

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374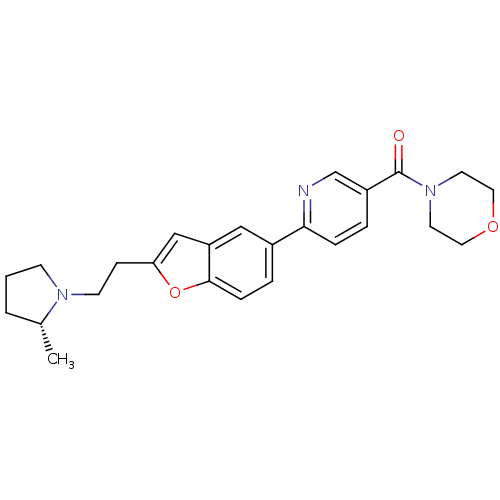 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139403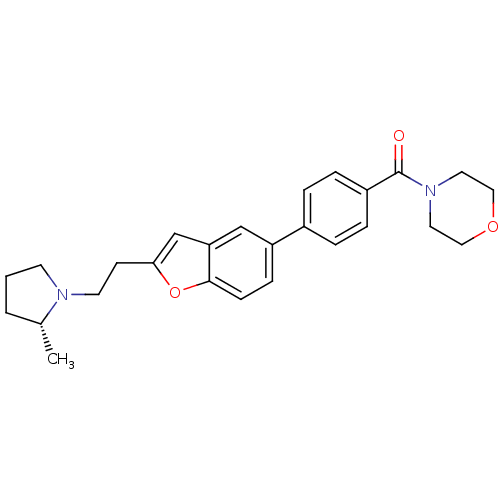 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139389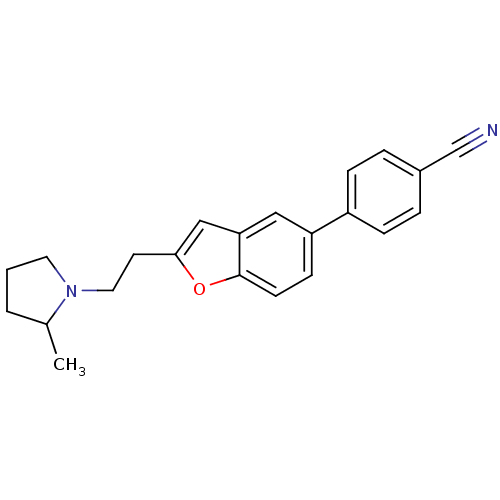 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139389 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139382 ((4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139382 ((4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139408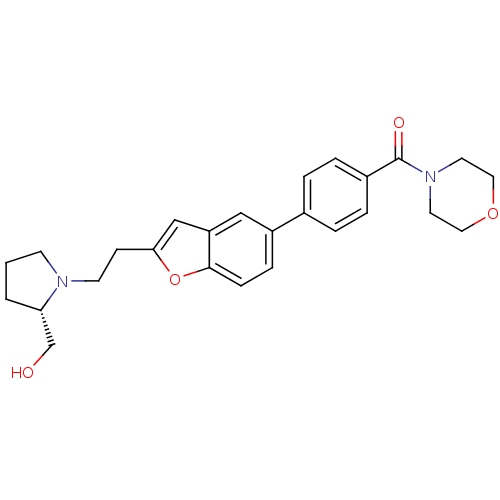 ((4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139408 ((4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description pBinding potency of the compound was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139380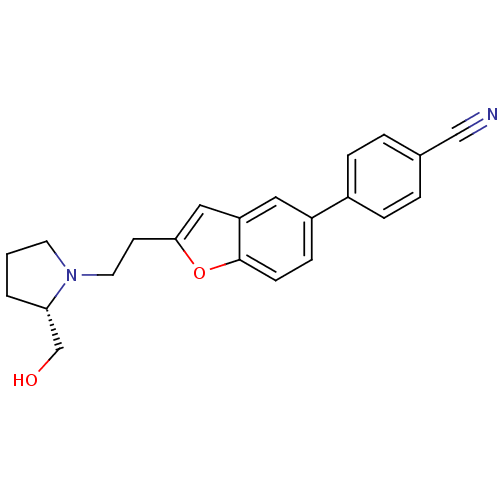 (4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139383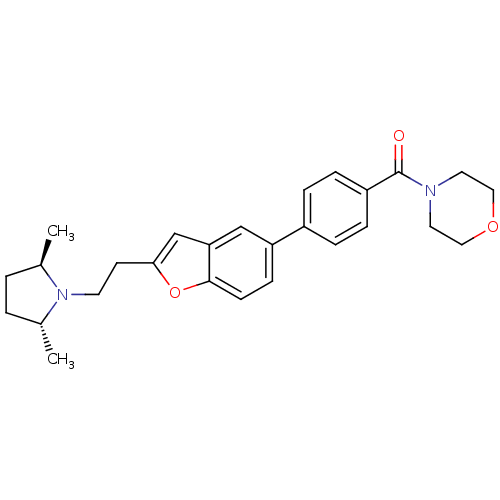 ((4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139383 ((4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139380 (4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139388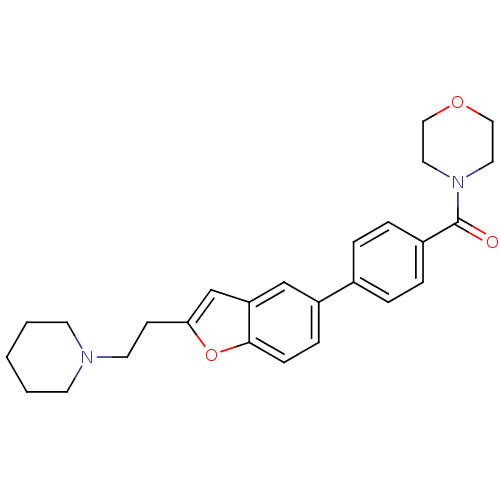 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139388 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139407 (4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139407 (4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139402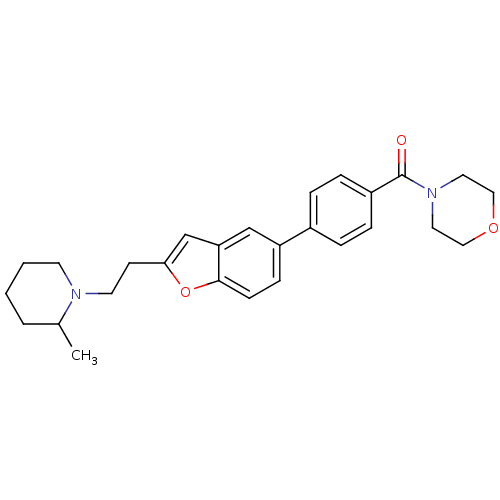 ((4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139402 ((4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139385 (CHEMBL346562 | {4-[2-(2-Azepan-1-yl-ethyl)-benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139385 (CHEMBL346562 | {4-[2-(2-Azepan-1-yl-ethyl)-benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139392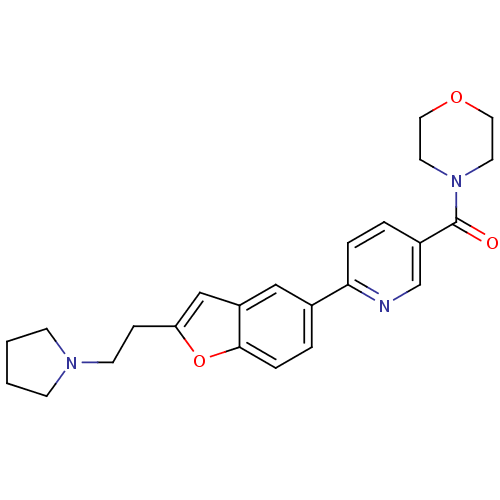 (CHEMBL422360 | Morpholin-4-yl-{6-[2-(2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139392 (CHEMBL422360 | Morpholin-4-yl-{6-[2-(2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139377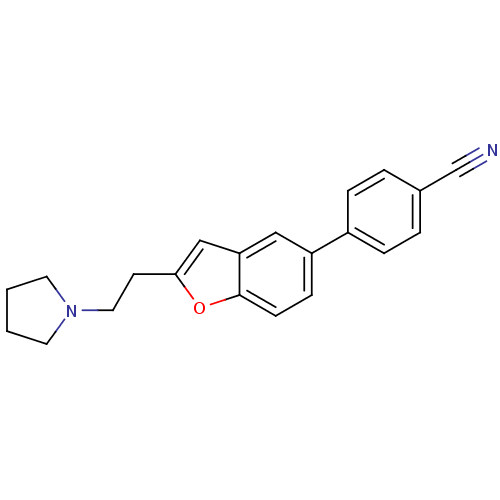 (4-[2-(2-Pyrrolidin-1-yl-ethyl)-benzofuran-5-yl]-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139377 (4-[2-(2-Pyrrolidin-1-yl-ethyl)-benzofuran-5-yl]-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139387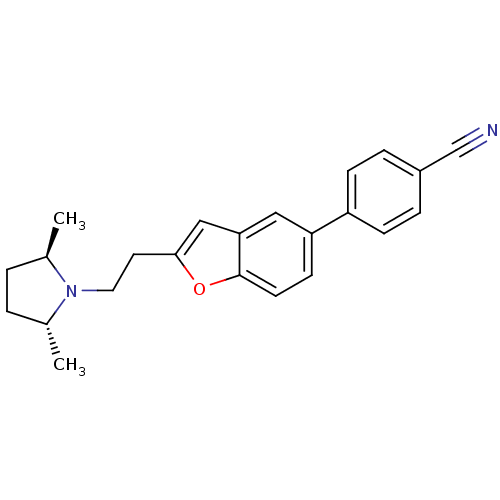 (4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139387 (4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139410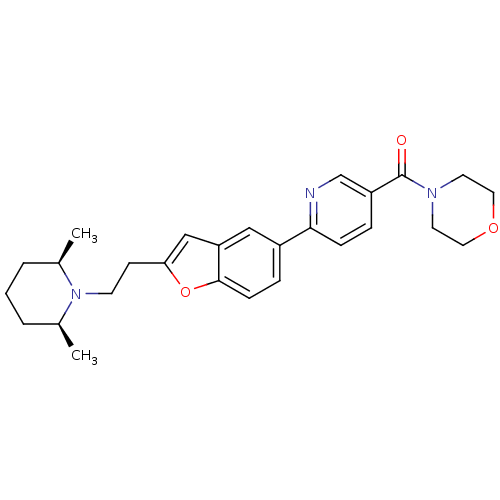 ((6-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139410 ((6-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139394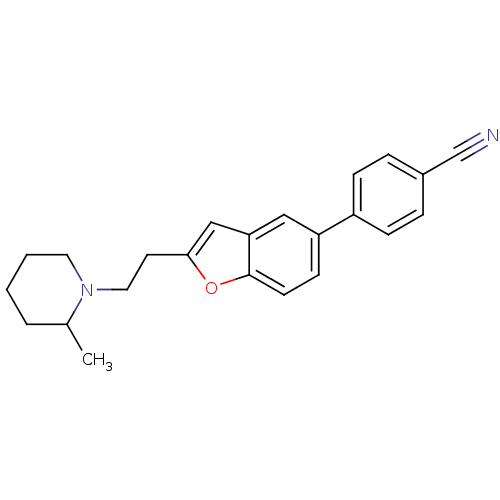 (4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139411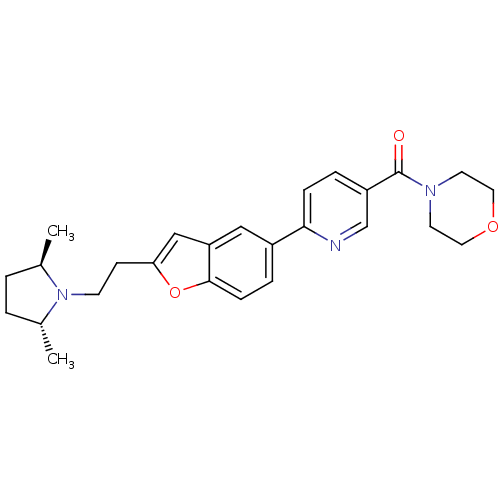 ((6-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139411 ((6-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139398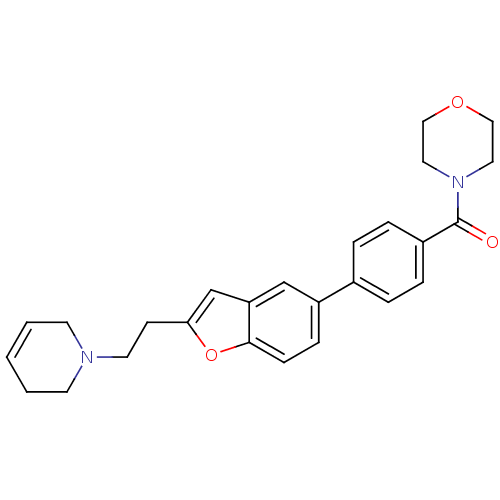 ((4-{2-[2-(3,6-Dihydro-2H-pyridin-1-yl)-ethyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139394 (4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139398 ((4-{2-[2-(3,6-Dihydro-2H-pyridin-1-yl)-ethyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139388 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50404283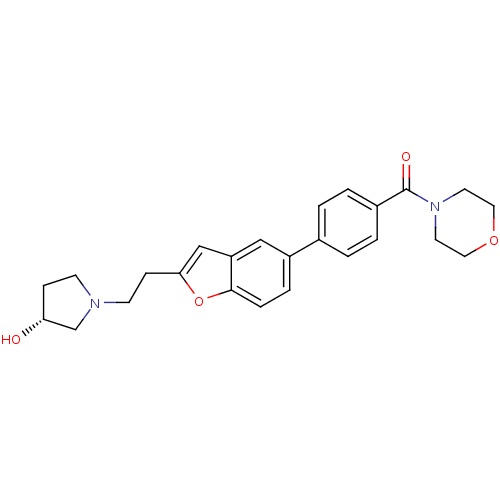 (CHEMBL2112921) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139388 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50404283 (CHEMBL2112921) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139389 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139389 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 152 total ) | Next | Last >> |