 Found 307 hits of ki for UniProtKB: P00491
Found 307 hits of ki for UniProtKB: P00491 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293089
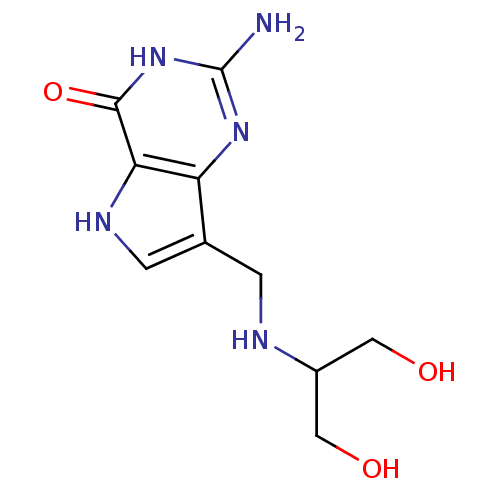
(2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...)Show InChI InChI=1S/C10H15N5O3/c11-10-14-7-5(1-12-6(3-16)4-17)2-13-8(7)9(18)15-10/h2,6,12-13,16-17H,1,3-4H2,(H3,11,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293090
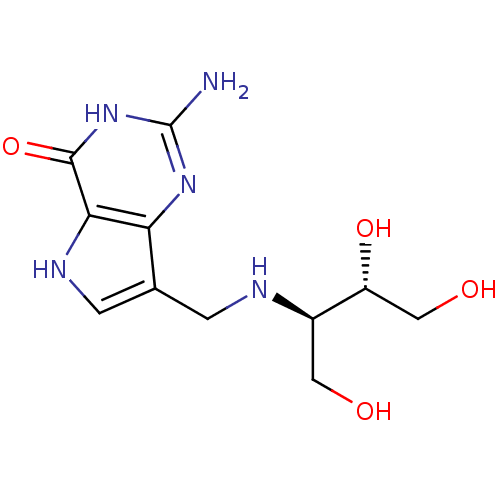
(2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...)Show SMILES Nc1nc2c(CN[C@H](CO)[C@H](O)CO)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C11H17N5O4/c12-11-15-8-5(2-14-9(8)10(20)16-11)1-13-6(3-17)7(19)4-18/h2,6-7,13-14,17-19H,1,3-4H2,(H3,12,15,16,20)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246593
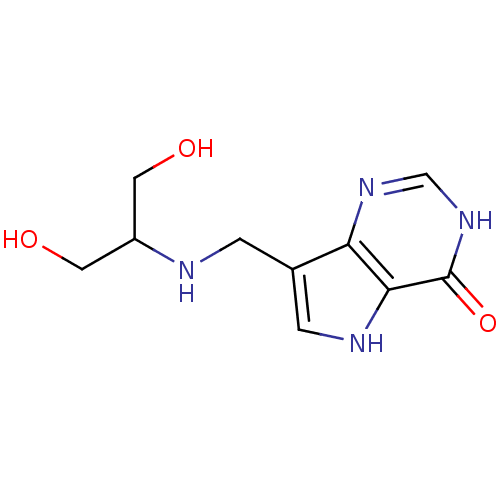
(7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...)Show InChI InChI=1S/C10H14N4O3/c15-3-7(4-16)11-1-6-2-12-9-8(6)13-5-14-10(9)17/h2,5,7,11-12,15-16H,1,3-4H2,(H,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246593

(7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...)Show InChI InChI=1S/C10H14N4O3/c15-3-7(4-16)11-1-6-2-12-9-8(6)13-5-14-10(9)17/h2,5,7,11-12,15-16H,1,3-4H2,(H,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Equilibrium binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920
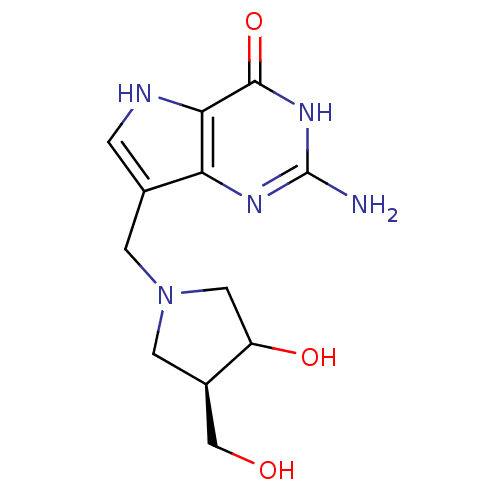
(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087
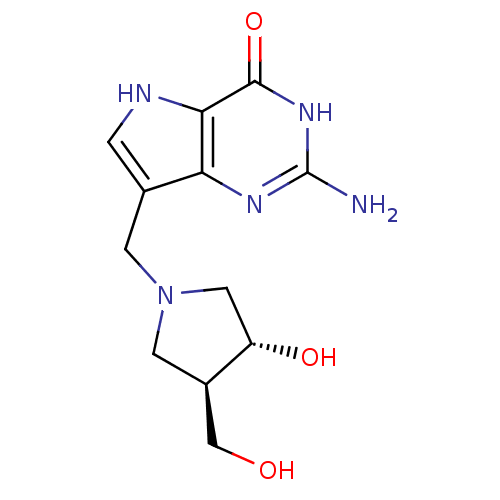
(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087

(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
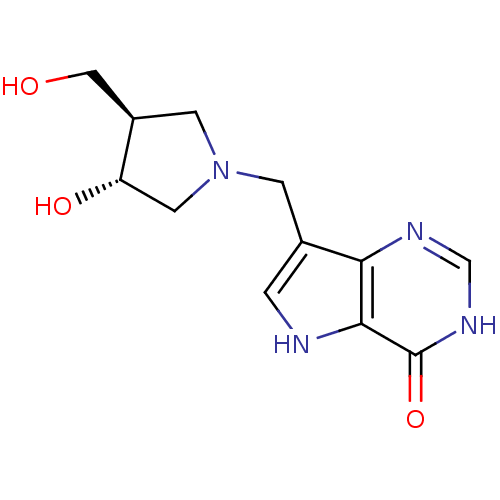
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293091

(7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...)Show SMILES OC[C@@H](O)[C@@H](CO)NCc1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H16N4O4/c16-3-7(8(18)4-17)12-1-6-2-13-10-9(6)14-5-15-11(10)19/h2,5,7-8,12-13,16-18H,1,3-4H2,(H,14,15,19)/t7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246590
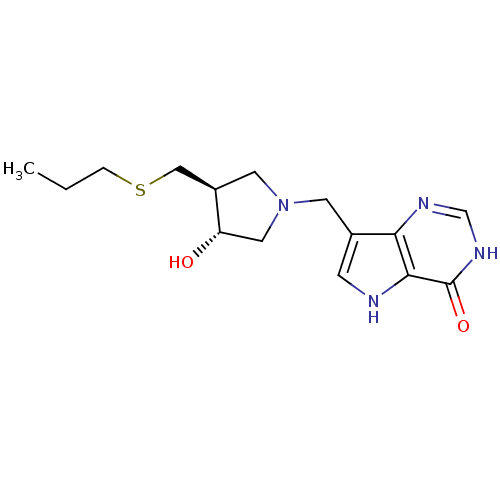
(7-(((3R,4S)-3-hydroxy-4-(propylthiomethyl)pyrrolid...)Show SMILES CCCSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C15H22N4O2S/c1-2-3-22-8-11-6-19(7-12(11)20)5-10-4-16-14-13(10)17-9-18-15(14)21/h4,9,11-12,16,20H,2-3,5-8H2,1H3,(H,17,18,21)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Equilibrium binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Equilibrium binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay |
J Med Chem 51: 5880-4 (2008)
Article DOI: 10.1021/jm800792b
BindingDB Entry DOI: 10.7270/Q2VQ32H9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247151
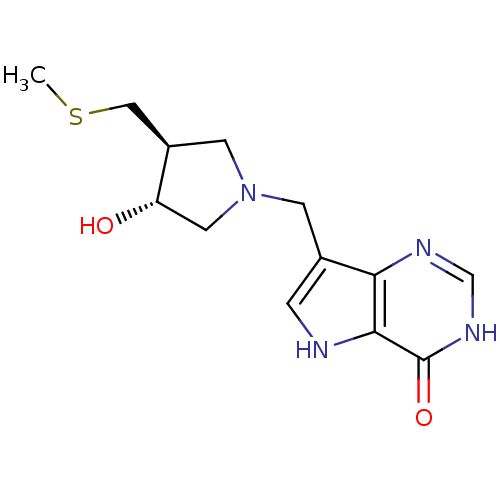
(7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C13H18N4O2S/c1-20-6-9-4-17(5-10(9)18)3-8-2-14-12-11(8)15-7-16-13(12)19/h2,7,9-10,14,18H,3-6H2,1H3,(H,15,16,19)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Equilibrium binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086
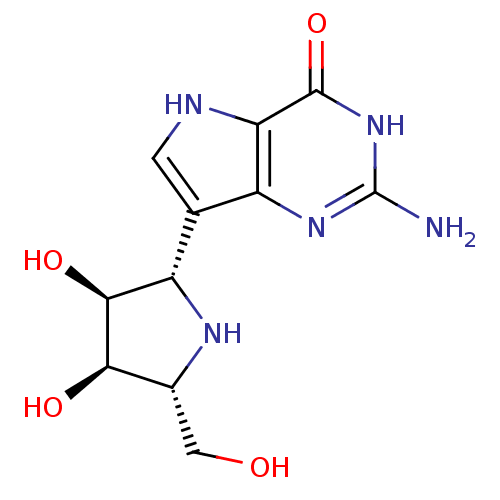
((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) |
J Med Chem 46: 155-60 (2002)
Article DOI: 10.1021/jm0203332
BindingDB Entry DOI: 10.7270/Q2DF6QJZ |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhbitory activity of compound against human purine nucleoside phosphorylase (PNP) |
J Med Chem 47: 3275-81 (2004)
Article DOI: 10.1021/jm0306475
BindingDB Entry DOI: 10.7270/Q2PZ59J8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0579 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Equilibrium binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50252865
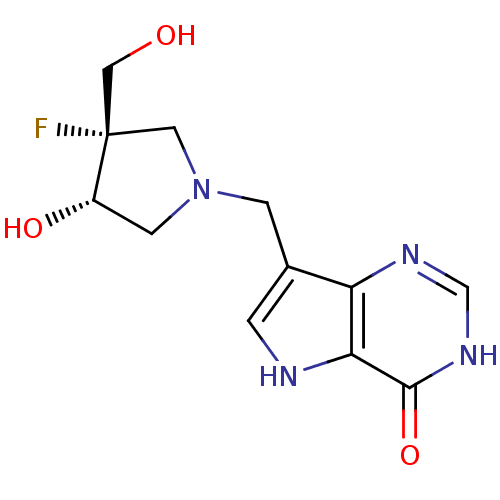
((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...)Show SMILES OC[C@@]1(F)CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H15FN4O3/c13-12(5-18)4-17(3-8(12)19)2-7-1-14-10-9(7)15-6-16-11(10)20/h1,6,8,14,18-19H,2-5H2,(H,15,16,20)/t8-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay |
J Med Chem 51: 5880-4 (2008)
Article DOI: 10.1021/jm800792b
BindingDB Entry DOI: 10.7270/Q2VQ32H9 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50252865

((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...)Show SMILES OC[C@@]1(F)CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H15FN4O3/c13-12(5-18)4-17(3-8(12)19)2-7-1-14-10-9(7)15-6-16-11(10)20/h1,6,8,14,18-19H,2-5H2,(H,15,16,20)/t8-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay |
J Med Chem 51: 5880-4 (2008)
Article DOI: 10.1021/jm800792b
BindingDB Entry DOI: 10.7270/Q2VQ32H9 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247151

(7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C13H18N4O2S/c1-20-6-9-4-17(5-10(9)18)3-8-2-14-12-11(8)15-7-16-13(12)19/h2,7,9-10,14,18H,3-6H2,1H3,(H,15,16,19)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... |
Bioorg Med Chem 23: 5326-33 (2015)
Article DOI: 10.1016/j.bmc.2015.07.059
BindingDB Entry DOI: 10.7270/Q2HX1FF5 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50122726
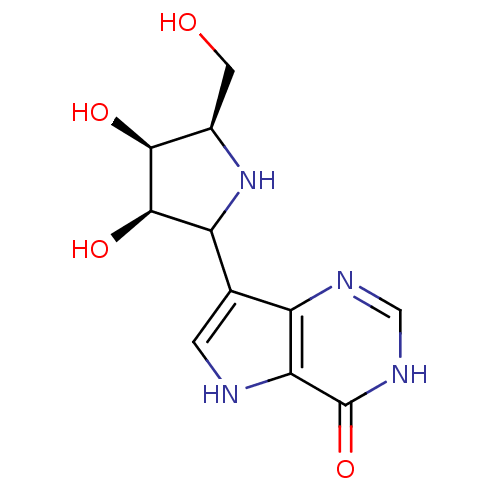
(2-Hydroxymethyl-5-(4-hydroxy-5H-pyrrolo[3,2-d]pyri...)Show SMILES OC[C@H]1NC([C@@H](O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7?,9+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) |
J Med Chem 46: 155-60 (2002)
Article DOI: 10.1021/jm0203332
BindingDB Entry DOI: 10.7270/Q2DF6QJZ |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50122724
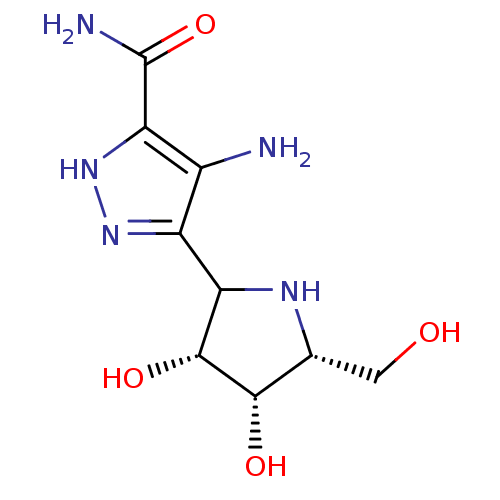
(4-Amino-5-(3,4-dihydroxy-5-hydroxymethyl-pyrrolidi...)Show SMILES NC(=O)c1[nH]nc(C2N[C@H](CO)[C@H](O)[C@@H]2O)c1N Show InChI InChI=1S/C9H15N5O4/c10-3-4(13-14-5(3)9(11)18)6-8(17)7(16)2(1-15)12-6/h2,6-8,12,15-17H,1,10H2,(H2,11,18)(H,13,14)/t2-,6?,7+,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) |
J Med Chem 46: 155-60 (2002)
Article DOI: 10.1021/jm0203332
BindingDB Entry DOI: 10.7270/Q2DF6QJZ |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247151

(7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C13H18N4O2S/c1-20-6-9-4-17(5-10(9)18)3-8-2-14-12-11(8)15-7-16-13(12)19/h2,7,9-10,14,18H,3-6H2,1H3,(H,15,16,19)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Initial binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435
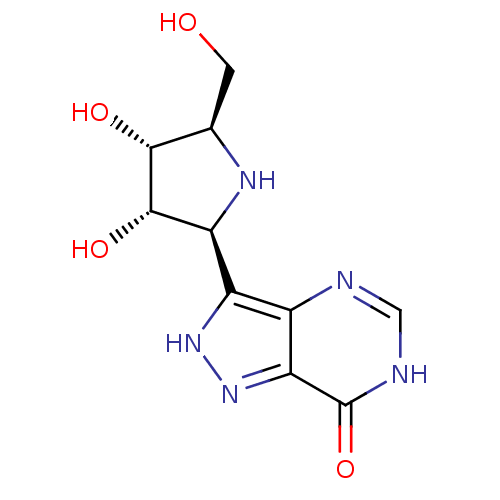
(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) |
J Med Chem 46: 155-60 (2002)
Article DOI: 10.1021/jm0203332
BindingDB Entry DOI: 10.7270/Q2DF6QJZ |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246593

(7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...)Show InChI InChI=1S/C10H14N4O3/c15-3-7(4-16)11-1-6-2-12-9-8(6)13-5-14-10(9)17/h2,5,7,11-12,15-16H,1,3-4H2,(H,13,14,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Initial binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50246590

(7-(((3R,4S)-3-hydroxy-4-(propylthiomethyl)pyrrolid...)Show SMILES CCCSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C15H22N4O2S/c1-2-3-22-8-11-6-19(7-12(11)20)5-10-4-16-14-13(10)17-9-18-15(14)21/h4,9,11-12,16,20H,2-3,5-8H2,1H3,(H,17,18,21)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Initial binding affinity to wild type human PNP |
Bioorg Med Chem Lett 18: 5900-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.047
BindingDB Entry DOI: 10.7270/Q2SX6F4H |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087

(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation by measuring reduction in uric acid formatio... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920

(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293060
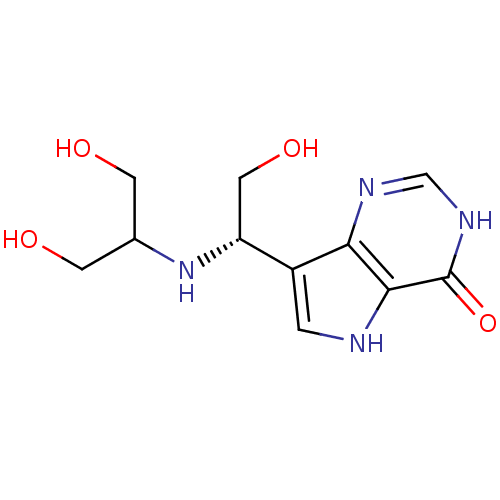
(7-{(1S)-1-[(1,3-Dihydroxypropan-2-yl)amino]-2-hydr...)Show InChI InChI=1S/C11H16N4O4/c16-2-6(3-17)15-8(4-18)7-1-12-10-9(7)13-5-14-11(10)19/h1,5-6,8,12,15-18H,2-4H2,(H,13,14,19)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
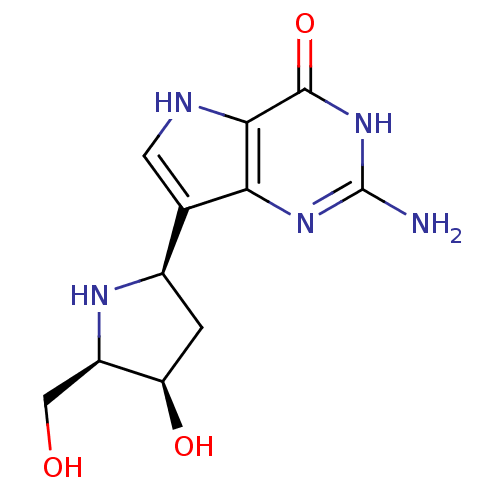
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370253
(CHEMBL114781)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@H]1C[C@@H](O)[C@@H](CO)N1 Show InChI InChI=1S/C11H15N5O3/c12-11-15-8-4(2-13-9(8)10(19)16-11)5-1-7(18)6(3-17)14-5/h2,5-7,13-14,17-18H,1,3H2,(H3,12,15,16,19)/t5-,6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22103
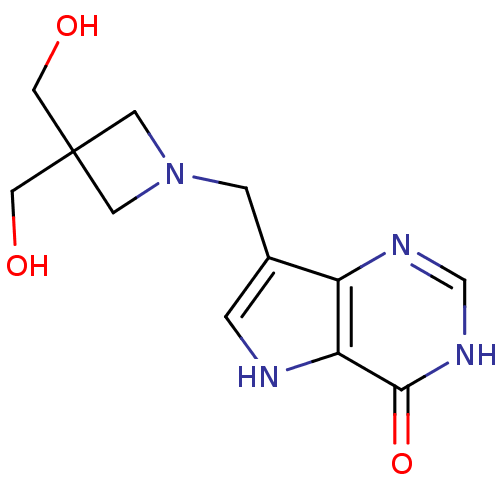
(7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...)Show InChI InChI=1S/C12H16N4O3/c17-5-12(6-18)3-16(4-12)2-8-1-13-10-9(8)14-7-15-11(10)19/h1,7,13,17-18H,2-6H2,(H,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22103

