 Found 395 hits with Last Name = 'webb' and Initial = 'j'
Found 395 hits with Last Name = 'webb' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM86054
(5'-IODORESINIFERATOXIN | I-RTX)Show SMILES COc1cc(CC(=O)OCC2=CC[C@H]3[C@@H](C=C(C)C3=O)[C@]34O[C@]5(Cc6ccccc6)O[C@H]([C@H]23)[C@](C[C@H]4C)(O5)C(C)=C)cc(I)c1O |t:10,15,THB:37:22:32:35.34.33,23:22:32:35.34.33| Show InChI InChI=1S/C37H39IO8/c1-20(2)35-17-22(4)37-27-13-21(3)32(40)26(27)12-11-25(19-43-30(39)16-24-14-28(38)33(41)29(15-24)42-5)31(37)34(35)44-36(45-35,46-37)18-23-9-7-6-8-10-23/h6-11,13-15,22,26-27,31,34,41H,1,12,16-19H2,2-5H3/t22-,26+,27-,31+,34-,35-,36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1052-60 (2002)
Article DOI: 10.1124/jpet.102.040394
BindingDB Entry DOI: 10.7270/Q279437S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM86054

(5'-IODORESINIFERATOXIN | I-RTX)Show SMILES COc1cc(CC(=O)OCC2=CC[C@H]3[C@@H](C=C(C)C3=O)[C@]34O[C@]5(Cc6ccccc6)O[C@H]([C@H]23)[C@](C[C@H]4C)(O5)C(C)=C)cc(I)c1O |t:10,15,THB:37:22:32:35.34.33,23:22:32:35.34.33| Show InChI InChI=1S/C37H39IO8/c1-20(2)35-17-22(4)37-27-13-21(3)32(40)26(27)12-11-25(19-43-30(39)16-24-14-28(38)33(41)29(15-24)42-5)31(37)34(35)44-36(45-35,46-37)18-23-9-7-6-8-10-23/h6-11,13-15,22,26-27,31,34,41H,1,12,16-19H2,2-5H3/t22-,26+,27-,31+,34-,35-,36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1052-60 (2002)
Article DOI: 10.1124/jpet.102.040394
BindingDB Entry DOI: 10.7270/Q279437S |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50273099
(3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...)Show InChI InChI=1S/C7H8N6/c1-2-4-5(3-1)8-9-6(4)7-10-12-13-11-7/h1-3H2,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20284
(CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...)Show InChI InChI=1S/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1052-60 (2002)
Article DOI: 10.1124/jpet.102.040394
BindingDB Entry DOI: 10.7270/Q279437S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50423388
(CHEMBL251024)Show SMILES CCCCNc1nc(SCCC)nc2n(nnc12)[C@@H]1O[C@H](\C=C\C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C22H31N7O8S/c1-3-5-8-23-18-15-19(26-22(25-18)38-9-4-2)29(28-27-15)20-17(34)16(33)12(37-20)6-7-13(30)24-11(21(35)36)10-14(31)32/h6-7,11-12,16-17,20,33-34H,3-5,8-10H2,1-2H3,(H,24,30)(H,31,32)(H,35,36)(H,23,25,26)/b7-6+/t11-,12+,16+,17+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation |
Bioorg Med Chem Lett 17: 6013-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.057
BindingDB Entry DOI: 10.7270/Q2JD4Z2Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50423387
(CHEMBL437204)Show SMILES CCCCNc1nc(SCCC)nc2n(nnc12)[C@@H]1C[C@H](\C=C\C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C23H33N7O7S/c1-3-5-8-24-20-17-21(27-23(26-20)38-9-4-2)30(29-28-17)14-10-12(18(34)19(14)35)6-7-15(31)25-13(22(36)37)11-16(32)33/h6-7,12-14,18-19,34-35H,3-5,8-11H2,1-2H3,(H,25,31)(H,32,33)(H,36,37)(H,24,26,27)/b7-6+/t12-,13-,14+,18+,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation |
Bioorg Med Chem Lett 17: 6013-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.057
BindingDB Entry DOI: 10.7270/Q2JD4Z2Q |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50261965
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(N)(=O)=O |(20.38,-43.46,;21.92,-43.46,;22.82,-42.22,;24.28,-42.69,;24.28,-44.23,;22.82,-44.71,;22.35,-46.17,;23.26,-47.43,;24.8,-47.43,;25.57,-48.76,;24.8,-50.09,;25.57,-51.42,;27.11,-51.43,;27.88,-50.08,;29.41,-50.07,;30.17,-48.74,;29.39,-47.41,;30.15,-46.07,;27.86,-47.43,;27.11,-48.75,;22.35,-48.68,;20.87,-48.2,;19.53,-48.97,;19.52,-50.51,;18.19,-51.28,;16.65,-51.28,;17.42,-52.62,;18.19,-48.2,;16.86,-48.97,;18.19,-46.65,;16.86,-45.88,;19.53,-45.87,;19.52,-44.33,;20.87,-46.65,;25.52,-41.78,;26.85,-41,;24.68,-40.49,;26.37,-43.07,)| Show InChI InChI=1S/C25H24ClN7O4S/c1-30-13-17(38(27,36)37)10-20(30)22-21-23(32(11-14-3-4-14)25(35)31(2)24(21)34)29-33(22)12-15-7-8-28-19-6-5-16(26)9-18(15)19/h5-10,13-14H,3-4,11-12H2,1-2H3,(H2,27,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262113
(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cyclopropylm...)Show SMILES Cn1cc(cc1-c1n(Cc2c[nH]c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)=O |(20.02,-45.88,;21.56,-45.88,;22.46,-44.63,;23.92,-45.1,;23.92,-46.64,;22.46,-47.12,;21.99,-48.58,;22.9,-49.83,;24.44,-49.83,;25.21,-51.17,;24.59,-52.57,;25.74,-53.6,;27.06,-52.83,;28.52,-53.3,;29.67,-52.28,;29.34,-50.77,;30.48,-49.74,;27.88,-50.3,;26.75,-51.33,;21.99,-51.09,;20.51,-50.61,;19.17,-51.38,;19.17,-52.92,;17.83,-53.68,;16.3,-53.69,;17.07,-55.02,;17.84,-50.61,;16.51,-51.38,;17.84,-49.06,;16.5,-48.29,;19.17,-48.28,;19.17,-46.74,;20.51,-49.06,;25.16,-44.19,;25.15,-42.65,;26.57,-44.81,)| Show InChI InChI=1S/C25H25ClN6O3S/c1-29-13-17(36(3)35)9-20(29)22-21-23(31(11-14-4-5-14)25(34)30(2)24(21)33)28-32(22)12-15-10-27-19-7-6-16(26)8-18(15)19/h6-10,13-14,27H,4-5,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262115
(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cyclopropylm...)Show SMILES Cn1cc(cc1-c1n(Cc2c[nH]c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(19.34,2.59,;20.88,2.59,;21.78,3.84,;23.24,3.37,;23.25,1.83,;21.78,1.35,;21.31,-.11,;22.22,-1.37,;23.76,-1.37,;24.53,-2.7,;23.91,-4.1,;25.06,-5.13,;26.39,-4.36,;27.85,-4.84,;28.99,-3.81,;28.66,-2.3,;29.81,-1.27,;27.21,-1.83,;26.07,-2.86,;21.31,-2.62,;19.84,-2.14,;18.5,-2.91,;18.49,-4.45,;17.16,-5.22,;15.62,-5.22,;16.39,-6.55,;17.16,-2.14,;15.83,-2.91,;17.16,-.59,;15.83,.18,;18.5,.19,;18.49,1.73,;19.84,-.59,;24.49,4.28,;25.73,5.19,;23.64,5.56,;25.34,2.99,)| Show InChI InChI=1S/C25H25ClN6O4S/c1-29-13-17(37(3,35)36)9-20(29)22-21-23(31(11-14-4-5-14)25(34)30(2)24(21)33)28-32(22)12-15-10-27-19-7-6-16(26)8-18(15)19/h6-10,13-14,27H,4-5,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50215445
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262011
(5-(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cycloprop...)Show SMILES CNS(=O)(=O)c1cc(-c2n(Cc3c[nH]c4ccc(Cl)cc34)nc3n(CC4CC4)c(=O)n(C)c(=O)c23)n(C)c1 |(26.84,5.41,;25.5,6.17,;24.18,5.39,;23.33,6.68,;25.03,4.1,;22.94,4.48,;22.94,2.94,;21.48,2.46,;21.01,1,;21.92,-.25,;23.46,-.25,;24.23,-1.59,;23.61,-2.99,;24.75,-4.02,;26.08,-3.25,;27.54,-3.72,;28.68,-2.7,;28.36,-1.19,;29.5,-.16,;26.9,-.72,;25.76,-1.74,;21.01,-1.51,;19.53,-1.03,;18.19,-1.8,;18.19,-3.34,;16.85,-4.1,;15.31,-4.11,;16.08,-5.44,;16.85,-1.03,;15.52,-1.8,;16.85,.52,;15.52,1.29,;18.19,1.3,;18.19,2.84,;19.53,.52,;20.58,3.71,;19.04,3.71,;21.48,4.95,)| Show InChI InChI=1S/C25H26ClN7O4S/c1-27-38(36,37)17-9-20(30(2)13-17)22-21-23(32(11-14-4-5-14)25(35)31(3)24(21)34)29-33(22)12-15-10-28-19-7-6-16(26)8-18(15)19/h6-10,13-14,27-28H,4-5,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262060
(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cyclopropylm...)Show SMILES Cn1cc(nc1-c1n(Cc2c[nH]c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(18.81,-30.49,;20.35,-30.49,;21.25,-29.25,;22.71,-29.72,;22.71,-31.25,;21.25,-31.74,;20.78,-33.2,;21.69,-34.45,;23.23,-34.45,;24,-35.79,;23.38,-37.19,;24.52,-38.22,;25.85,-37.45,;27.31,-37.92,;28.45,-36.9,;28.13,-35.39,;29.27,-34.36,;26.67,-34.92,;25.54,-35.94,;20.78,-35.71,;19.3,-35.23,;17.96,-36,;17.96,-37.54,;16.62,-38.3,;15.08,-38.31,;15.85,-39.64,;16.63,-35.23,;15.29,-36,;16.63,-33.68,;15.29,-32.91,;17.96,-32.9,;17.96,-31.36,;19.3,-33.68,;23.95,-28.81,;25.19,-27.9,;23.1,-27.52,;24.8,-30.1,)| Show InChI InChI=1S/C24H24ClN7O4S/c1-29-12-18(37(3,35)36)27-22(29)20-19-21(31(10-13-4-5-13)24(34)30(2)23(19)33)28-32(20)11-14-9-26-17-7-6-15(25)8-16(14)17/h6-9,12-13,26H,4-5,10-11H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262163

