

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21842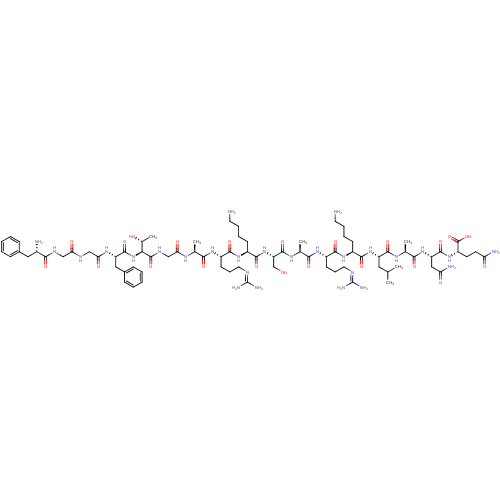 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21844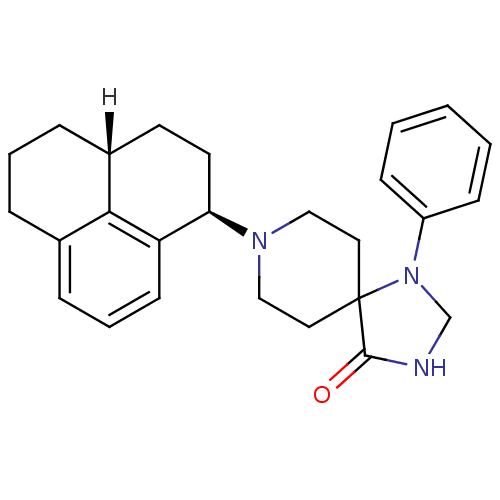 (8-[(1R,3aR)-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21845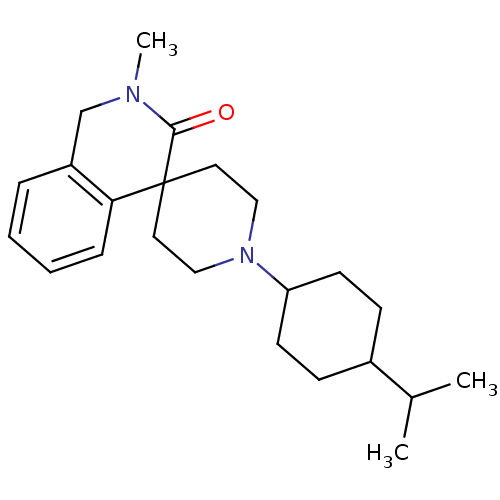 (2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21857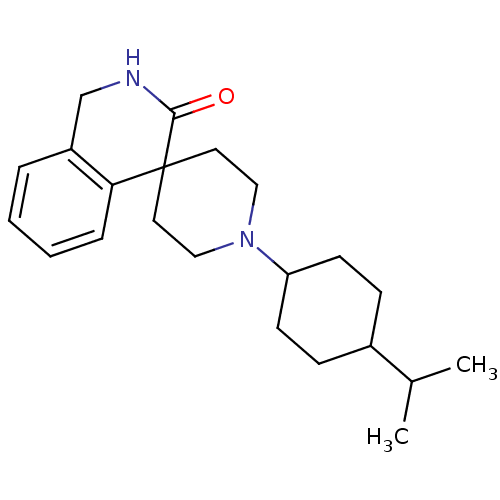 (1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydro-1H-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21851 (2-benzyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21847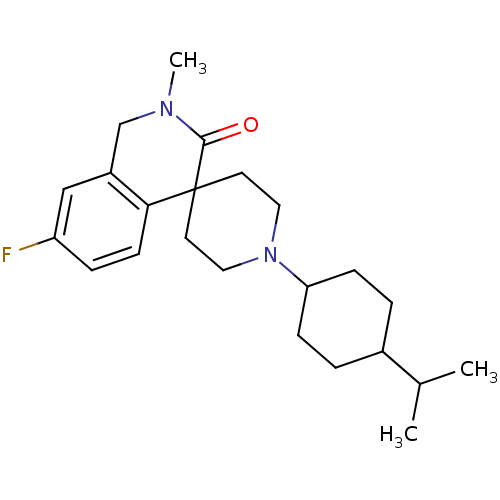 (7-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21852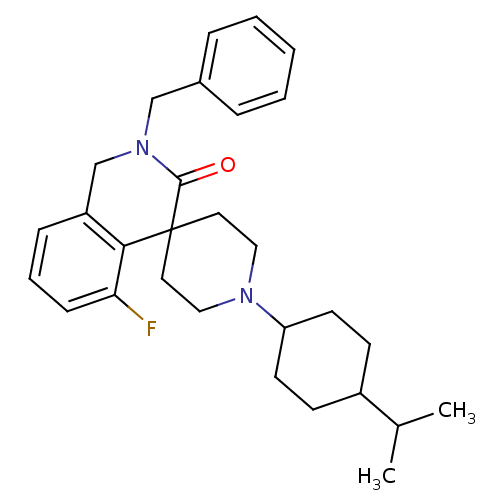 (2-benzyl-5-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21848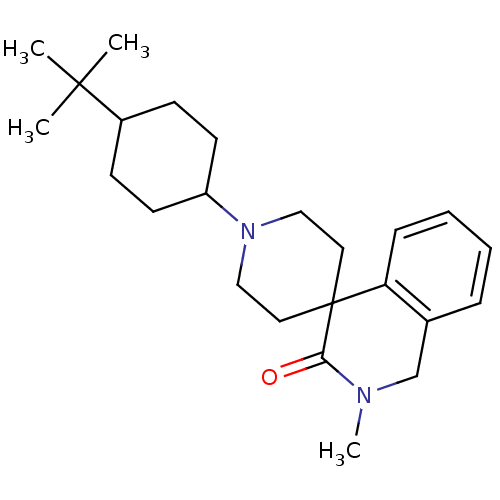 (1'-(4-tert-butylcyclohexyl)-2-methyl-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21858 (7-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21853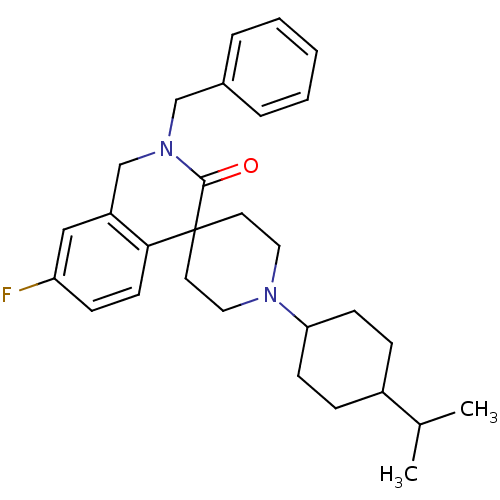 (2-benzyl-7-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21859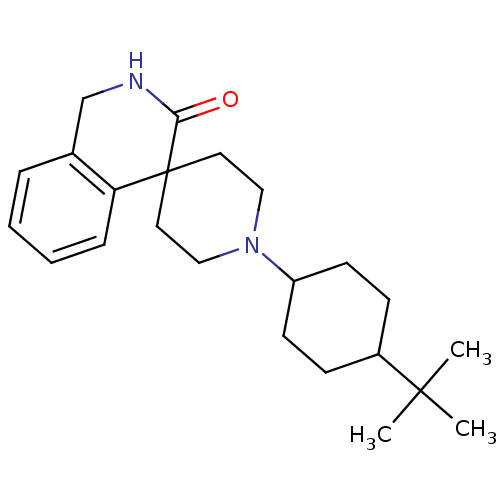 (1'-(4-tert-butylcyclohexyl)-2,3-dihydro-1H-spiro[i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21844 (8-[(1R,3aR)-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21854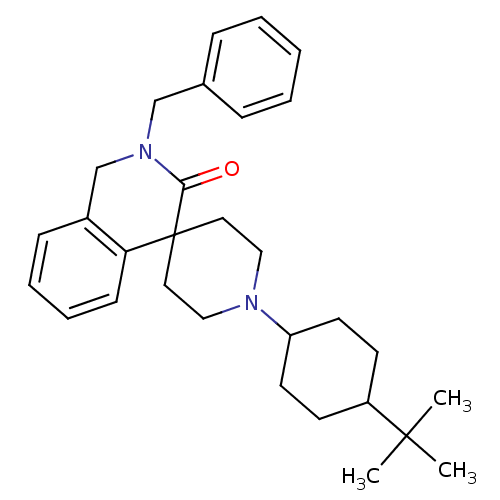 (2-benzyl-1'-(4-tert-butylcyclohexyl)-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21862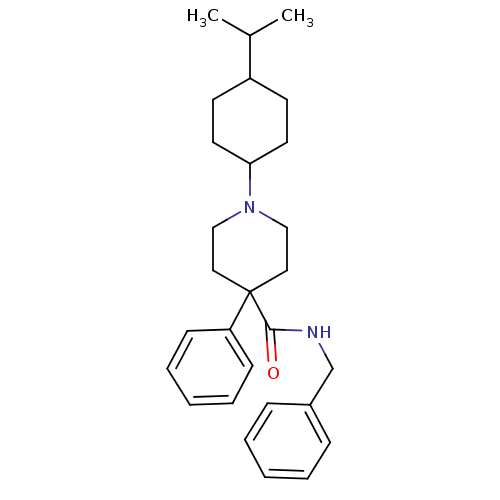 (N-benzyl-4-phenyl-1-[4-(propan-2-yl)cyclohexyl]pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21855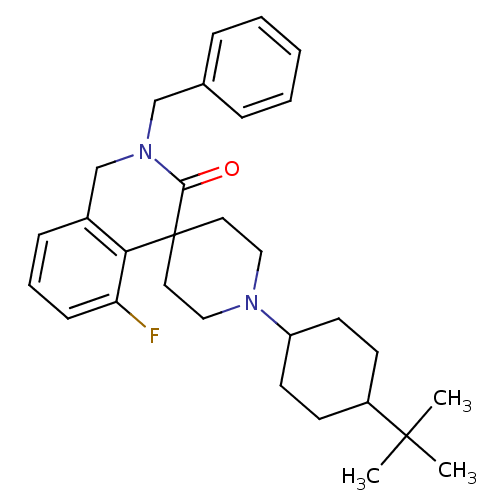 (2-benzyl-1'-(4-tert-butylcyclohexyl)-5-fluoro-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21844 (8-[(1R,3aR)-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21860 (1'-(4-tert-butylcyclohexyl)-7-fluoro-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21863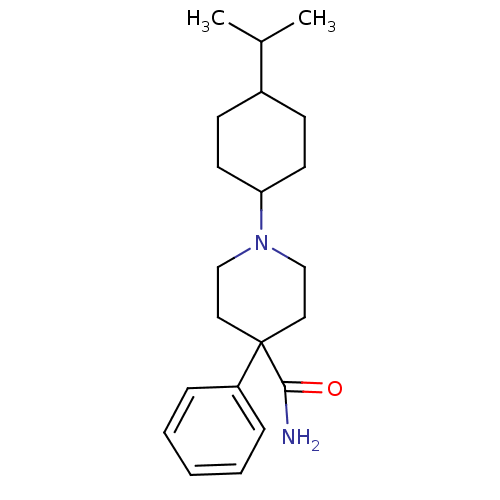 (4-phenyl-1-[4-(propan-2-yl)cyclohexyl]piperidine-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 92 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21854 (2-benzyl-1'-(4-tert-butylcyclohexyl)-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21847 (7-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21852 (2-benzyl-5-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21861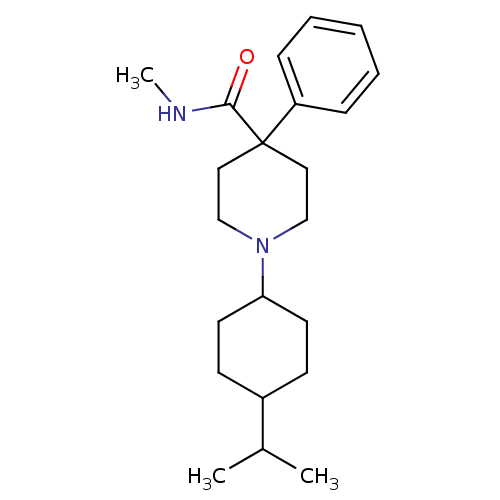 (N-methyl-4-phenyl-1-[4-(propan-2-yl)cyclohexyl]pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21856 (2-benzyl-1'-(4-tert-butylcyclohexyl)-7-fluoro-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 136 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21850 (1'-(4-tert-butylcyclohexyl)-7-fluoro-2-methyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 