(7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...)Show InChI InChI=1S/C12H16N4O3/c17-5-12(6-18)3-16(4-12)2-8-1-13-10-9(8)14-7-15-11(10)19/h1,7,13,17-18H,2-6H2,(H,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.229 | -13.0 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited
| Assay Description
PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... |
J Med Chem 51: 948-56 (2008)
Article DOI: 10.1021/jm701265n
BindingDB Entry DOI: 10.7270/Q2QC01T3 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370255
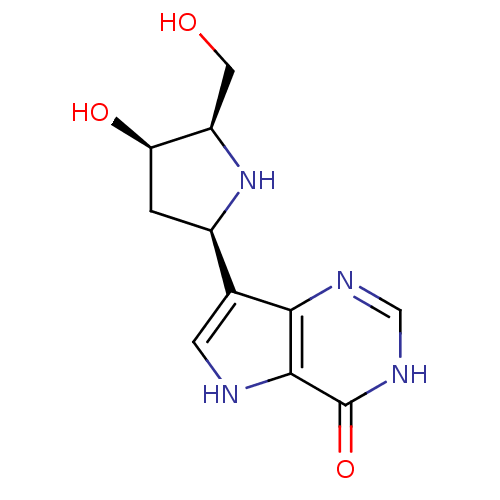
(CHEMBL115146)Show SMILES OC[C@H]1N[C@H](C[C@H]1O)c1c[nH]c2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O3/c16-3-7-8(17)1-6(15-7)5-2-12-10-9(5)13-4-14-11(10)18/h2,4,6-8,12,15-17H,1,3H2,(H,13,14,18)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase; Initial rate. |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247151

(7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C13H18N4O2S/c1-20-6-9-4-17(5-10(9)18)3-8-2-14-12-11(8)15-7-16-13(12)19/h2,7,9-10,14,18H,3-6H2,1H3,(H,15,16,19)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay |
Bioorg Med Chem 23: 5326-33 (2015)
Article DOI: 10.1016/j.bmc.2015.07.059
BindingDB Entry DOI: 10.7270/Q2HX1FF5 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay |
J Med Chem 51: 5880-4 (2008)
Article DOI: 10.1021/jm800792b
BindingDB Entry DOI: 10.7270/Q2VQ32H9 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50370251
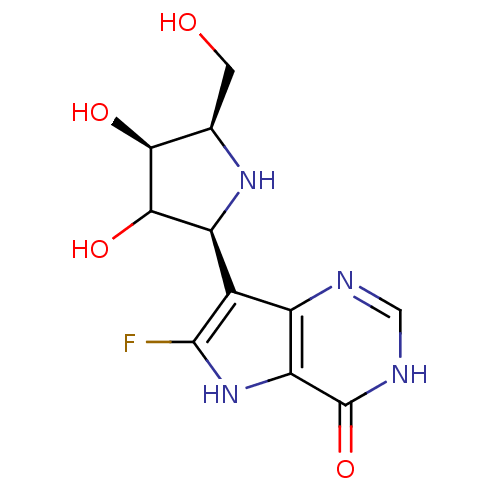
(CHEMBL542455)Show SMILES OC[C@H]1N[C@H](C(O)[C@H]1O)c1c(F)[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H13FN4O4/c12-10-4(5-7(16-10)11(20)14-2-13-5)6-9(19)8(18)3(1-17)15-6/h2-3,6,8-9,15-19H,1H2,(H,13,14,20)/t3-,6+,8+,9?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition |
J Med Chem 46: 3412-23 (2003)
Article DOI: 10.1021/jm030145r
BindingDB Entry DOI: 10.7270/Q2X92C29 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293061

