

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50169035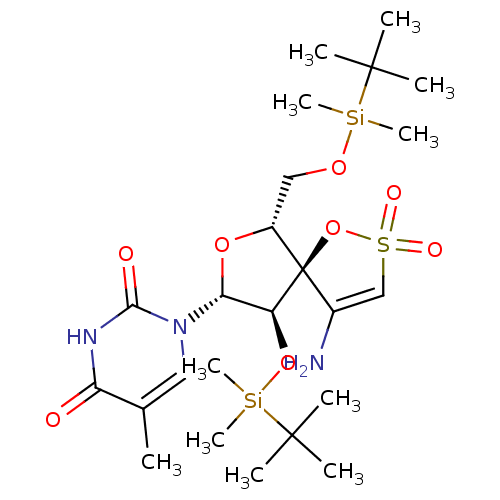 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106919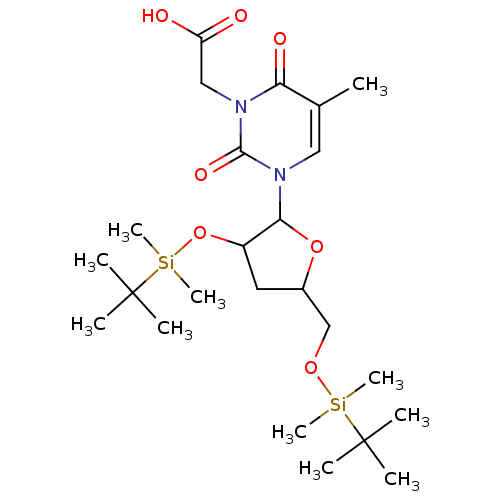 (CHEMBL105018 | {3-[3-(tert-Butyl-dimethyl-silanylo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106919 (CHEMBL105018 | {3-[3-(tert-Butyl-dimethyl-silanylo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50024581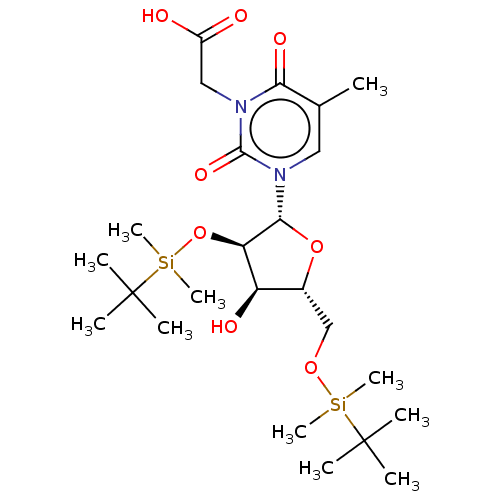 (CHEMBL3142813) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50024581 (CHEMBL3142813) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 mutant GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106903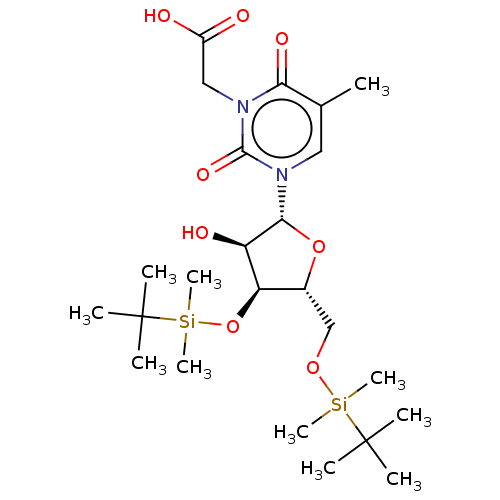 (CHEMBL3142817 | {3-[4-(tert-Butyl-dimethyl-silanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 mutant GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106903 (CHEMBL3142817 | {3-[4-(tert-Butyl-dimethyl-silanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 mutant GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50169035 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 mutant GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403887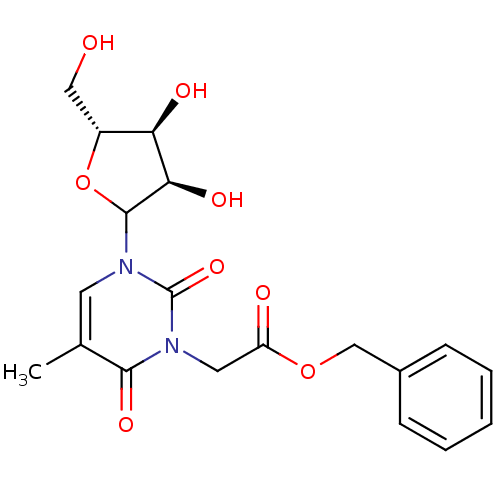 (CHEMBL611868) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403888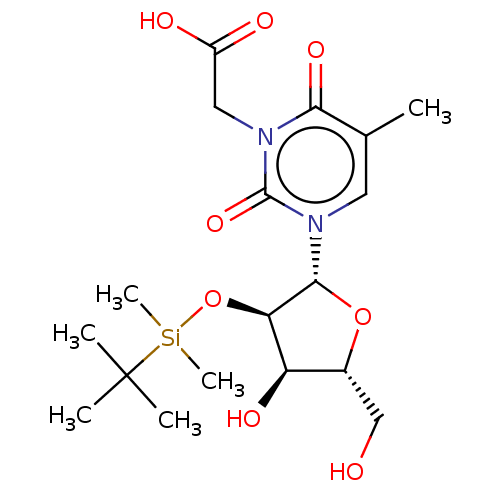 (CHEMBL3142812 | CHEMBL321344) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403889 (CHEMBL318807) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403890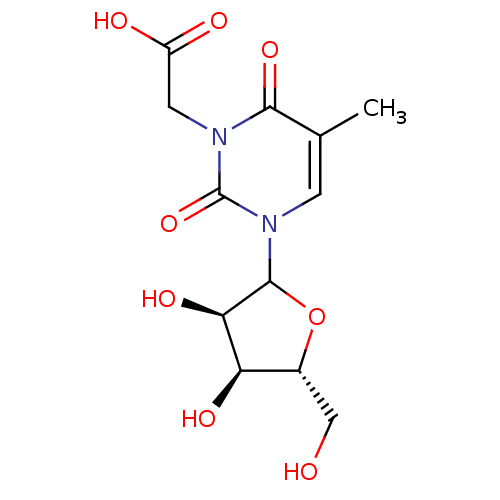 (CHEMBL608622) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403891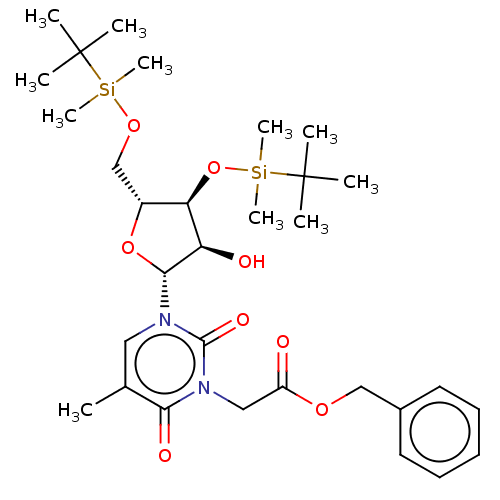 (CHEMBL105831 | CHEMBL3142816) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403892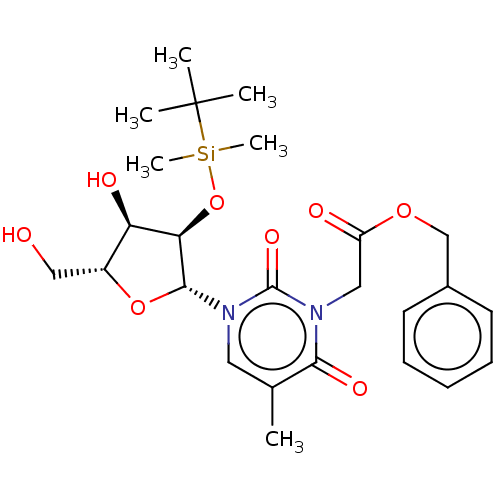 (CHEMBL3142818 | CHEMBL321135) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403893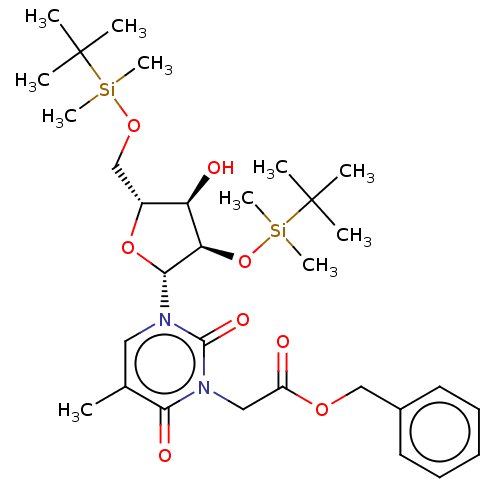 (CHEMBL107000 | CHEMBL3142820) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403894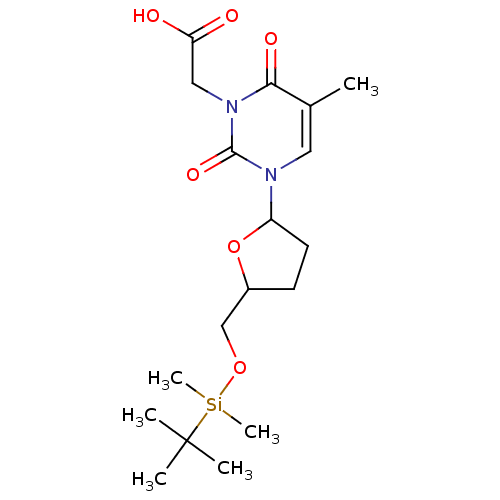 (CHEMBL322101) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106919 (CHEMBL105018 | {3-[3-(tert-Butyl-dimethyl-silanylo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403895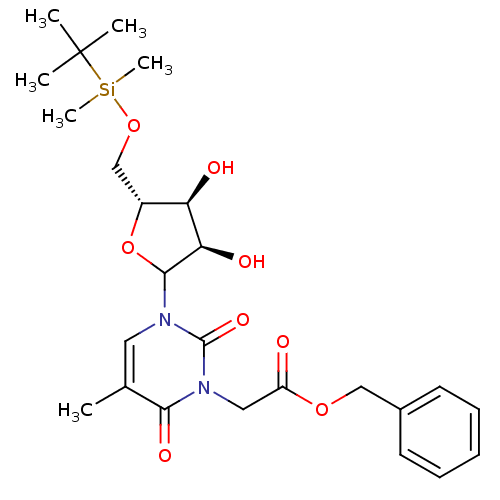 (CHEMBL608621) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403886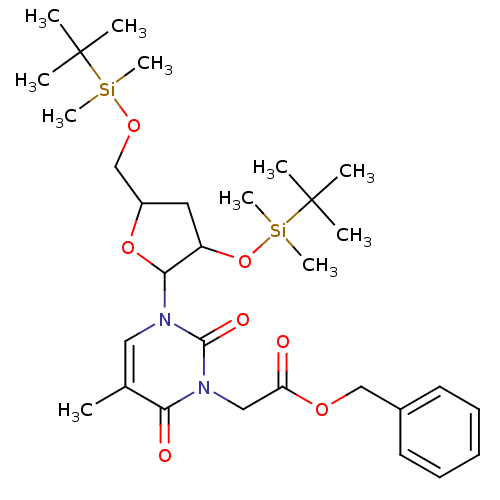 (CHEMBL102269) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403885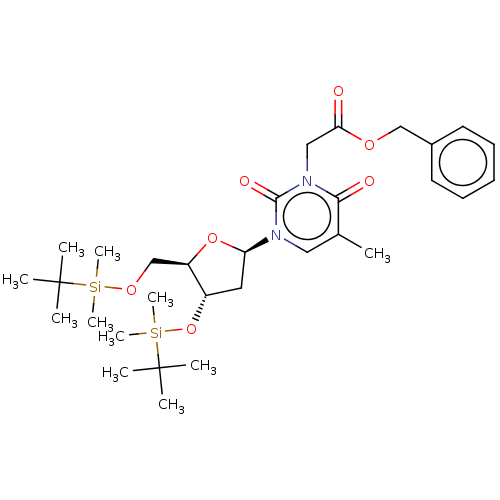 (CHEMBL104540 | CHEMBL3142811) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403896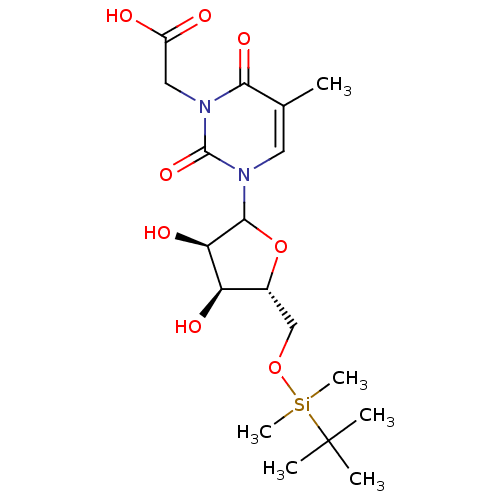 (CHEMBL607754) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403883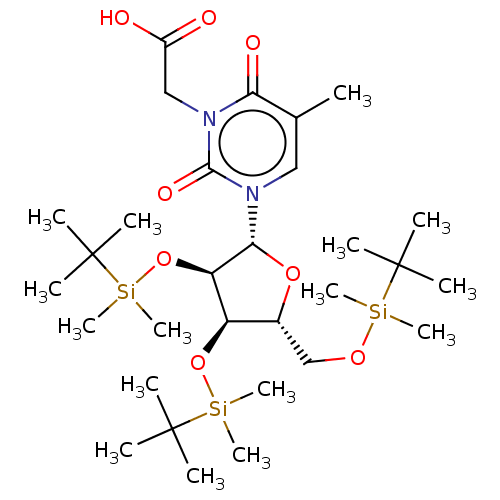 (CHEMBL3142815 | CHEMBL431822) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403882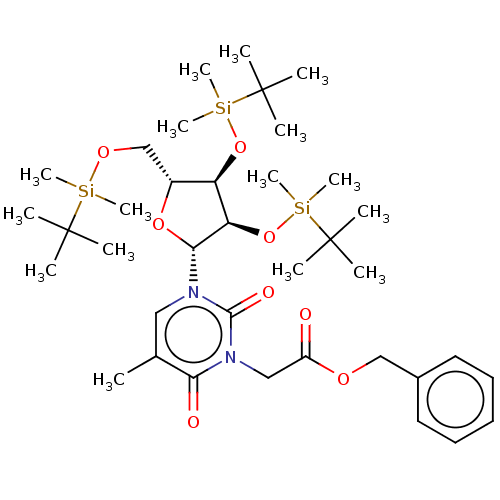 (CHEMBL3142814 | CHEMBL321101) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50024581 (CHEMBL3142813) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106903 (CHEMBL3142817 | {3-[4-(tert-Butyl-dimethyl-silanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50169035 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50403884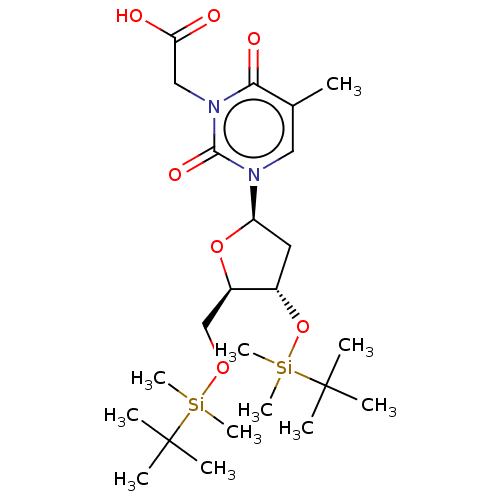 (CHEMBL3142819 | CHEMBL323339) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||