147 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21848 (1'-(4-tert-butylcyclohexyl)-2-methyl-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21846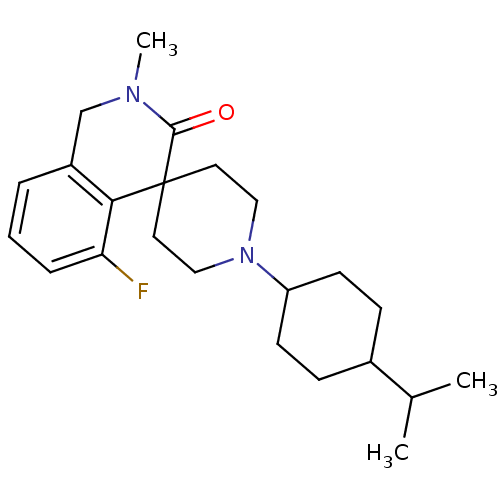 (5-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 186 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21855 (2-benzyl-1'-(4-tert-butylcyclohexyl)-5-fluoro-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21854 (2-benzyl-1'-(4-tert-butylcyclohexyl)-2,3-dihydro-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21855 (2-benzyl-1'-(4-tert-butylcyclohexyl)-5-fluoro-2,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21849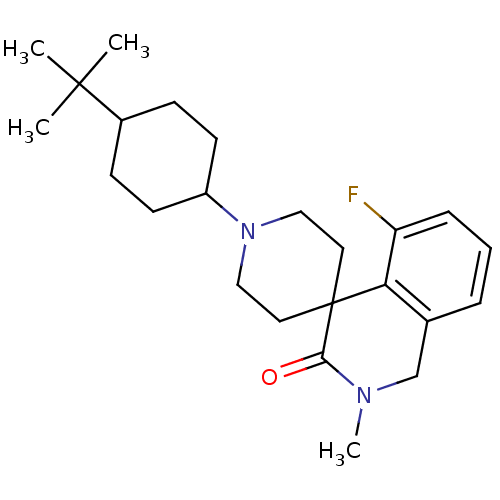 (1'-(4-tert-butylcyclohexyl)-5-fluoro-2-methyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 395 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21846 (5-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 412 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21845 (2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21847 (7-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21845 (2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 557 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21851 (2-benzyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 928 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21848 (1'-(4-tert-butylcyclohexyl)-2-methyl-2,3-dihydro-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21856 (2-benzyl-1'-(4-tert-butylcyclohexyl)-7-fluoro-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21850 (1'-(4-tert-butylcyclohexyl)-7-fluoro-2-methyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21851 (2-benzyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21844 (8-[(1R,3aR)-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21856 (2-benzyl-1'-(4-tert-butylcyclohexyl)-7-fluoro-2,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21853 (2-benzyl-7-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21853 (2-benzyl-7-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21846 (5-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21862 (N-benzyl-4-phenyl-1-[4-(propan-2-yl)cyclohexyl]pip...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21842 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21850 (1'-(4-tert-butylcyclohexyl)-7-fluoro-2-methyl-2,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21849 (1'-(4-tert-butylcyclohexyl)-5-fluoro-2-methyl-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21860 (1'-(4-tert-butylcyclohexyl)-7-fluoro-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21857 (1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydro-1H-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |