 Found 507 hits of ic50 data for polymerid = 50003850
Found 507 hits of ic50 data for polymerid = 50003850 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50067697
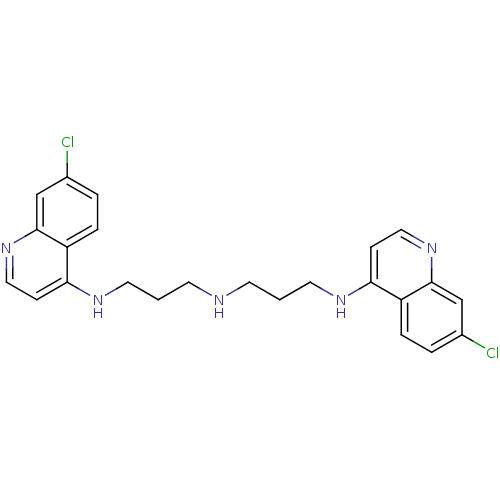
(7-chloro-N-(3-(3-(7-chloroquinolin-4-ylamino)propy...)Show SMILES Clc1ccc2c(NCCCNCCCNc3ccnc4cc(Cl)ccc34)ccnc2c1 Show InChI InChI=1S/C24H25Cl2N5/c25-17-3-5-19-21(7-13-30-23(19)15-17)28-11-1-9-27-10-2-12-29-22-8-14-31-24-16-18(26)4-6-20(22)24/h3-8,13-16,27H,1-2,9-12H2,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain |
J Med Chem 56: 5860-71 (2014)
Article DOI: 10.1021/jm4006077
BindingDB Entry DOI: 10.7270/Q2QF8V8K |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429171
(CHEMBL2336714)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc(Cl)cc1 |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C18H22ClNO2/c19-15-3-1-14(2-4-15)18-8-12-5-13(9-18)7-17(6-12,11-18)10-16(21)20-22/h1-4,12-13,22H,5-11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429170
(CHEMBL2336715)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc(Br)cc1 |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C18H22BrNO2/c19-15-3-1-14(2-4-15)18-8-12-5-13(9-18)7-17(6-12,11-18)10-16(21)20-22/h1-4,12-13,22H,5-11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447108

(CHEMBL3112881)Show InChI InChI=1S/C11H7ClFNO2S/c12-11-7-2-1-6(13)5-9(7)17-8(11)3-4-10(15)14-16/h1-5,16H,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429175
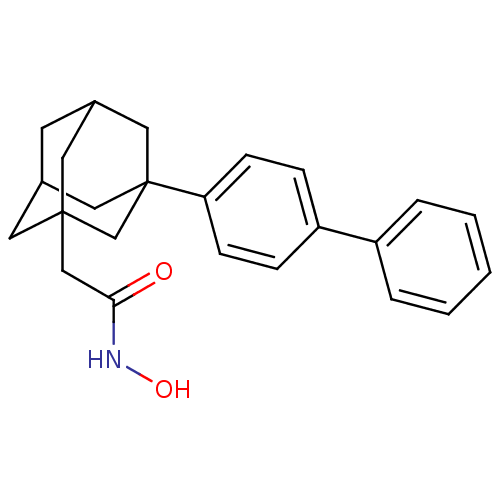
(CHEMBL2336718)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc(cc1)-c1ccccc1 |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C24H27NO2/c26-22(25-27)15-23-11-17-10-18(12-23)14-24(13-17,16-23)21-8-6-20(7-9-21)19-4-2-1-3-5-19/h1-9,17-18,27H,10-16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383525
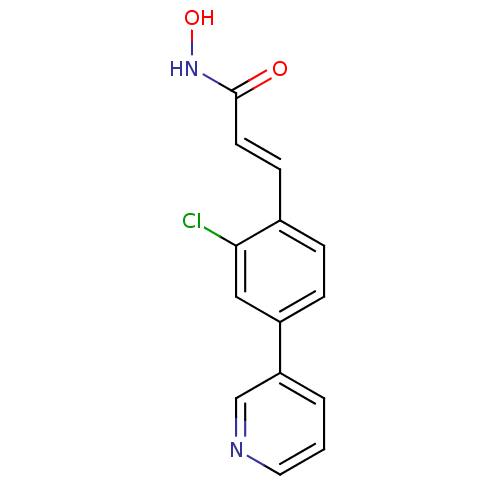
(CHEMBL2032269)Show InChI InChI=1S/C14H11ClN2O2/c15-13-8-11(12-2-1-7-16-9-12)4-3-10(13)5-6-14(18)17-19/h1-9,19H,(H,17,18)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429178
(CHEMBL2336712)Show SMILES Cc1ccc(cc1)C12CC3CC(CC(CC(=O)NO)(C3)C1)C2 |TLB:14:13:10:21.8.7,4:7:10:19.13.12,THB:8:9:12:21.7.20,8:7:10.9.19:12,20:7:10:19.13.12,20:13:10:21.8.7| Show InChI InChI=1S/C19H25NO2/c1-13-2-4-16(5-3-13)19-9-14-6-15(10-19)8-18(7-14,12-19)11-17(21)20-22/h2-5,14-15,22H,6-12H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429172
(CHEMBL2336713)Show SMILES COc1ccc(cc1)C12CC3CC(CC(CC(=O)NO)(C3)C1)C2 |TLB:15:14:11:22.9.8,5:8:11:20.14.13,THB:9:10:13:22.8.21,9:8:11.10.20:13,21:8:11:20.14.13,21:14:11:22.9.8| Show InChI InChI=1S/C19H25NO3/c1-23-16-4-2-15(3-5-16)19-9-13-6-14(10-19)8-18(7-13,12-19)11-17(21)20-22/h2-5,13-14,22H,6-12H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50589492

(CHEMBL5171667)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCN)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@@H](C)O |r| | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128913
BindingDB Entry DOI: 10.7270/Q24B3583 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50576781

(CHEMBL4860434) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429180
(CHEMBL2336710)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc2ccccc2c1 |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C22H25NO2/c24-20(23-25)13-21-9-15-7-16(10-21)12-22(11-15,14-21)19-6-5-17-3-1-2-4-18(17)8-19/h1-6,8,15-16,25H,7,9-14H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50576781

(CHEMBL4860434) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain using SNAPtide as substrate by FRET based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50576781

(CHEMBL4860434) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429176
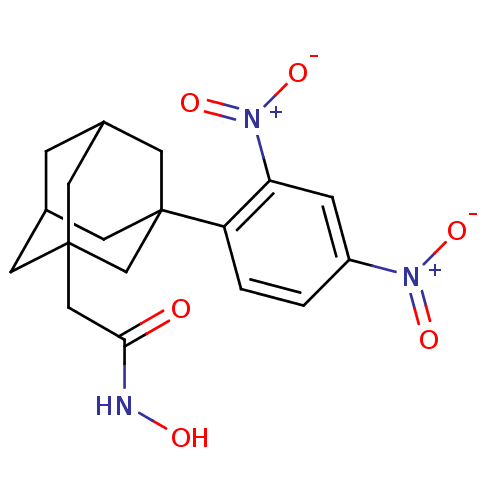
(CHEMBL2336717)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc(cc1[N+]([O-])=O)[N+]([O-])=O |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C18H21N3O6/c22-16(19-23)9-17-5-11-3-12(6-17)8-18(7-11,10-17)14-2-1-13(20(24)25)4-15(14)21(26)27/h1-2,4,11-12,23H,3,5-10H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Clostridium botulinum BoNT/A |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01006
BindingDB Entry DOI: 10.7270/Q2WD446P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of Clostridium botulinum BoNT/A using SNAP-25 as substrate by FRET analysis |
Bioorg Med Chem 24: 4875-4889 (2016)
Article DOI: 10.1016/j.bmc.2016.07.031
BindingDB Entry DOI: 10.7270/Q2JH3QPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum toxin BoNT/A light chain |
J Med Chem 53: 2264-76 (2010)
Article DOI: 10.1021/jm901852f
BindingDB Entry DOI: 10.7270/Q2HQ40V7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A |
J Med Chem 56: 7997-8007 (2013)
Article DOI: 10.1021/jm401053m
BindingDB Entry DOI: 10.7270/Q2N29ZC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50589504

(CHEMBL5202691)Show SMILES [#8]-[#6](=O)-[#6]-[#6]-c1ccc(cc1)-n1[se;v2]c2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128913
BindingDB Entry DOI: 10.7270/Q24B3583 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429177
(CHEMBL2336716)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccc(cc1)[N+]([O-])=O |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C18H22N2O4/c21-16(19-22)10-17-6-12-5-13(7-17)9-18(8-12,11-17)14-1-3-15(4-2-14)20(23)24/h1-4,12-13,22H,5-11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50589500

(CHEMBL5208729)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N1)C(=O)NCCNC(=O)Cc1ccc(cc1)-n1[se]c2ccccc2c1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128913
BindingDB Entry DOI: 10.7270/Q24B3583 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50429173
(CHEMBL2336709)Show SMILES ONC(=O)CC12CC3CC(C1)CC(C3)(C2)c1ccccc1 |TLB:13:12:8.7.6:10,4:5:8:11.13.12,15:12:8:6.5.10,THB:13:7:10:11.12.14,14:12:8:6.5.10,14:5:8:11.13.12| Show InChI InChI=1S/C18H23NO2/c20-16(19-21)11-17-7-13-6-14(8-17)10-18(9-13,12-17)15-4-2-1-3-5-15/h1-5,13-14,21H,6-12H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAPtide as substrate by FRET assay |
Bioorg Med Chem 21: 1344-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.001
BindingDB Entry DOI: 10.7270/Q2N58NQJ |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50308030

(5-chloro-7-((4-ethoxyphenyl)(pyridin-3-ylamino)met...)Show SMILES CCOc1ccc(cc1)C(Nc1cccnc1)c1cc(Cl)c2cccnc2c1O Show InChI InChI=1S/C23H20ClN3O2/c1-2-29-17-9-7-15(8-10-17)21(27-16-5-3-11-25-14-16)19-13-20(24)18-6-4-12-26-22(18)23(19)28/h3-14,21,27-28H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum toxin BoNT/A light chain |
J Med Chem 53: 2264-76 (2010)
Article DOI: 10.1021/jm901852f
BindingDB Entry DOI: 10.7270/Q2HQ40V7 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383533

(CHEMBL2032254)Show InChI InChI=1S/C15H13ClN2O2/c16-13-9-11(12-3-1-2-4-14(12)17)6-5-10(13)7-8-15(19)18-20/h1-9,20H,17H2,(H,18,19)/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383550

(CHEMBL2032381)Show InChI InChI=1S/C15H13ClN2O3/c1-21-15-7-5-12(9-17-15)11-3-2-10(13(16)8-11)4-6-14(19)18-20/h2-9,20H,1H3,(H,18,19)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50561055

(CHEMBL4742073)Show SMILES CC[C@@H](C)[C@@H](NS(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(=O)NO |r| | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 587 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain using SNAPtide as substrate preincubated with enzyme for 2 mins followed by substrate addition... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115659
BindingDB Entry DOI: 10.7270/Q2S18669 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50445604

(CHEMBL3103448)Show InChI InChI=1S/C18H18Cl2N2O3/c1-11-4-2-3-5-16(11)21-17(23)8-12(9-18(24)22-25)14-7-6-13(19)10-15(14)20/h2-7,10,12,25H,8-9H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50445604

(CHEMBL3103448)Show InChI InChI=1S/C18H18Cl2N2O3/c1-11-4-2-3-5-16(11)21-17(23)8-12(9-18(24)22-25)14-7-6-13(19)10-15(14)20/h2-7,10,12,25H,8-9H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234245
(CHEMBL4060854)Show SMILES Oc1c(cc(Cl)c2cccnc12)C(Nc1cccnc1)c1ccc(F)cc1F Show InChI InChI=1S/C21H14ClF2N3O/c22-17-10-16(21(28)20-14(17)4-2-8-26-20)19(27-13-3-1-7-25-11-13)15-6-5-12(23)9-18(15)24/h1-11,19,27-28H | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234255
(CHEMBL4091467)Show SMILES Oc1c(cc(Cl)c2cccnc12)C(Nc1ccccn1)c1ccc(F)cc1 Show InChI InChI=1S/C21H15ClFN3O/c22-17-12-16(21(27)20-15(17)4-3-11-25-20)19(13-6-8-14(23)9-7-13)26-18-5-1-2-10-24-18/h1-12,19,27H,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383556

(CHEMBL2032387)Show InChI InChI=1S/C14H11ClN2O2/c15-13-9-12(10-5-7-16-8-6-10)2-1-11(13)3-4-14(18)17-19/h1-9,19H,(H,17,18)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013665

(CHEMBL3264507)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](N)CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)[C@H](C)CCCN(CCCNc1ccnc2cc(Cl)ccc12)CCC[C@@H](C)[C@@]1([H])CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](N)CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O |r| Show InChI InChI=1S/C68H104ClN5O8/c1-39(51-18-20-53-63-55(37-61(67(51,53)9)81-43(5)77)65(7)25-22-48(70)32-45(65)34-59(63)79-41(3)75)14-11-29-74(31-13-27-72-57-24-28-73-58-36-47(69)16-17-50(57)58)30-12-15-40(2)52-19-21-54-64-56(38-62(68(52,54)10)82-44(6)78)66(8)26-23-49(71)33-46(66)35-60(64)80-42(4)76/h16-17,24,28,36,39-40,45-46,48-49,51-56,59-64H,11-15,18-23,25-27,29-35,37-38,70-71H2,1-10H3,(H,72,73)/t39-,40-,45+,46+,48-,49-,51-,52-,53+,54+,55+,56+,59-,60-,61+,62+,63+,64+,65+,66+,67-,68-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383532
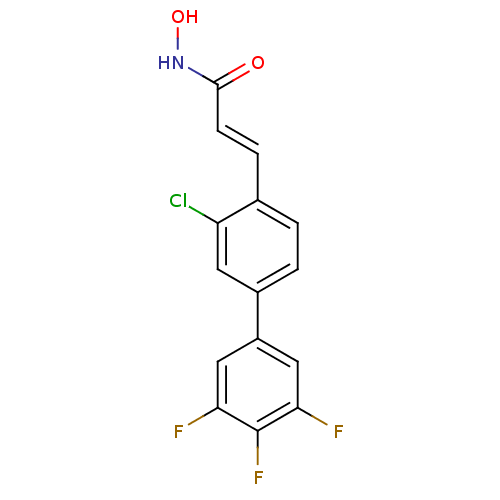
(CHEMBL2032249)Show InChI InChI=1S/C15H9ClF3NO2/c16-11-5-9(2-1-8(11)3-4-14(21)20-22)10-6-12(17)15(19)13(18)7-10/h1-7,22H,(H,20,21)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383552
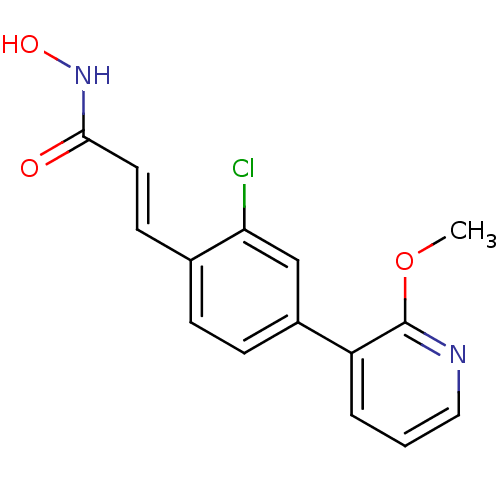
(CHEMBL2032383)Show InChI InChI=1S/C15H13ClN2O3/c1-21-15-12(3-2-8-17-15)11-5-4-10(13(16)9-11)6-7-14(19)18-20/h2-9,20H,1H3,(H,18,19)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383527
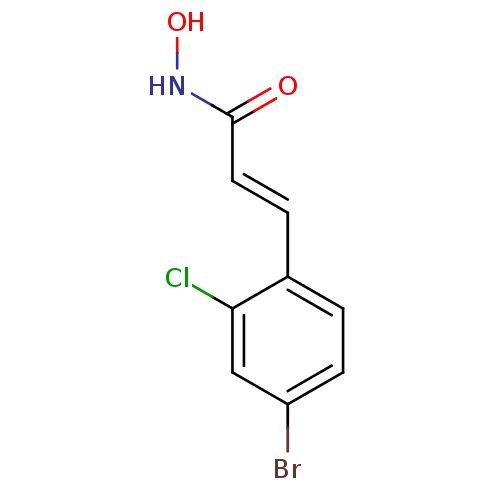
(CHEMBL2032250)Show InChI InChI=1S/C9H7BrClNO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234272
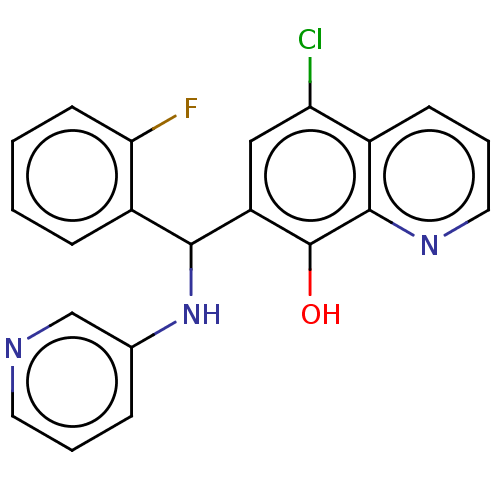
(CHEMBL4084638)Show InChI InChI=1S/C21H15ClFN3O/c22-17-11-16(21(27)20-14(17)7-4-10-25-20)19(15-6-1-2-8-18(15)23)26-13-5-3-9-24-12-13/h1-12,19,26-27H | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234270
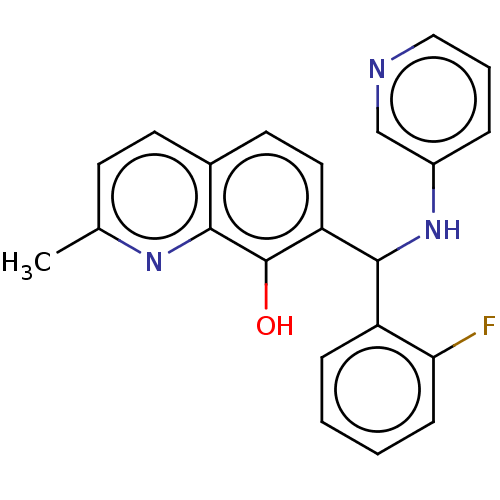
(CHEMBL4071334)Show InChI InChI=1S/C22H18FN3O/c1-14-8-9-15-10-11-18(22(27)20(15)25-14)21(17-6-2-3-7-19(17)23)26-16-5-4-12-24-13-16/h2-13,21,26-27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM52237
(2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-qui...)Show InChI InChI=1S/C22H19N3O/c1-15-10-11-17-12-13-18(22(26)21(17)24-15)20(16-7-3-2-4-8-16)25-19-9-5-6-14-23-19/h2-14,20,26H,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234257
(CHEMBL4089872)Show SMILES Oc1c(cc(Cl)c2cccnc12)C(Nc1ccccn1)c1ccc(F)cc1F Show InChI InChI=1S/C21H14ClF2N3O/c22-16-11-15(21(28)20-13(16)4-3-9-26-20)19(27-18-5-1-2-8-25-18)14-7-6-12(23)10-17(14)24/h1-11,19,28H,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234273
(CHEMBL4100165)Show SMILES Oc1c(cc(Cl)c2cccnc12)C(Nc1ncccn1)c1ccc(F)cc1 Show InChI InChI=1S/C20H14ClFN4O/c21-16-11-15(19(27)18-14(16)3-1-8-23-18)17(12-4-6-13(22)7-5-12)26-20-24-9-2-10-25-20/h1-11,17,27H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50247828

