,;29.24,-24.48,;27.88,-23.72,;27.87,-22.19,;26.55,-21.44,;25.22,-22.22,;23.88,-21.46,;25.24,-23.72,;26.57,-24.49,;30.58,-26.78,;30.58,-28.32,;31.91,-26.01,;33.24,-26.77,;34.58,-26,;35.91,-26.77,;37.24,-26.01,;37.24,-24.46,;35.91,-23.69,;34.57,-24.47,;33.25,-28.32,;31.92,-29.09,;34.59,-29.08,)| Show InChI InChI=1S/C25H26ClN3O5/c1-33-18-9-6-10-19(34-2)20(18)22-16-13-15(26)11-12-17(16)27-23(28-22)24(30)29-21(25(31)32)14-7-4-3-5-8-14/h6,9-14,21H,3-5,7-8H2,1-2H3,(H,29,30)(H,31,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041429
(CHEMBL3356854)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](CC(C)C)C(O)=O |r,wU:24.27,(2.86,-22.1,;4.19,-22.87,;4.2,-24.41,;2.87,-25.18,;2.87,-26.72,;4.2,-27.49,;5.54,-26.72,;6.87,-27.49,;6.87,-29.03,;5.53,-25.17,;6.86,-24.4,;8.19,-25.17,;9.52,-24.39,;9.52,-22.85,;8.17,-22.09,;8.16,-20.57,;6.83,-19.81,;5.51,-20.59,;4.17,-19.84,;5.52,-22.09,;6.85,-22.87,;10.86,-25.16,;10.87,-26.7,;12.19,-24.38,;13.53,-25.15,;14.86,-24.38,;16.2,-25.14,;17.53,-24.37,;16.2,-26.68,;13.54,-26.69,;12.2,-27.47,;14.87,-27.46,)| Show InChI InChI=1S/C23H24ClN3O5/c1-12(2)10-16(23(29)30)26-22(28)21-25-15-9-8-13(24)11-14(15)20(27-21)19-17(31-3)6-5-7-18(19)32-4/h5-9,11-12,16H,10H2,1-4H3,(H,26,28)(H,29,30)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS2 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103095
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103101
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50240845
((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25+,26+,27+,28+,29+,30+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103100
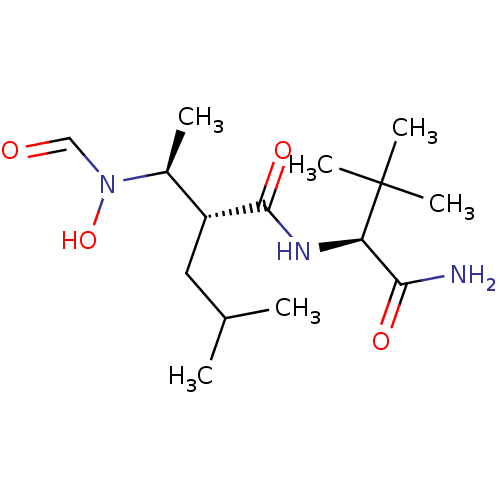
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(N)=O)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-11(10(3)18(22)8-19)14(21)17-12(13(16)20)15(4,5)6/h8-12,22H,7H2,1-6H3,(H2,16,20)(H,17,21)/t10-,11+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50019405