(2-((5-chloro-1-methyl-1H-indol-3-yl)methyl)-7-(cyc...)Show SMILES Cn1cc(cc1-c1n(Cc2cn(C)c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.41,-16.12,;-1.87,-16.12,;-.98,-14.87,;.49,-15.34,;.49,-16.88,;-.97,-17.36,;-1.44,-18.82,;-.53,-20.08,;1.01,-20.08,;1.78,-21.41,;1.16,-22.81,;2.3,-23.84,;2.14,-25.38,;3.63,-23.07,;5.09,-23.55,;6.23,-22.52,;5.91,-21.01,;7.05,-19.98,;4.45,-20.54,;3.31,-21.57,;-1.44,-21.33,;-2.92,-20.85,;-4.26,-21.62,;-4.26,-23.16,;-5.6,-23.93,;-7.14,-23.93,;-6.37,-25.27,;-5.6,-20.85,;-6.93,-21.62,;-5.6,-19.3,;-6.93,-18.54,;-4.26,-18.52,;-4.26,-16.98,;-2.92,-19.3,;1.73,-14.44,;2.97,-13.53,;.88,-13.15,;2.58,-15.72,)| Show InChI InChI=1S/C26H27ClN6O4S/c1-29-12-16(19-9-17(27)7-8-20(19)29)13-33-23(21-10-18(14-30(21)2)38(4,36)37)22-24(28-33)32(11-15-5-6-15)26(35)31(3)25(22)34/h7-10,12,14-15H,5-6,11,13H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262114
(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylme...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.7,2.05,;-2.16,2.05,;-1.26,3.29,;.2,2.82,;.21,1.29,;-1.26,.8,;-1.73,-.66,;-.82,-1.91,;.72,-1.91,;1.5,-3.25,;.72,-4.58,;1.49,-5.91,;3.04,-5.92,;3.8,-4.57,;5.33,-4.56,;6.1,-3.23,;5.31,-1.9,;6.07,-.56,;3.79,-1.92,;3.03,-3.24,;-1.73,-3.17,;-3.2,-2.69,;-4.55,-3.46,;-4.55,-5,;-5.89,-5.77,;-7.42,-5.77,;-6.66,-7.11,;-5.88,-2.69,;-7.22,-3.46,;-5.88,-1.14,;-7.22,-.37,;-4.55,-.36,;-4.55,1.18,;-3.2,-1.14,;1.45,3.73,;2.69,4.64,;.6,5.02,;2.3,2.45,)| Show InChI InChI=1S/C26H25ClN6O4S/c1-30-14-18(38(3,36)37)11-21(30)23-22-24(32(12-15-4-5-15)26(35)31(2)25(22)34)29-33(23)13-16-8-9-28-20-7-6-17(27)10-19(16)20/h6-11,14-15H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262012
(5-(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cycloprop...)Show SMILES CONS(=O)(=O)c1cc(-c2n(Cc3c[nH]c4ccc(Cl)cc34)nc3n(CC4CC4)c(=O)n(C)c(=O)c23)n(C)c1 |(3.98,-13.58,;3.97,-12.04,;2.63,-11.28,;1.3,-12.06,;.46,-10.78,;2.15,-13.35,;.06,-12.97,;.06,-14.51,;-1.4,-14.99,;-1.87,-16.45,;-.96,-17.71,;.58,-17.71,;1.35,-19.04,;.73,-20.44,;1.87,-21.47,;3.2,-20.7,;4.66,-21.18,;5.8,-20.15,;5.48,-18.64,;6.62,-17.61,;4.02,-18.17,;2.89,-19.2,;-1.87,-18.96,;-3.35,-18.48,;-4.69,-19.25,;-4.69,-20.79,;-6.03,-21.56,;-7.56,-21.56,;-6.8,-22.9,;-6.02,-18.48,;-7.36,-19.25,;-6.02,-16.93,;-7.36,-16.16,;-4.69,-16.15,;-4.69,-14.61,;-3.35,-16.93,;-2.3,-13.75,;-3.84,-13.75,;-1.4,-12.5,)| Show InChI InChI=1S/C25H26ClN7O5S/c1-30-13-17(39(36,37)29-38-3)9-20(30)22-21-23(32(11-14-4-5-14)25(35)31(2)24(21)34)28-33(22)12-15-10-27-19-7-6-16(26)8-18(15)19/h6-10,13-14,27,29H,4-5,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262061
(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylme...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)=O |(-3.28,-44.41,;-1.74,-44.41,;-.84,-43.17,;.62,-43.64,;.63,-45.17,;-.84,-45.66,;-1.31,-47.12,;-.4,-48.37,;1.14,-48.37,;1.92,-49.71,;1.14,-51.04,;1.91,-52.37,;3.46,-52.37,;4.22,-51.03,;5.75,-51.02,;6.52,-49.69,;5.73,-48.36,;6.49,-47.02,;4.21,-48.38,;3.45,-49.7,;-1.31,-49.63,;-2.78,-49.15,;-4.13,-49.92,;-4.13,-51.46,;-5.47,-52.23,;-7,-52.23,;-6.24,-53.57,;-5.46,-49.15,;-6.8,-49.92,;-5.46,-47.6,;-6.8,-46.83,;-4.13,-46.82,;-4.13,-45.28,;-2.78,-47.6,;1.87,-42.73,;1.86,-41.18,;3.28,-43.35,)| Show InChI InChI=1S/C26H25ClN6O3S/c1-30-14-18(37(3)36)11-21(30)23-22-24(32(12-15-4-5-15)26(35)31(2)25(22)34)29-33(23)13-16-8-9-28-20-7-6-17(27)10-19(16)20/h6-11,14-15H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50261964
(2-(2-((5-chloro-1H-indol-3-yl)methyl)-7-(cycloprop...)Show SMILES Cn1cc(nc1-c1n(Cc2c[nH]c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(N)(=O)=O |(-4.37,-43.05,;-2.83,-43.05,;-1.93,-41.81,;-.47,-42.28,;-.47,-43.81,;-1.93,-44.3,;-2.4,-45.76,;-1.49,-47.01,;.05,-47.01,;.82,-48.34,;.2,-49.75,;1.34,-50.78,;2.67,-50.01,;4.13,-50.48,;5.27,-49.45,;4.95,-47.95,;6.09,-46.91,;3.49,-47.48,;2.36,-48.5,;-2.4,-48.27,;-3.88,-47.79,;-5.22,-48.55,;-5.22,-50.09,;-6.56,-50.86,;-8.09,-50.86,;-7.33,-52.2,;-6.55,-47.78,;-7.89,-48.55,;-6.55,-46.24,;-7.89,-45.47,;-5.22,-45.46,;-5.22,-43.92,;-3.88,-46.24,;.77,-41.37,;2.1,-40.59,;-.07,-40.08,;1.62,-42.65,)| Show InChI InChI=1S/C23H23ClN8O4S/c1-29-11-17(37(25,35)36)27-21(29)19-18-20(31(9-12-3-4-12)23(34)30(2)22(18)33)28-32(19)10-13-8-26-16-6-5-14(24)7-15(13)16/h5-8,11-12,26H,3-4,9-10H2,1-2H3,(H2,25,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
| Assay Description
Demethylase reactions and AlphaScreen assays were performed as described (Sayegh et al., 2013), with a few exceptions. All demethylase buffers contai... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080087
(3-sec-Butoxy-4-(4-methanesulfonyl-phenyl)-5,5-dime...)Show SMILES CCC(C)OC1=C(c2ccc(cc2)S(C)(=O)=O)C(C)(C)OC1=O |c:5| Show InChI InChI=1S/C17H22O5S/c1-6-11(2)21-15-14(17(3,4)22-16(15)18)12-7-9-13(10-8-12)23(5,19)20/h7-11H,6H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080083

(3-Cyclohexyloxy-4-(4-methanesulfonyl-phenyl)-5,5-d...)Show SMILES CC1(C)OC(=O)C(OC2CCCCC2)=C1c1ccc(cc1)S(C)(=O)=O |c:14| Show InChI InChI=1S/C19H24O5S/c1-19(2)16(13-9-11-15(12-10-13)25(3,21)22)17(18(20)24-19)23-14-7-5-4-6-8-14/h9-12,14H,4-8H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50261966
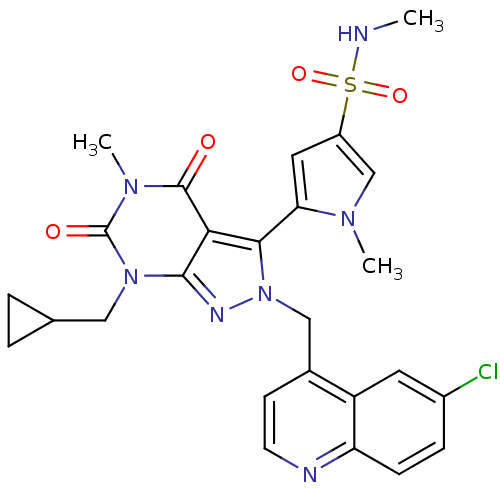
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES CNS(=O)(=O)c1cc(-c2n(Cc3ccnc4ccc(Cl)cc34)nc3n(CC4CC4)c(=O)n(C)c(=O)c23)n(C)c1 |(3.07,6.21,;3.08,4.67,;1.76,3.89,;.91,5.17,;2.61,2.6,;.51,2.98,;.52,1.44,;-.95,.96,;-1.42,-.51,;-.51,-1.76,;1.03,-1.76,;1.8,-3.1,;1.03,-4.43,;1.8,-5.76,;3.34,-5.76,;4.11,-4.42,;5.64,-4.41,;6.4,-3.08,;5.62,-1.75,;6.38,-.41,;4.09,-1.76,;3.34,-3.09,;-1.42,-3.02,;-2.9,-2.54,;-4.24,-3.3,;-4.24,-4.85,;-5.58,-5.61,;-7.11,-5.62,;-6.35,-6.95,;-5.57,-2.53,;-6.91,-3.3,;-5.57,-.98,;-6.91,-.22,;-4.24,-.21,;-4.24,1.34,;-2.9,-.98,;-1.85,2.2,;-3.39,2.2,;-.95,3.45,)| Show InChI InChI=1S/C26H26ClN7O4S/c1-28-39(37,38)18-11-21(31(2)14-18)23-22-24(33(12-15-4-5-15)26(36)32(3)25(22)35)30-34(23)13-16-8-9-29-20-7-6-17(27)10-19(16)20/h6-11,14-15,28H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
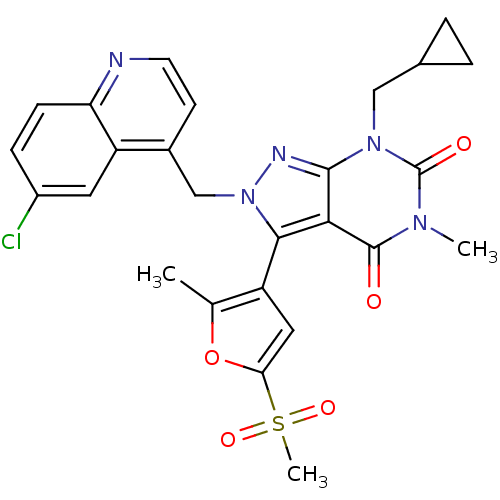
(Helicobacter pylori) | BDBM50261912
(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylme...)Show SMILES Cc1oc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.85,-28.84,;-2.31,-28.84,;-1.41,-27.59,;.05,-28.06,;.05,-29.6,;-1.41,-30.08,;-1.88,-31.55,;-.97,-32.8,;.57,-32.8,;1.34,-34.14,;.57,-35.47,;1.34,-36.8,;2.88,-36.8,;3.65,-35.46,;5.18,-35.45,;5.94,-34.12,;5.16,-32.79,;5.92,-31.45,;3.63,-32.8,;2.88,-34.13,;-1.88,-34.06,;-3.36,-33.58,;-4.7,-34.34,;-4.7,-35.89,;-6.04,-36.65,;-7.58,-36.66,;-6.81,-37.99,;-6.04,-33.57,;-7.37,-34.34,;-6.04,-32.02,;-7.37,-31.26,;-4.7,-31.25,;-4.7,-29.7,;-3.36,-32.02,;1.29,-27.15,;2.62,-26.37,;.45,-25.87,;2.14,-28.44,)| Show InChI InChI=1S/C26H24ClN5O5S/c1-14-18(11-21(37-14)38(3,35)36)23-22-24(31(12-15-4-5-15)26(34)30(2)25(22)33)29-32(23)13-16-8-9-28-20-7-6-17(27)10-19(16)20/h6-11,15H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262013

