 Found 1821 hits with Last Name = 'yuan' and Initial = 's'
Found 1821 hits with Last Name = 'yuan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053
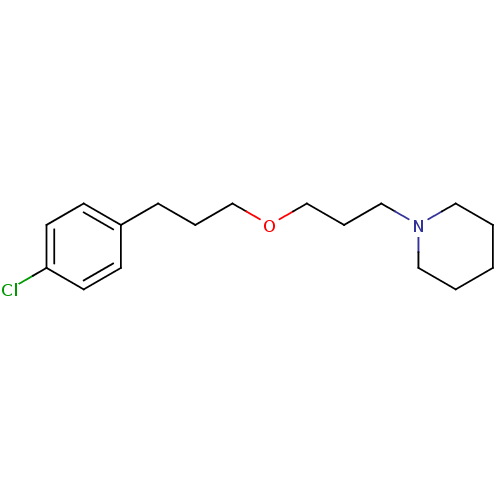
(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human H3 receptor expressed in CHO-K1 cell membranes incubated for 60 mins by [35S]GTPgammaS binding assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50556205

(BAY 1841788 | BAY-1841788 | BAY1841788 | Darolutam...)Show SMILES C[C@@H](Cn1ccc(n1)-c1ccc(C#N)c(Cl)c1)NC(=O)c1cc([nH]n1)C(C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051436
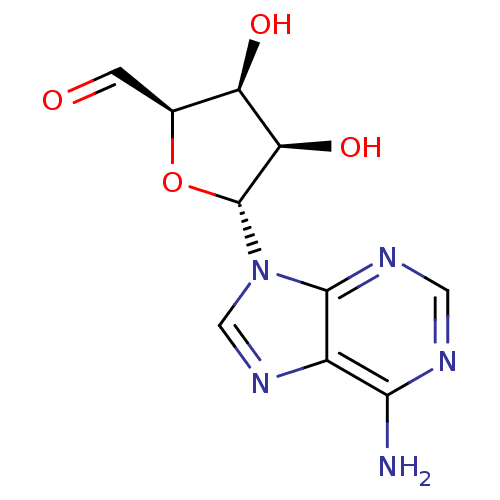
((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435
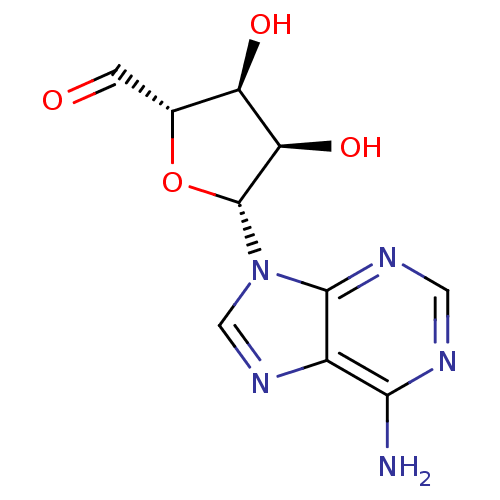
(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051436

((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732
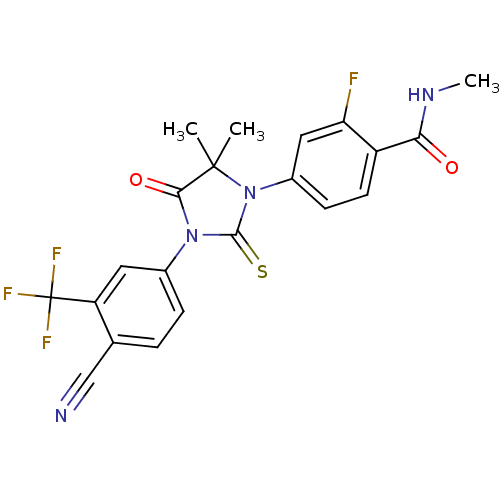
(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50094975

(956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C11CCC1)c1cnc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H15F4N5O2S/c1-27-17(31)13-4-3-11(8-15(13)22)30-19(33)29(18(32)20(30)5-2-6-20)12-7-14(21(23,24)25)16(9-26)28-10-12/h3-4,7-8,10H,2,5-6H2,1H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112667
BindingDB Entry DOI: 10.7270/Q2HD80BX |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369258
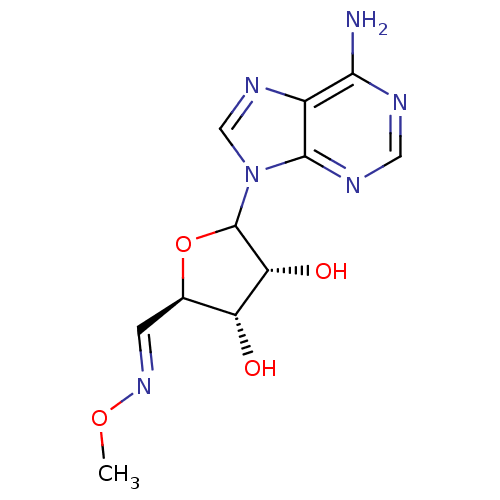
(CHEMBL606276)Show SMILES CO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-20-16-2-5-7(18)8(19)11(21-5)17-4-15-6-9(12)13-3-14-10(6)17/h2-5,7-8,11,18-19H,1H3,(H2,12,13,14)/b16-2+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50368896
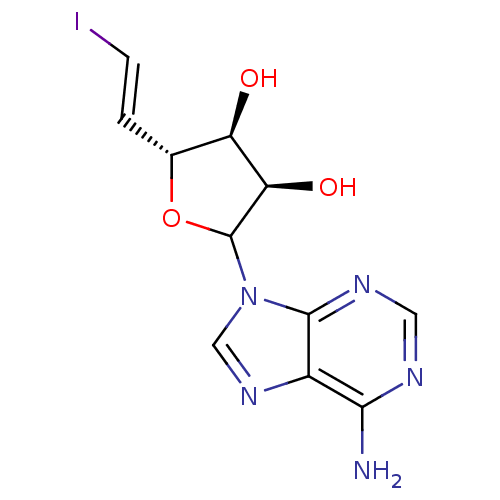
(CHEMBL608056)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C\I)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12IN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369257
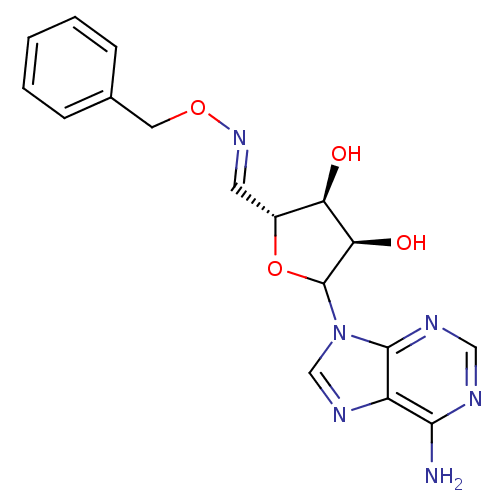
(CHEMBL605902)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=N\OCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H18N6O4/c18-15-12-16(20-8-19-15)23(9-21-12)17-14(25)13(24)11(27-17)6-22-26-7-10-4-2-1-3-5-10/h1-6,8-9,11,13-14,17,24-25H,7H2,(H2,18,19,20)/b22-6+/t11-,13-,14-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407233
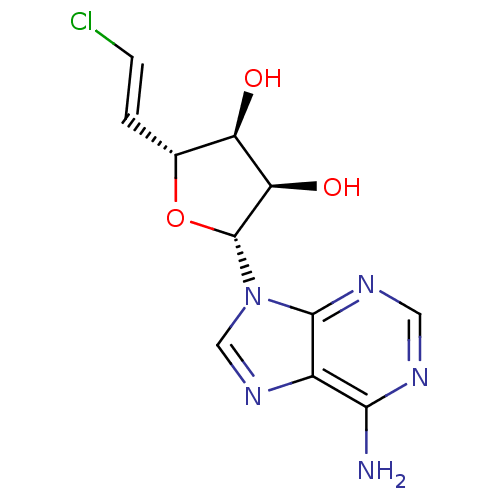
(CHEMBL2092790)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Cl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12ClN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369255

(CHEMBL605900)Show SMILES CCO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H16N6O4/c1-2-21-17-3-6-8(19)9(20)12(22-6)18-5-16-7-10(13)14-4-15-11(7)18/h3-6,8-9,12,19-20H,2H2,1H3,(H2,13,14,15)/b17-3+/t6-,8-,9-,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407232

(CHEMBL2092789)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Br)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12BrN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50408149
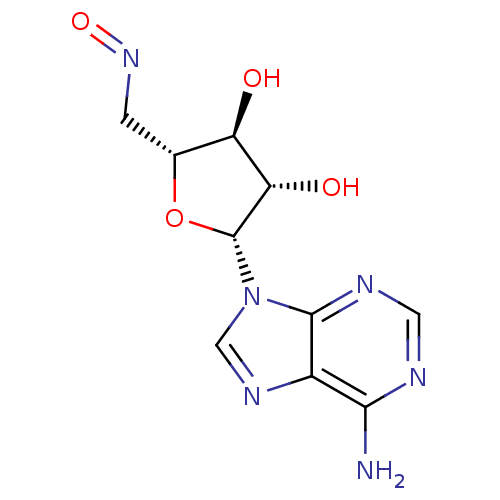
(CHEMBL2093112)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CN=O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C10H12N6O4/c11-8-5-9(13-2-12-8)16(3-14-5)10-7(18)6(17)4(20-10)1-15-19/h2-4,6-7,10,17-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051438

((1R,2R,3S,4R)-4-(6-Amino-purin-9-yl)-2,3-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h2-6,8-9,18-19H,1H2,(H2,12,13,14)/t5-,6+,8+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369159
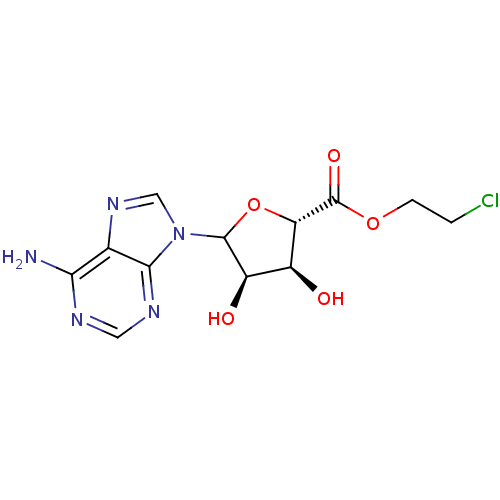
(CHEMBL610148)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCCCl |r| Show InChI InChI=1S/C12H14ClN5O5/c13-1-2-22-12(21)8-6(19)7(20)11(23-8)18-4-17-5-9(14)15-3-16-10(5)18/h3-4,6-8,11,19-20H,1-2H2,(H2,14,15,16)/t6-,7+,8-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369158
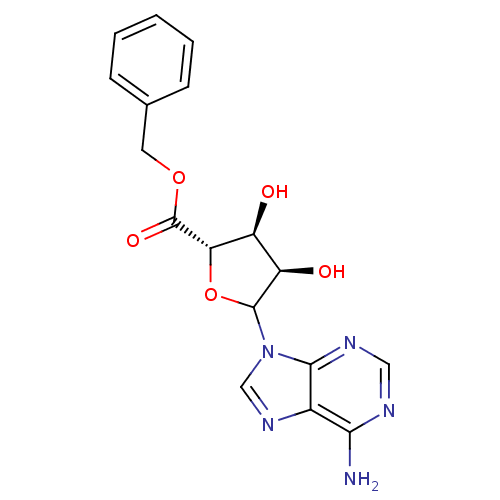
(CHEMBL612224)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C17H17N5O5/c18-14-10-15(20-7-19-14)22(8-21-10)16-12(24)11(23)13(27-16)17(25)26-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,16,23-24H,6H2,(H2,18,19,20)/t11-,12+,13-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50331791
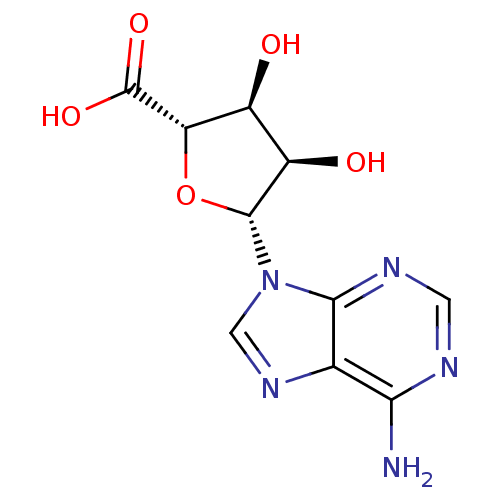
((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(O)=O |r| Show InChI InChI=1S/C10H11N5O5/c11-7-3-8(13-1-12-7)15(2-14-3)9-5(17)4(16)6(20-9)10(18)19/h1-2,4-6,9,16-17H,(H,18,19)(H2,11,12,13)/t4-,5+,6-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369156
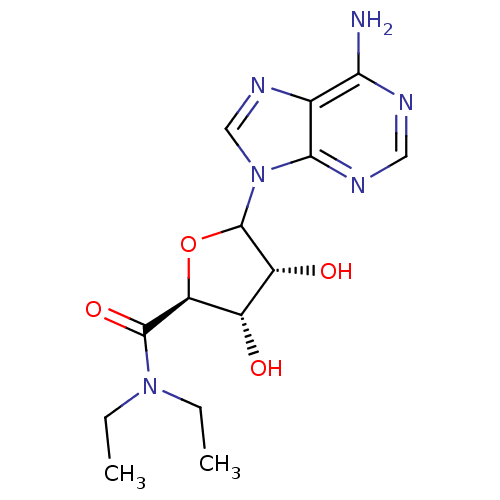
(CHEMBL608072)Show SMILES CCN(CC)C(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-3-19(4-2)13(23)10-8(21)9(22)14(24-10)20-6-18-7-11(15)16-5-17-12(7)20/h5-6,8-10,14,21-22H,3-4H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369161
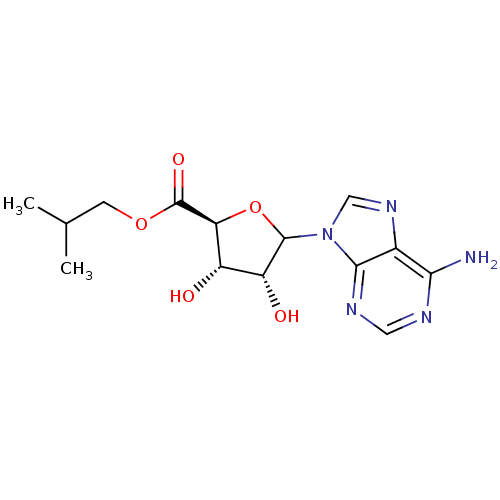
(CHEMBL610383)Show SMILES CC(C)COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-6(2)3-23-14(22)10-8(20)9(21)13(24-10)19-5-18-7-11(15)16-4-17-12(7)19/h4-6,8-10,13,20-21H,3H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50408148
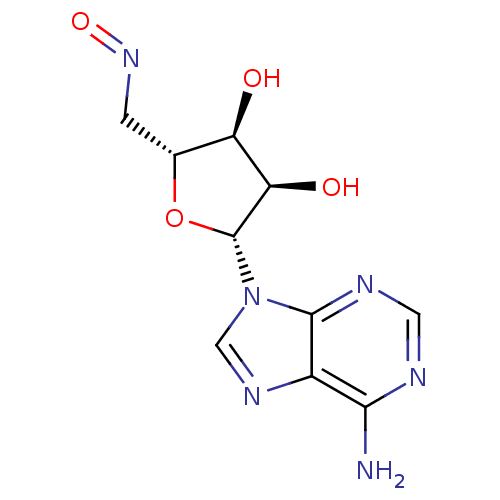
(CHEMBL1288616)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CN=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12N6O4/c11-8-5-9(13-2-12-8)16(3-14-5)10-7(18)6(17)4(20-10)1-15-19/h2-4,6-7,10,17-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50368898
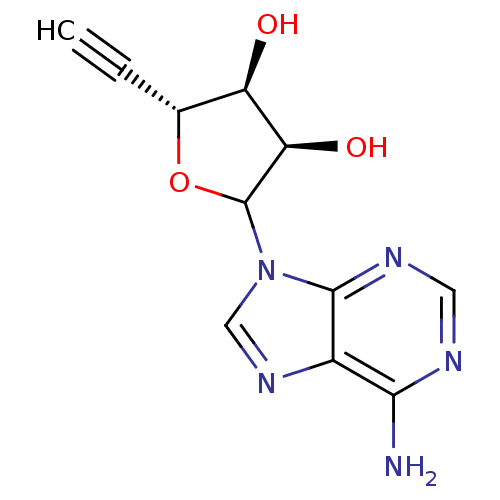
(CHEMBL604208)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H11N5O3/c1-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h1,3-5,7-8,11,17-18H,(H2,12,13,14)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 681 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369162
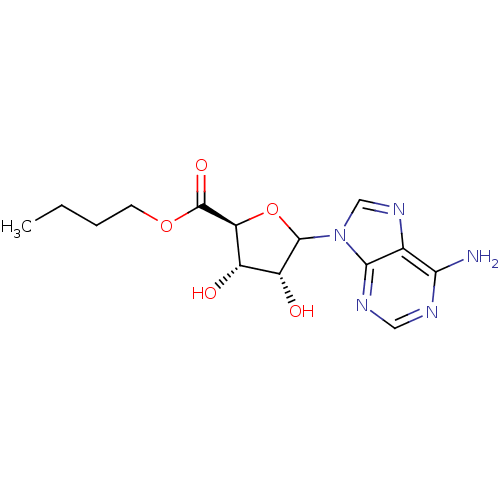
(CHEMBL610384)Show SMILES CCCCOC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-2-3-4-23-14(22)10-8(20)9(21)13(24-10)19-6-18-7-11(15)16-5-17-12(7)19/h5-6,8-10,13,20-21H,2-4H2,1H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369157
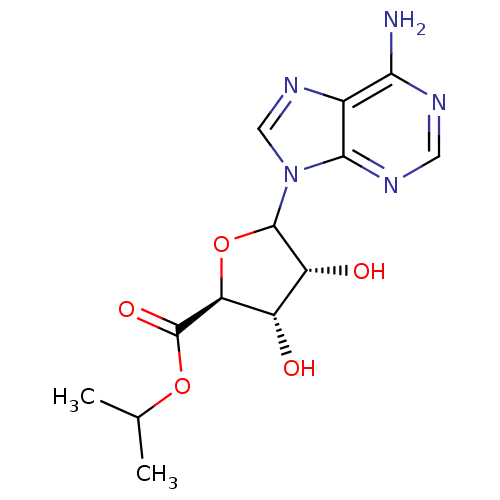
(CHEMBL608915)Show SMILES CC(C)OC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C13H17N5O5/c1-5(2)22-13(21)9-7(19)8(20)12(23-9)18-4-17-6-10(14)15-3-16-11(6)18/h3-5,7-9,12,19-20H,1-2H3,(H2,14,15,16)/t7-,8+,9-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369393
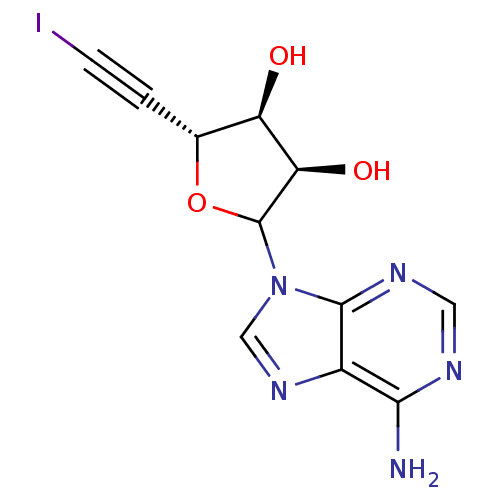
(CHEMBL608312)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CI)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10IN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50368895
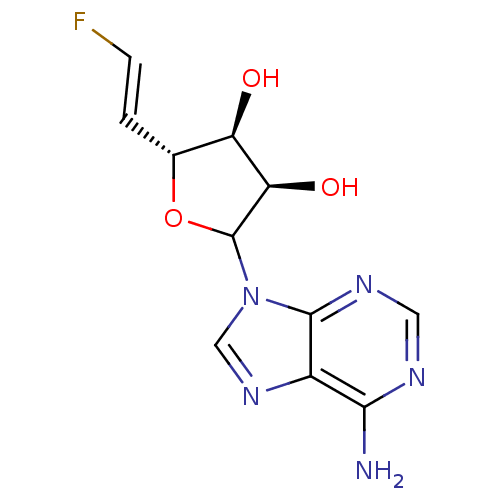
(CHEMBL610125)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C\F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12FN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369160
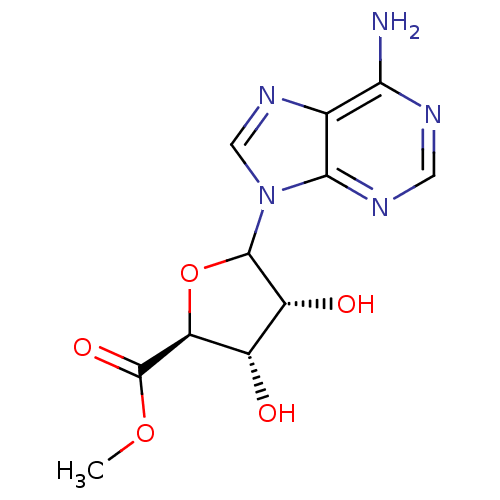
(CHEMBL608025)Show SMILES COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13N5O5/c1-20-11(19)7-5(17)6(18)10(21-7)16-3-15-4-8(12)13-2-14-9(4)16/h2-3,5-7,10,17-18H,1H3,(H2,12,13,14)/t5-,6+,7-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
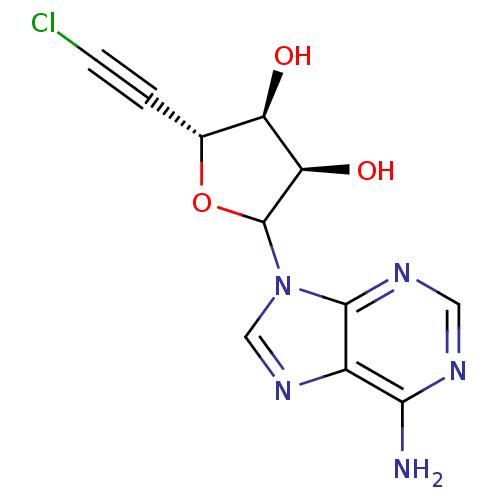