(LEVOCABASTINE | R-50547)Show SMILES C[C@@H]1CN(CC[C@]1(C(O)=O)c1ccccc1)[C@H]1CC[C@](CC1)(C#N)c1ccc(F)cc1 |wU:6.7,16.17,19.24,wD:1.0,6.10,19.26,(9.61,3.85,;8.28,3.08,;8.28,1.54,;6.95,.77,;5.61,1.54,;5.61,3.08,;6.95,3.85,;7.67,5.21,;9.21,5.26,;7.78,6.75,;6.22,5.21,;4.68,5.26,;3.96,6.62,;4.78,7.93,;6.32,7.88,;7.04,6.52,;6.95,-.77,;8.28,-1.54,;8.28,-3.08,;6.95,-3.85,;5.61,-3.08,;5.61,-1.54,;6.22,-5.21,;5.5,-6.57,;7.67,-5.21,;9.21,-5.26,;9.93,-6.62,;9.11,-7.93,;9.84,-9.29,;7.57,-7.88,;6.85,-6.52,)| Show InChI InChI=1S/C26H29FN2O2/c1-19-17-29(16-15-26(19,24(30)31)21-5-3-2-4-6-21)23-11-13-25(18-28,14-12-23)20-7-9-22(27)10-8-20/h2-10,19,23H,11-17H2,1H3,(H,30,31)/t19-,23-,25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103095

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103096

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50248034
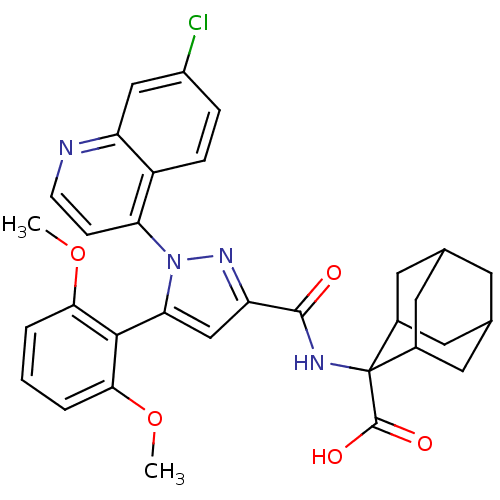
(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50019426
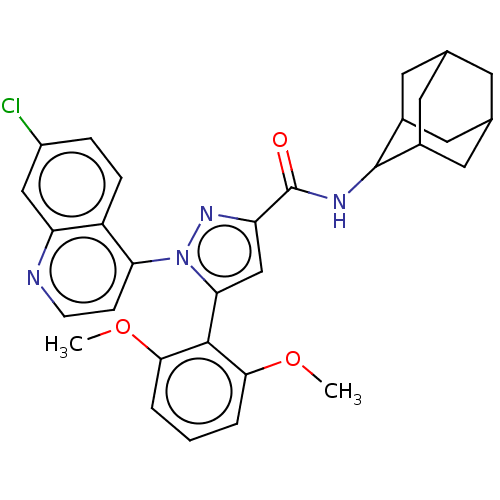
(CHEMBL3290089)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:38:37:35:31.32.33,THB:28:29:35:31.32.33,38:32:29.37.36:35,33:32:29:36.34.35,33:34:29:31.38.32,(32.46,-36.27,;32.94,-34.8,;34.44,-34.48,;35.47,-35.63,;36.98,-35.3,;37.45,-33.84,;36.42,-32.7,;36.9,-31.23,;38.4,-30.91,;34.92,-33.02,;33.58,-31.53,;33.9,-30.02,;32.57,-29.25,;31.42,-30.28,;32.05,-31.69,;31.04,-33.42,;31.81,-34.76,;31.04,-36.09,;29.51,-36.09,;28.74,-34.76,;27.19,-34.76,;26.43,-33.42,;24.89,-33.42,;27.19,-32.09,;28.74,-32.09,;29.51,-33.42,;32.41,-27.72,;33.65,-26.82,;31,-27.09,;30.84,-25.56,;30.47,-23.79,;31.67,-22.96,;32.22,-21.58,;30.93,-20.66,;29.62,-21.49,;29.21,-22.91,;30.03,-23.1,;31.38,-24.05,;32.69,-23.2,)| Show InChI InChI=1S/C31H31ClN4O3/c1-38-27-4-3-5-28(39-2)29(27)26-16-24(31(37)34-30-19-11-17-10-18(13-19)14-20(30)12-17)35-36(26)25-8-9-33-23-15-21(32)6-7-22(23)25/h3-9,15-20,30H,10-14H2,1-2H3,(H,34,37) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data