(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES CONS(=O)(=O)c1cc(-c2n(Cc3ccnc4ccc(Cl)cc34)nc3n(CC4CC4)c(=O)n(C)c(=O)c23)n(C)c1 |(24.98,-9.2,;26.32,-9.96,;26.33,-11.5,;25,-12.28,;24.16,-10.99,;25.85,-13.57,;23.76,-13.19,;23.76,-14.73,;22.3,-15.21,;21.83,-16.67,;22.74,-17.93,;24.28,-17.93,;25.05,-19.26,;24.28,-20.59,;25.05,-21.93,;26.59,-21.93,;27.36,-20.58,;28.89,-20.58,;29.65,-19.24,;28.87,-17.92,;29.63,-16.58,;27.34,-17.93,;26.59,-19.26,;21.83,-19.18,;20.35,-18.7,;19.01,-19.47,;19.01,-21.01,;17.67,-21.78,;16.13,-21.78,;16.9,-23.12,;17.67,-18.7,;16.34,-19.47,;17.67,-17.15,;16.34,-16.38,;19.01,-16.37,;19.01,-14.83,;20.35,-17.15,;21.4,-13.96,;19.86,-13.97,;22.3,-12.72,)| Show InChI InChI=1S/C26H26ClN7O5S/c1-31-14-18(40(37,38)30-39-3)11-21(31)23-22-24(33(12-15-4-5-15)26(36)32(2)25(22)35)29-34(23)13-16-8-9-28-20-7-6-17(27)10-19(16)20/h6-11,14-15,30H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50261913

(2-((5-chloro-1-methyl-1H-indol-3-yl)methyl)-7-(cyc...)Show SMILES Cc1oc(cc1-c1n(Cc2cn(C)c3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(19.66,-27.53,;21.2,-27.53,;22.1,-26.29,;23.56,-26.76,;23.57,-28.29,;22.1,-28.77,;21.63,-30.24,;22.54,-31.49,;24.08,-31.49,;24.85,-32.82,;24.23,-34.23,;25.38,-35.26,;25.22,-36.79,;26.71,-34.49,;28.16,-34.96,;29.31,-33.93,;28.98,-32.43,;30.12,-31.39,;27.52,-31.96,;26.39,-32.98,;21.63,-32.74,;20.16,-32.27,;18.81,-33.03,;18.81,-34.57,;17.47,-35.34,;15.94,-35.34,;16.71,-36.68,;17.48,-32.26,;16.15,-33.03,;17.48,-30.71,;16.14,-29.95,;18.81,-29.94,;18.81,-28.4,;20.16,-30.71,;24.8,-25.85,;26.13,-25.07,;23.96,-24.56,;25.65,-27.13,)| Show InChI InChI=1S/C26H26ClN5O5S/c1-14-18(10-21(37-14)38(4,35)36)23-22-24(31(11-15-5-6-15)26(34)30(3)25(22)33)28-32(23)13-16-12-29(2)20-8-7-17(27)9-19(16)20/h7-10,12,15H,5-6,11,13H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Glutamate racemase
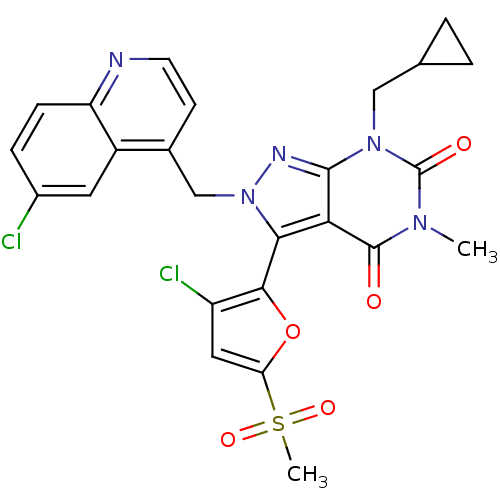
(Helicobacter pylori) | BDBM50261911
(3-(3-chloro-5-(methylsulfonyl)furan-2-yl)-2-((6-ch...)Show SMILES Cn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3oc(cc3Cl)S(C)(=O)=O)c2c1=O |(15.9,-13.52,;17.24,-14.28,;17.24,-15.83,;15.9,-16.6,;18.57,-16.6,;18.57,-18.15,;17.23,-18.91,;15.7,-18.92,;16.46,-20.25,;19.92,-15.84,;21.39,-16.31,;22.3,-15.06,;23.85,-15.06,;24.62,-16.39,;23.84,-17.73,;24.61,-19.06,;26.16,-19.06,;26.92,-17.71,;28.46,-17.71,;29.22,-16.38,;28.44,-15.05,;29.2,-13.71,;26.91,-15.06,;26.15,-16.39,;21.39,-13.8,;21.86,-12.34,;23.33,-11.86,;23.32,-10.32,;21.86,-9.85,;20.96,-11.1,;19.42,-11.1,;24.57,-9.41,;25.89,-8.63,;23.72,-8.12,;25.42,-10.7,;19.92,-14.28,;18.57,-13.5,;18.57,-11.96,)| Show InChI InChI=1S/C25H21Cl2N5O5S/c1-30-24(33)20-21(22-17(27)10-19(37-22)38(2,35)36)32(29-23(20)31(25(30)34)11-13-3-4-13)12-14-7-8-28-18-6-5-15(26)9-16(14)18/h5-10,13H,3-4,11-12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
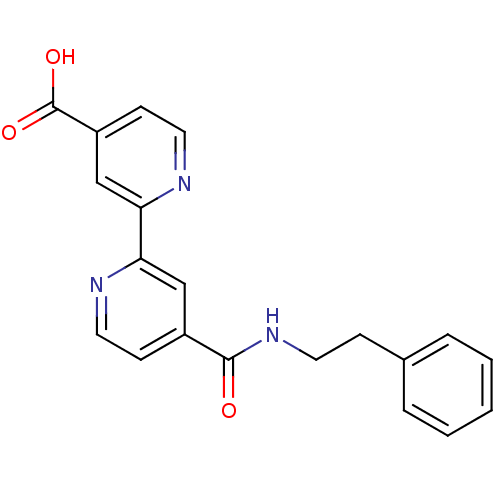
(Homo sapiens (Human)) | BDBM50396019
(4'-(phenethylcarbamoyl)-[2,2'-bipyridine]-...)Show SMILES OC(=O)c1ccnc(c1)-c1cc(ccn1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-9-6-14-4-2-1-3-5-14)15-7-10-21-17(12-15)18-13-16(20(25)26)8-11-22-18/h1-5,7-8,10-13H,6,9H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191597
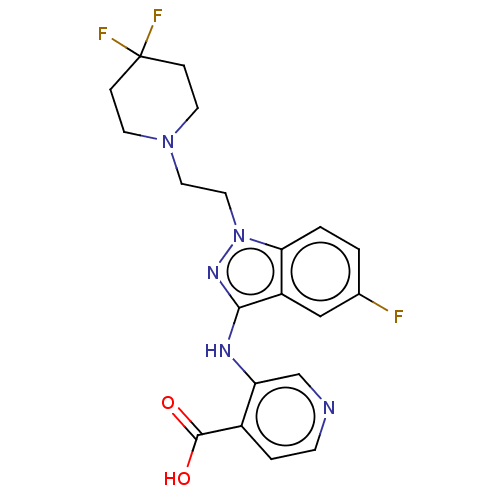
(3-((1-(2-(4,4-difluoropiperidin-1-yl)ethyl)-5-fluo...)Show SMILES OC(=O)c1ccncc1Nc1nn(CCN2CCC(F)(F)CC2)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N5O2/c21-13-1-2-17-15(11-13)18(25-16-12-24-6-3-14(16)19(29)30)26-28(17)10-9-27-7-4-20(22,23)5-8-27/h1-3,6,11-12H,4-5,7-10H2,(H,25,26)(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191597