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369392
(CHEMBL608911)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10ClN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407975
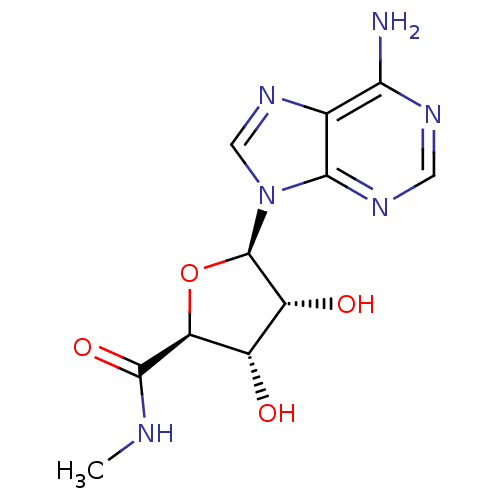
(CHEMBL519809)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-13-10(20)7-5(18)6(19)11(21-7)17-3-16-4-8(12)14-2-15-9(4)17/h2-3,5-7,11,18-19H,1H3,(H,13,20)(H2,12,14,15)/t5-,6+,7-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
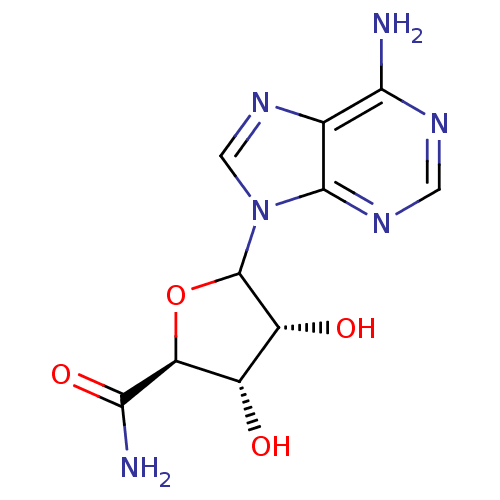
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367376
(CHEMBL605866)Show SMILES NC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C10H12N6O4/c11-7-3-9(14-1-13-7)16(2-15-3)10-5(18)4(17)6(20-10)8(12)19/h1-2,4-6,10,17-18H,(H2,12,19)(H2,11,13,14)/t4-,5+,6-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369380
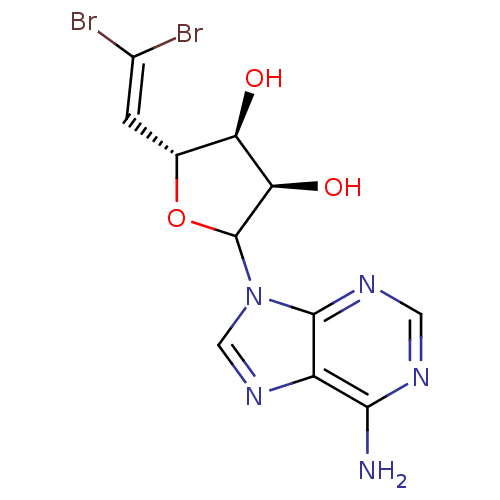
(CHEMBL606502)Show SMILES [#7]-c1ncnc2n(cnc12)-[#6]-1-[#8]-[#6@H](\[#6]=[#6](\Br)Br)-[#6@@H](-[#8])-[#6@H]-1-[#8] |r| Show InChI InChI=1S/C11H11Br2N5O3/c12-5(13)1-4-7(19)8(20)11(21-4)18-3-17-6-9(14)15-2-16-10(6)18/h1-4,7-8,11,19-20H,(H2,14,15,16)/t4-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369381

(CHEMBL612194)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(\Br)=C/Br |r| Show InChI InChI=1S/C11H11Br2N5O3/c12-1-4(13)8-6(19)7(20)11(21-8)18-3-17-5-9(14)15-2-16-10(5)18/h1-3,6-8,11,19-20H,(H2,14,15,16)/b4-1+/t6-,7+,8+,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369394

(CHEMBL608943)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CBr)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10BrN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
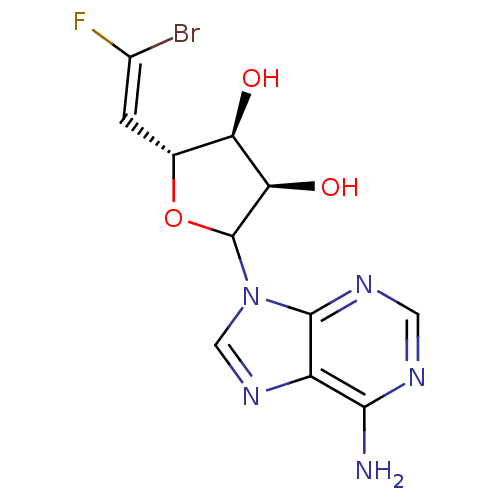
(Homo sapiens (Human)) | BDBM50369379