(CHEMBL4065254)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](N)CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)[C@H](C)CCCN(C)CCCC(C)Nc1ccnc2ccccc12 |r| Show InChI InChI=1S/C43H66N4O4/c1-27(12-10-22-47(7)23-11-13-28(2)46-38-19-21-45-37-15-9-8-14-33(37)38)34-16-17-35-41-36(26-40(43(34,35)6)51-30(4)49)42(5)20-18-32(44)24-31(42)25-39(41)50-29(3)48/h8-9,14-15,19,21,27-28,31-32,34-36,39-41H,10-13,16-18,20,22-26,44H2,1-7H3,(H,45,46)/t27-,28?,31+,32-,34-,35+,36+,39-,40+,41+,42+,43-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain using P39 as substrate preincubated for 10 mins followed by substrate addition measured after ... |
J Med Chem 61: 1595-1608 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01710
BindingDB Entry DOI: 10.7270/Q2T15617 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50383551

(CHEMBL2032382)Show InChI InChI=1S/C16H15ClN2O3/c1-10-7-16(22-2)18-9-13(10)12-4-3-11(14(17)8-12)5-6-15(20)19-21/h3-9,21H,1-2H3,(H,19,20)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysis |
Bioorg Med Chem Lett 22: 3754-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.019
BindingDB Entry DOI: 10.7270/Q21C1XWX |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50576777
(CHEMBL4854965) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50576777

(CHEMBL4854965) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal 6XHis-tagged Clostridium botulinum BoNT/A light chain C-terminal truncation mutant (1 to 425 residues) expressed in Escheric... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00325
BindingDB Entry DOI: 10.7270/Q29Z98Q6 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50234248

(CHEMBL4068440)Show SMILES Cc1ccc2ccc(C(Nc3cccnc3)c3ccc(F)cc3)c(O)c2n1 Show InChI InChI=1S/C22H18FN3O/c1-14-4-5-16-8-11-19(22(27)21(16)25-14)20(15-6-9-17(23)10-7-15)26-18-3-2-12-24-13-18/h2-13,20,26-27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... |
Bioorg Med Chem Lett 27: 675-678 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.019
BindingDB Entry DOI: 10.7270/Q2NP26PM |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23289

((2E)-3-(4-chloro-2-methylphenyl)-N-hydroxyprop-2-e...)Show InChI InChI=1S/C10H10ClNO2/c1-7-6-9(11)4-2-8(7)3-5-10(13)12-14/h2-6,14H,1H3,(H,12,13)/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A |
Bioorg Med Chem Lett 20: 206-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.129
BindingDB Entry DOI: 10.7270/Q2H41RJ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data