(3-((1-(2-(4,4-difluoropiperidin-1-yl)ethyl)-5-fluo...)Show SMILES OC(=O)c1ccncc1Nc1nn(CCN2CCC(F)(F)CC2)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N5O2/c21-13-1-2-17-15(11-13)18(25-16-12-24-6-3-14(16)19(29)30)26-28(17)10-9-27-7-4-20(22,23)5-8-27/h1-3,6,11-12H,4-5,7-10H2,(H,25,26)(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080080

(3-(1-Cyclopropyl-ethoxy)-4-(4-methanesulfonyl-phen...)Show SMILES CC(OC1=C(c2ccc(cc2)S(C)(=O)=O)C(C)(C)OC1=O)C1CC1 |c:3| Show InChI InChI=1S/C18H22O5S/c1-11(12-5-6-12)22-16-15(18(2,3)23-17(16)19)13-7-9-14(10-8-13)24(4,20)21/h7-12H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50423389
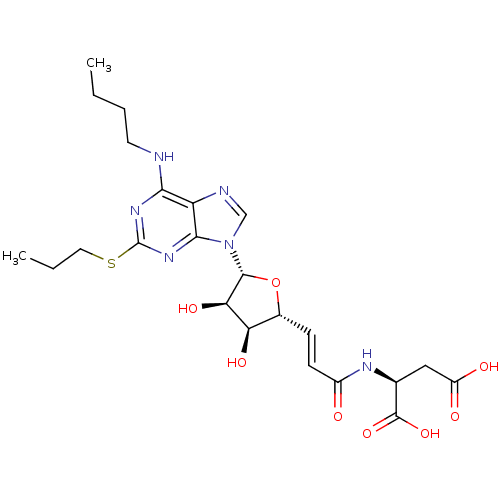
(CHEMBL250832)Show SMILES CCCCNc1nc(SCCC)nc2n(cnc12)[C@@H]1O[C@H](\C=C\C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C23H32N6O8S/c1-3-5-8-24-19-16-20(28-23(27-19)38-9-4-2)29(11-25-16)21-18(34)17(33)13(37-21)6-7-14(30)26-12(22(35)36)10-15(31)32/h6-7,11-13,17-18,21,33-34H,3-5,8-10H2,1-2H3,(H,26,30)(H,31,32)(H,35,36)(H,24,27,28)/b7-6+/t12-,13+,17+,18+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation |
Bioorg Med Chem Lett 17: 6013-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.057
BindingDB Entry DOI: 10.7270/Q2JD4Z2Q |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
| Assay Description
Demethylase reactions and AlphaScreen assays were performed as described (Sayegh et al., 2013), with a few exceptions. All demethylase buffers contai... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191596
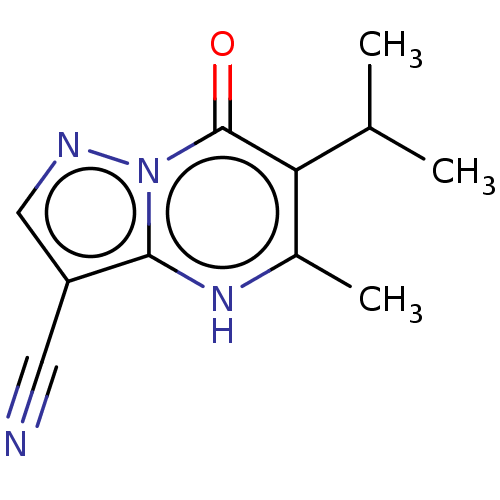
(6-isopropyl-5-methyl-7-oxo-4,7-dihydropyrazolo[1,5...)Show InChI InChI=1S/C11H12N4O/c1-6(2)9-7(3)14-10-8(4-12)5-13-15(10)11(9)16/h5-6,14H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50396019