(CHEMBL607755)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C(\F)Br)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H11BrFN5O3/c12-5(13)1-4-7(19)8(20)11(21-4)18-3-17-6-9(14)15-2-16-10(6)18/h1-4,7-8,11,19-20H,(H2,14,15,16)/b5-1+/t4-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051437
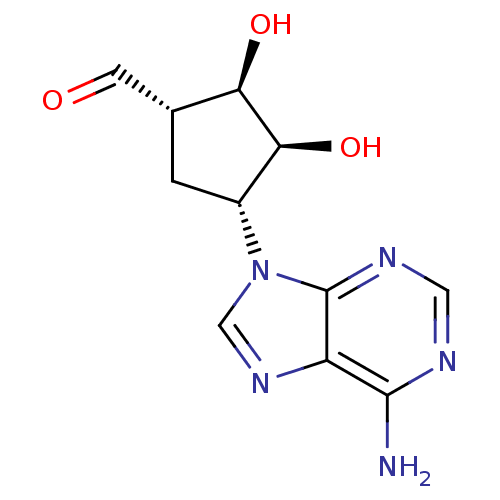
((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-2,3-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h2-6,8-9,18-19H,1H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase |
J Med Chem 39: 2347-53 (1996)
Article DOI: 10.1021/jm950916u
BindingDB Entry DOI: 10.7270/Q20Z72CP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369155
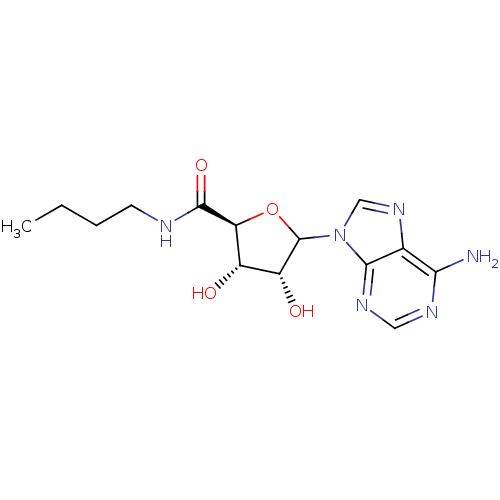
(CHEMBL609186)Show SMILES CCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-2-3-4-16-13(23)10-8(21)9(22)14(24-10)20-6-19-7-11(15)17-5-18-12(7)20/h5-6,8-10,14,21-22H,2-4H2,1H3,(H,16,23)(H2,15,17,18)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM85777
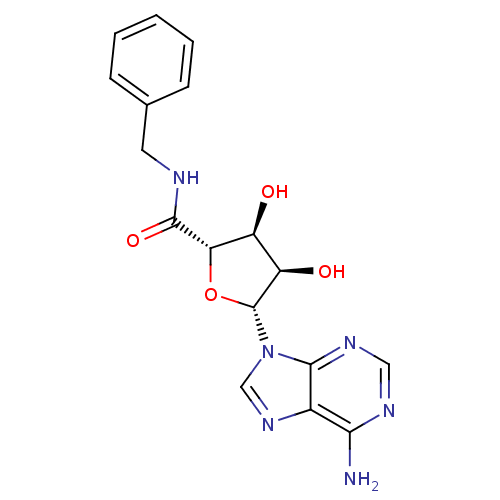
(B-NECA)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C17H18N6O4/c18-14-10-15(21-7-20-14)23(8-22-10)17-12(25)11(24)13(27-17)16(26)19-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,17,24-25H,6H2,(H,19,26)(H2,18,20,21)/t11-,12+,13-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369163
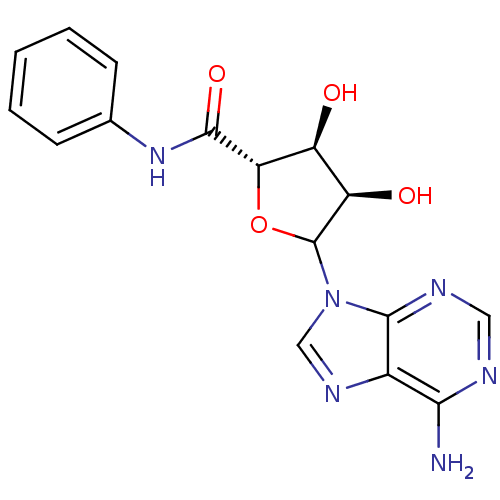
(CHEMBL610101)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C16H16N6O4/c17-13-9-14(19-6-18-13)22(7-20-9)16-11(24)10(23)12(26-16)15(25)21-8-4-2-1-3-5-8/h1-7,10-12,16,23-24H,(H,21,25)(H2,17,18,19)/t10-,11+,12-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81917
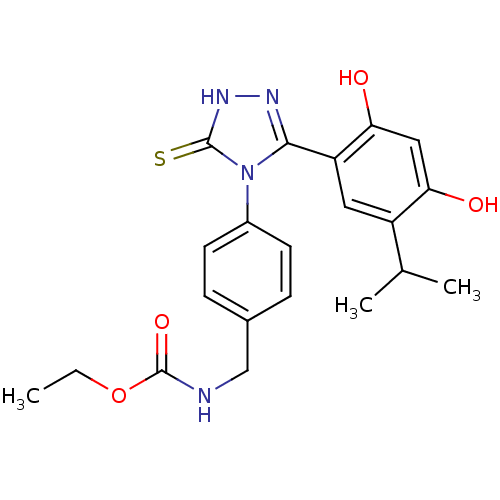