(2-Amino-7-({[1,3-dihydroxy-2-(hydroxymethyl)propan...)Show InChI InChI=1S/C11H17N5O4/c12-10-15-7-6(1-13-8(7)9(20)16-10)2-14-11(3-17,4-18)5-19/h1,13-14,17-19H,2-5H2,(H3,12,15,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293062
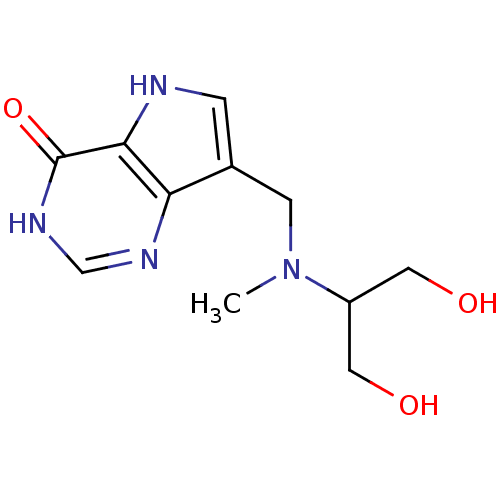
(7-{[(1,3-Dihydroxypropan-2-yl)(methyl)amino]methyl...)Show InChI InChI=1S/C11H16N4O3/c1-15(8(4-16)5-17)3-7-2-12-10-9(7)13-6-14-11(10)18/h2,6,8,12,16-17H,3-5H2,1H3,(H,13,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293063
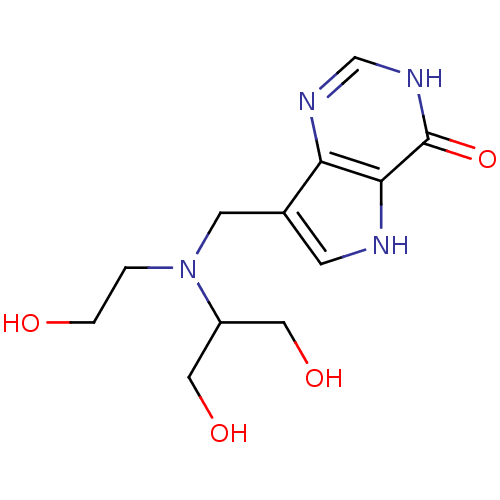
(7-{[(1,3-Dihydroxypropan-2-yl)(2-hydroxyethyl)amin...)Show InChI InChI=1S/C12H18N4O4/c17-2-1-16(9(5-18)6-19)4-8-3-13-11-10(8)14-7-15-12(11)20/h3,7,9,13,17-19H,1-2,4-6H2,(H,14,15,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PNP by xanthine-oxidase coupled assay |
J Med Chem 52: 1126-43 (2009)
Article DOI: 10.1021/jm801421q
BindingDB Entry DOI: 10.7270/Q2QR4Z18 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50252865

((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...)Show SMILES OC[C@@]1(F)CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H15FN4O3/c13-12(5-18)4-17(3-8(12)19)2-7-1-14-10-9(7)15-6-16-11(10)20/h1,6,8,14,18-19H,2-5H2,(H,15,16,20)/t8-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay |
J Med Chem 51: 5880-4 (2008)
Article DOI: 10.1021/jm800792b
BindingDB Entry DOI: 10.7270/Q2VQ32H9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data