(4'-(phenethylcarbamoyl)-[2,2'-bipyridine]-...)Show SMILES OC(=O)c1ccnc(c1)-c1cc(ccn1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-9-6-14-4-2-1-3-5-14)15-7-10-21-17(12-15)18-13-16(20(25)26)8-11-22-18/h1-5,7-8,10-13H,6,9H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM50396019

(4'-(phenethylcarbamoyl)-[2,2'-bipyridine]-...)Show SMILES OC(=O)c1ccnc(c1)-c1cc(ccn1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-9-6-14-4-2-1-3-5-14)15-7-10-21-17(12-15)18-13-16(20(25)26)8-11-22-18/h1-5,7-8,10-13H,6,9H2,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080082

(3-Cyclopropylmethoxy-4-(4-methanesulfonyl-phenyl)-...)Show SMILES CC1(C)OC(=O)C(OCC2CC2)=C1c1ccc(cc1)S(C)(=O)=O |c:12| Show InChI InChI=1S/C17H20O5S/c1-17(2)14(12-6-8-13(9-7-12)23(3,19)20)15(16(18)22-17)21-10-11-4-5-11/h6-9,11H,4-5,10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori) | BDBM50262059
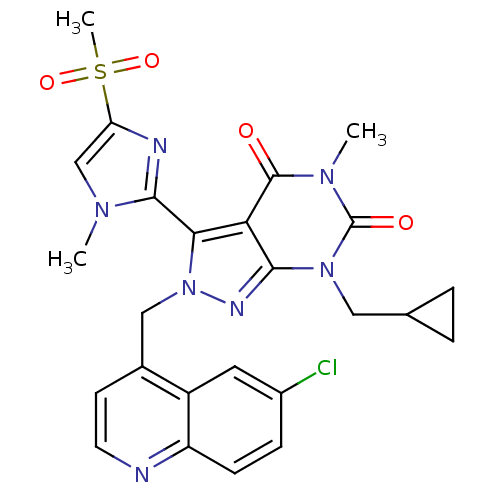
(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylme...)Show SMILES Cn1cc(nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.71,-29.79,;-2.17,-29.79,;-1.27,-28.54,;.2,-29.01,;.2,-30.55,;-1.27,-31.03,;-1.74,-32.49,;-.82,-33.75,;.72,-33.75,;1.49,-35.08,;.72,-36.41,;1.48,-37.75,;3.03,-37.75,;3.79,-36.4,;5.33,-36.4,;6.09,-35.07,;5.31,-33.74,;6.07,-32.4,;3.78,-33.75,;3.02,-35.08,;-1.74,-35,;-3.21,-34.52,;-4.56,-35.29,;-4.56,-36.83,;-5.9,-37.6,;-7.43,-37.61,;-6.66,-38.94,;-5.89,-34.52,;-7.23,-35.29,;-5.89,-32.97,;-7.23,-32.21,;-4.56,-32.19,;-4.56,-30.65,;-3.21,-32.97,;1.44,-28.1,;2.68,-27.19,;.59,-26.81,;2.29,-29.39,)| Show InChI InChI=1S/C25H24ClN7O4S/c1-30-13-19(38(3,36)37)28-23(30)21-20-22(32(11-14-4-5-14)25(35)31(2)24(20)34)29-33(21)12-15-8-9-27-18-7-6-16(26)10-17(15)18/h6-10,13-14H,4-5,11-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori MurI |
Bioorg Med Chem Lett 18: 4716-22 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.092
BindingDB Entry DOI: 10.7270/Q2N29WSP |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191598

(2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...)Show InChI InChI=1S/C15H24N4O3/c1-4-19(8-7-18(2)3)14(20)11-16-10-13-9-12(15(21)22)5-6-17-13/h5-6,9,16H,4,7-8,10-11H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
| Assay Description
Demethylase reactions and AlphaScreen assays were performed as described (Sayegh et al., 2013), with a few exceptions. All demethylase buffers contai... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191600
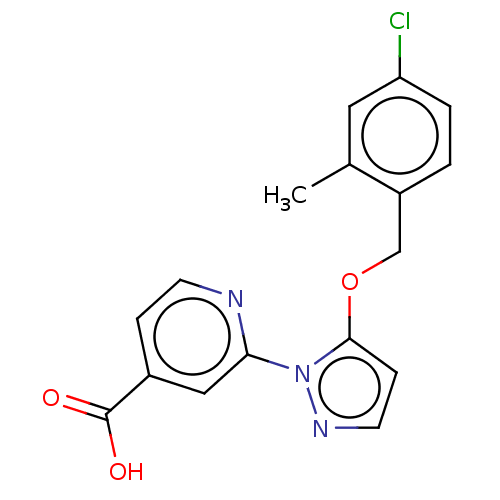
(2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl...)Show InChI InChI=1S/C17H14ClN3O3/c1-11-8-14(18)3-2-13(11)10-24-16-5-7-20-21(16)15-9-12(17(22)23)4-6-19-15/h2-9H,10H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM191601
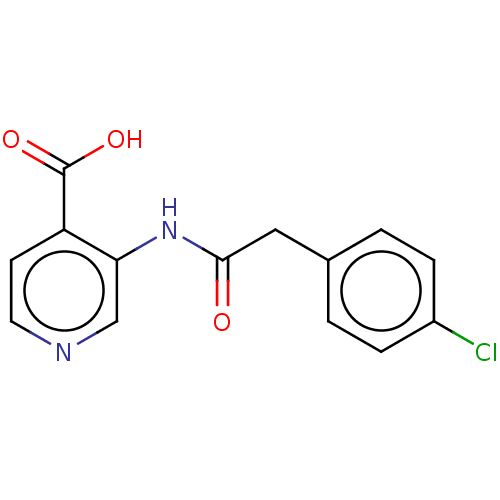
(3-(2-(4-chlorophenyl)acetamido)isonicotinic acid (...)Show InChI InChI=1S/C14H11ClN2O3/c15-10-3-1-9(2-4-10)7-13(18)17-12-8-16-6-5-11(12)14(19)20/h1-6,8H,7H2,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5C
(Homo sapiens (Human)) | BDBM191597