(BX-2819)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1cc(C(C)C)c(O)cc1O Show InChI InChI=1S/C21H24N4O4S/c1-4-29-21(28)22-11-13-5-7-14(8-6-13)25-19(23-24-20(25)30)16-9-15(12(2)3)17(26)10-18(16)27/h5-10,12,26-27H,4,11H2,1-3H3,(H,22,28)(H,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81914
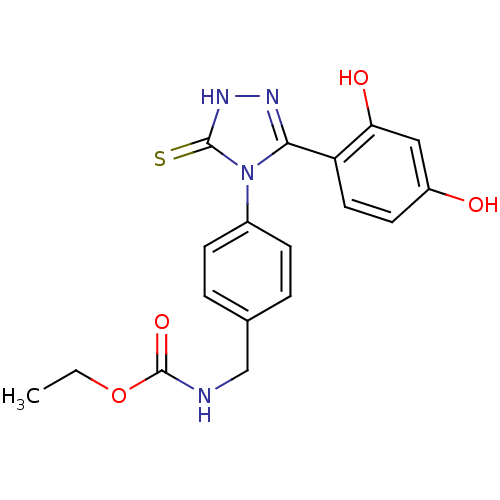
(Ethyl carbamate analog, 3)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(O)cc1O Show InChI InChI=1S/C18H18N4O4S/c1-2-26-18(25)19-10-11-3-5-12(6-4-11)22-16(20-21-17(22)27)14-8-7-13(23)9-15(14)24/h3-9,23-24H,2,10H2,1H3,(H,19,25)(H,21,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81916
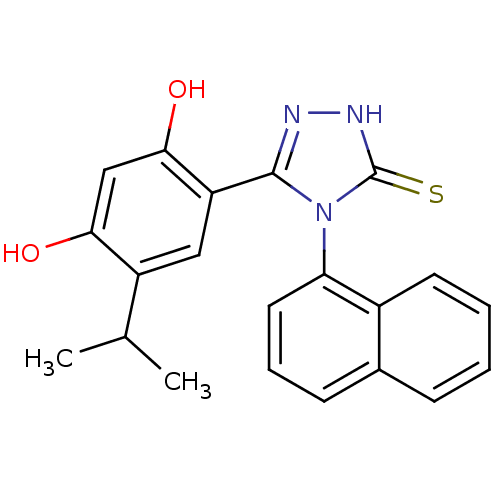
(lspropyl analog, 5)Show SMILES CC(C)c1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;.68,5.25,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C21H19N3O2S/c1-12(2)15-10-16(19(26)11-18(15)25)20-22-23-21(27)24(20)17-9-5-7-13-6-3-4-8-14(13)17/h3-12,25-26H,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50458169

(Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(Cc1ccc(cc1F)-c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81915
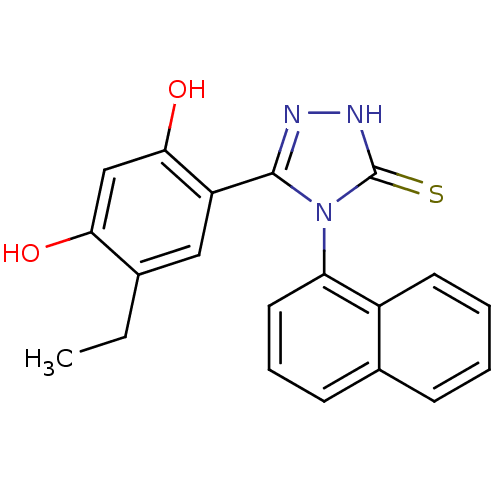
(Ethyl analog, 4)Show SMILES CCc1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C20H17N3O2S/c1-2-12-10-15(18(25)11-17(12)24)19-21-22-20(26)23(19)16-9-5-7-13-6-3-4-8-14(13)16/h3-11,24-25H,2H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492082

(CHEMBL2396959)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(c1)C(F)(F)F)c1c2cccc1Cl |r,THB:11:10:25.24:2.7.3| Show InChI InChI=1S/C19H13ClF3N3O2S/c20-14-6-2-5-12-16-8-15-13(9-24-25-15)18(17(12)14)26(16)29(27,28)11-4-1-3-10(7-11)19(21,22)23/h1-7,9,16,18H,8H2,(H,24,25)/t16-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492094
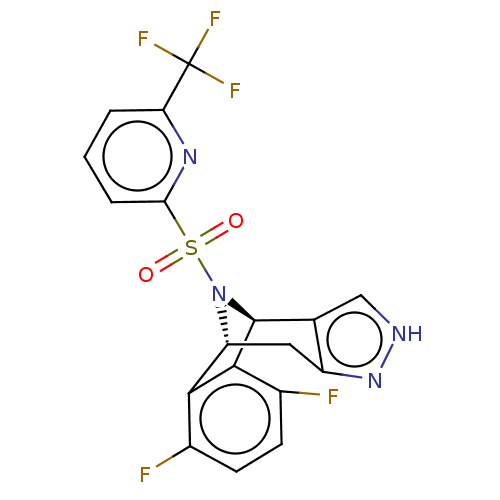
(CHEMBL2396964)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(n1)C(F)(F)F)c1c2c(F)ccc1F |r,THB:11:10:25.24:2.7.3| Show InChI InChI=1S/C18H11F5N4O2S/c19-9-4-5-10(20)16-15(9)12-6-11-8(7-24-26-11)17(16)27(12)30(28,29)14-3-1-2-13(25-14)18(21,22)23/h1-5,7,12,17H,6H2,(H,24,26)/t12-,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM549581
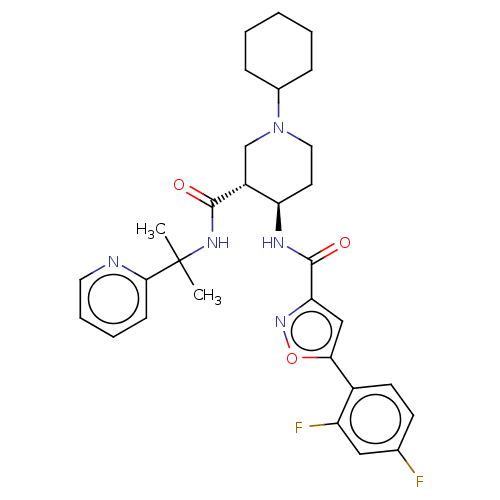
((3R*,4R*)-1-Cyclohexyl-4-{[5-(2,4-difluoro-phenyl)...)Show SMILES CC(C)(NC(=O)[C@@H]1CN(CC[C@H]1NC(=O)c1cc(on1)-c1ccc(F)cc1F)C1CCCCC1)c1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tango CXCR7-bla U2OS cells are detached from culture dishes with 0.05% trypsin-EDTA and collected in growing medium (McCoy's 5A 90% (v/v), dialyz... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S185P5 |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM549988

((3S,4S)-1-Cyclopropylmethyl-4-{[5-(2,4-difluoro-ph...)Show SMILES CC(C)(NC(=O)[C@H]1CN(CC2CC2)CC[C@@H]1NC(=O)c1cc(on1)-c1ccc(F)cc1F)c1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tango CXCR7-bla U2OS cells are detached from culture dishes with 0.05% trypsin-EDTA and collected in growing medium (McCoy's 5A 90% (v/v), dialyz... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S185P5 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81912
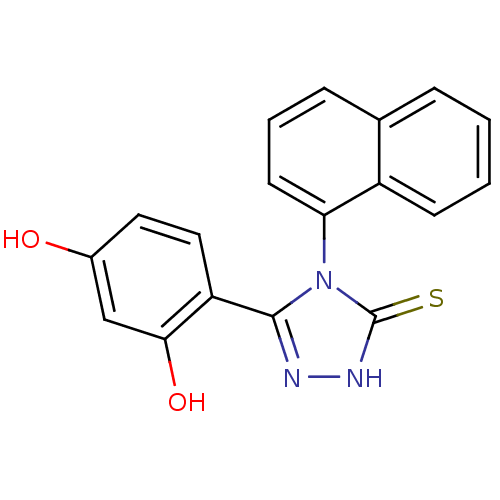
(DC23 | Resorcinol analog, 1)Show SMILES Oc1ccc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)c1 |(.68,.63,;2.01,1.4,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.06,;4.46,10.84,;5.8,10.07,;5.8,8.52,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,)| Show InChI InChI=1S/C18H13N3O2S/c22-12-8-9-14(16(23)10-12)17-19-20-18(24)21(17)15-7-3-5-11-4-1-2-6-13(11)15/h1-10,22-23H,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data