(3-((1-(2-(4,4-difluoropiperidin-1-yl)ethyl)-5-fluo...)Show SMILES OC(=O)c1ccncc1Nc1nn(CCN2CCC(F)(F)CC2)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N5O2/c21-13-1-2-17-15(11-13)18(25-16-12-24-6-3-14(16)19(29)30)26-28(17)10-9-27-7-4-20(22,23)5-8-27/h1-3,6,11-12H,4-5,7-10H2,(H,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191600

(2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl...)Show InChI InChI=1S/C17H14ClN3O3/c1-11-8-14(18)3-2-13(11)10-24-16-5-7-20-21(16)15-9-12(17(22)23)4-6-19-15/h2-9H,10H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080081
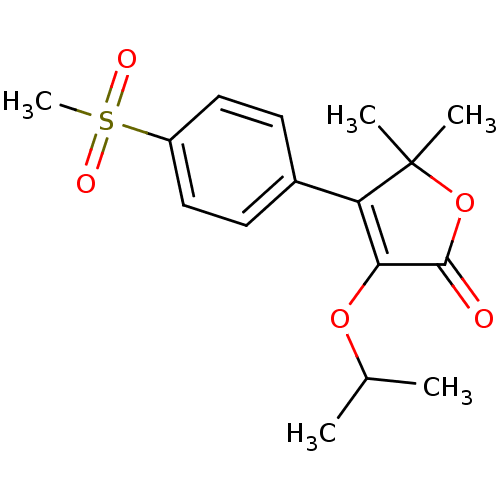
(3-Isopropoxy-4-(4-methanesulfonyl-phenyl)-5,5-dime...)Show SMILES CC(C)OC1=C(c2ccc(cc2)S(C)(=O)=O)C(C)(C)OC1=O |c:4| Show InChI InChI=1S/C16H20O5S/c1-10(2)20-14-13(16(3,4)21-15(14)17)11-6-8-12(9-7-11)22(5,18)19/h6-10H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 in human whole blood |
Bioorg Med Chem Lett 9: 2207-12 (1999)
BindingDB Entry DOI: 10.7270/Q2416W8T |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191601

(3-(2-(4-chlorophenyl)acetamido)isonicotinic acid (...)Show InChI InChI=1S/C14H11ClN2O3/c15-10-3-1-9(2-4-10)7-13(18)17-12-8-16-6-5-11(12)14(19)20/h1-6,8H,7H2,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50080081

(3-Isopropoxy-4-(4-methanesulfonyl-phenyl)-5,5-dime...)Show SMILES CC(C)OC1=C(c2ccc(cc2)S(C)(=O)=O)C(C)(C)OC1=O |c:4| Show InChI InChI=1S/C16H20O5S/c1-10(2)20-14-13(16(3,4)21-15(14)17)11-6-8-12(9-7-11)22(5,18)19/h6-10H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was determined against Prostaglandin G/H synthase 2 in human whole blood assay |
Bioorg Med Chem Lett 12: 3317-20 (2002)
BindingDB Entry DOI: 10.7270/Q2FQ9VZ6 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A [1-1090]
(Homo sapiens (Human)) | BDBM191599
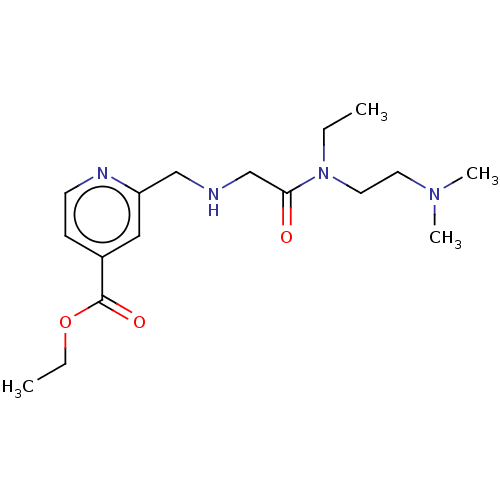
(KDOAM-21 | ethyl 2-(((2-((2-(dimethylamino)ethyl)(...)Show InChI InChI=1S/C17H28N4O3/c1-5-21(10-9-20(3)4)16(22)13-18-12-15-11-14(7-8-19-15)17(23)24-6-2/h7-8,11,18H,5-6,9-10,12-13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Emory University
| Assay Description
Reactions were performed in 50 mM HEPES [pH 7.2 for KDM5 or pH 7.5 for KDM4A(1-350)] containing 0.01% BSA and 0.01% Tween-20. To each well, 3 μL... |
Cell Chem Biol 23: 769-81 (2016)
Article DOI: 10.1016/j.chembiol.2016.06.006
BindingDB Entry DOI: 10.7270/Q2BZ64VH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data