

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50243294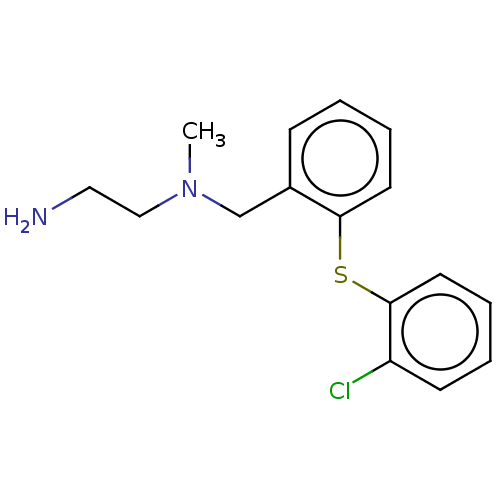 (CHEMBL4094513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of PRMT8 (unknown origin) incubated for 15 mins followed by substrate addition measured after 60 mins by AlphaLisa method | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50243294 (CHEMBL4094513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) incubated for 15 mins followed by substrate addition ... | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378746 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50095355 (CHEMBL3589029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged full-length human PRMT8 expressed in Escherichia coli (BL21(DE3) pre-incubated for 30 mins before addition of a [... | ACS Med Chem Lett 6: 655-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00071 BindingDB Entry DOI: 10.7270/Q20003V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143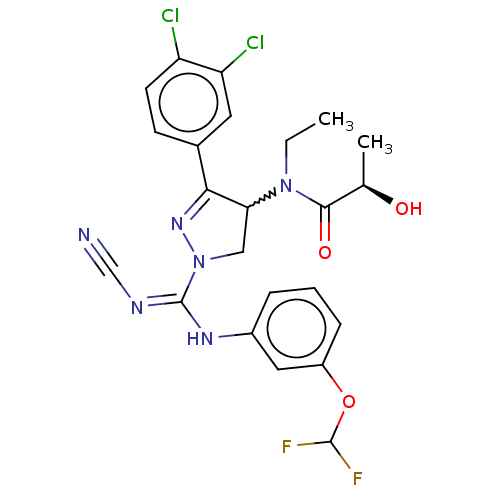 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095537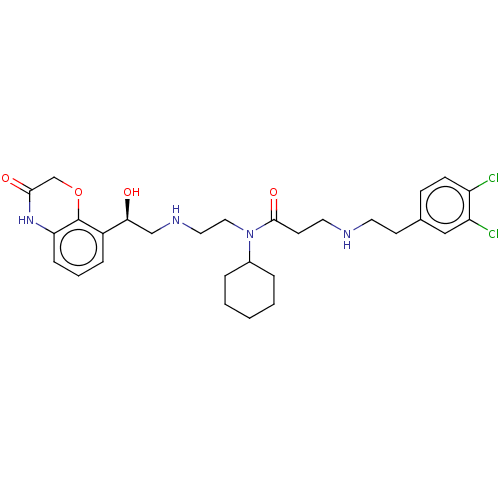 (CHEMBL3590526 | US9598381, 1a (S enantiomer)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378802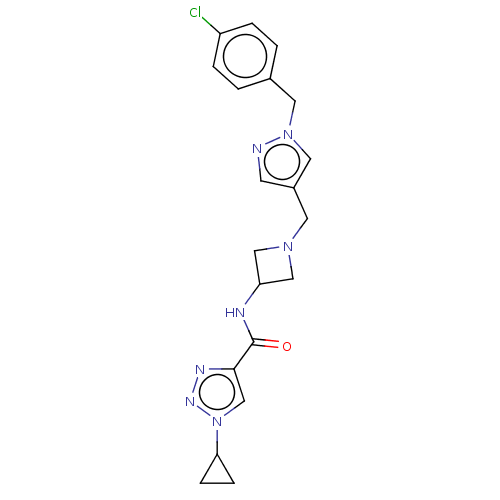 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00325 BindingDB Entry DOI: 10.7270/Q2M330R7 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50599376 (CHEMBL3819011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113880 BindingDB Entry DOI: 10.7270/Q2B56PS9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378747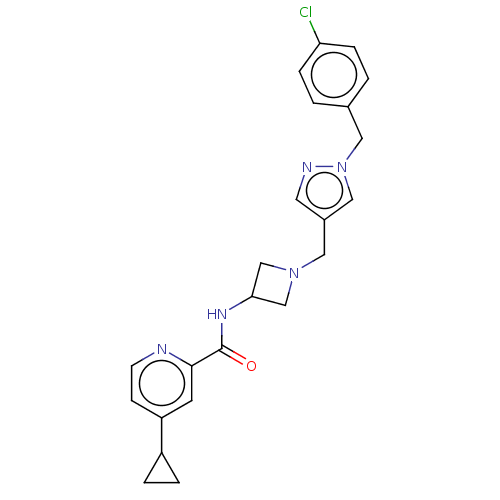 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378802 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378802 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00325 BindingDB Entry DOI: 10.7270/Q2M330R7 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PRMT8 (unknown origin) by scintillation proximity assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02160 BindingDB Entry DOI: 10.7270/Q20V8HN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM512404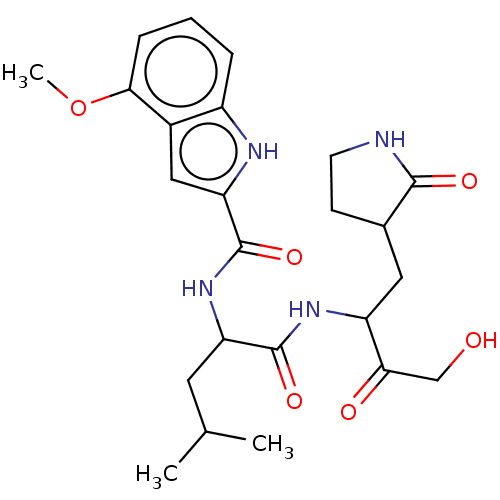 (acs.jmedchem.1c00409_ST.1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This is a review article. Please point to the original journal. | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00409 BindingDB Entry DOI: 10.7270/Q2J1069F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PRMT8 (unknown origin) using biotinylated histone H4 (1 to 24 residues) as substrate in presence of [3H]SAM by scintillation proximity ... | J Med Chem 62: 5414-5433 (2019) Article DOI: 10.1021/acs.jmedchem.9b00297 BindingDB Entry DOI: 10.7270/Q289199B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2005113580 | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description The SARS 3CLpro FRET assay measures the protease catalyzed cleavage of TAMRA- SITSAVLQSGFRKMK-(DABCYL)-OH to TAMRA - SITSAVLQ and SGFRKMK- (DABCYL)-O... | WIPO (2005) BindingDB Entry DOI: 10.7270/Q2PV6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT8 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
A*STAR | Assay Description The assays are from references cited in this article. | Bioorg Med Chem Lett 48: 128263 (2021) Article DOI: 10.1016/j.bmcl.2021.128263 BindingDB Entry DOI: 10.7270/Q27084H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50243294 (CHEMBL4094513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) using biotinylated H4 peptide (1 to 21 residues) as s... | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283194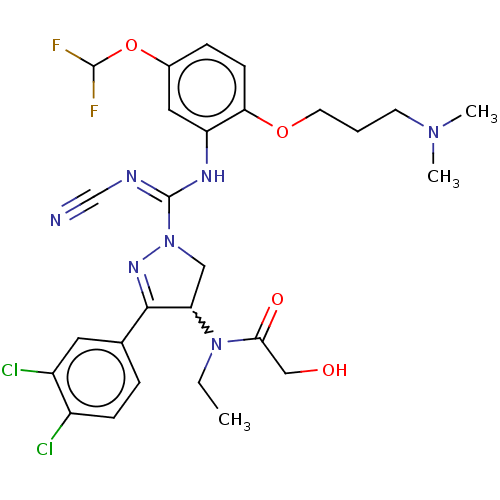 (Rac-N-[1-(N′-cyano-N-{5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.55 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095536 (CHEMBL3590527 | US9598381, 1 (racemate)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50519299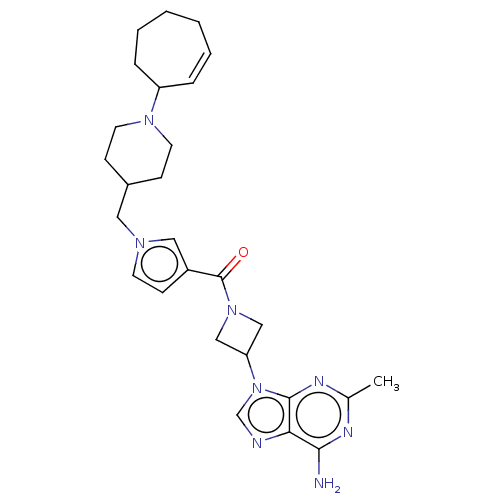 (CHEMBL4536230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SMYD2 (1 to 433 residues) expressed in baculovirus infected Sf9 insect cells using biotinylated-p53 peptide (361 to 3... | J Med Chem 62: 7669-7683 (2019) Article DOI: 10.1021/acs.jmedchem.9b00112 BindingDB Entry DOI: 10.7270/Q2R78JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 (Rattus norvegicus) | BDBM50243295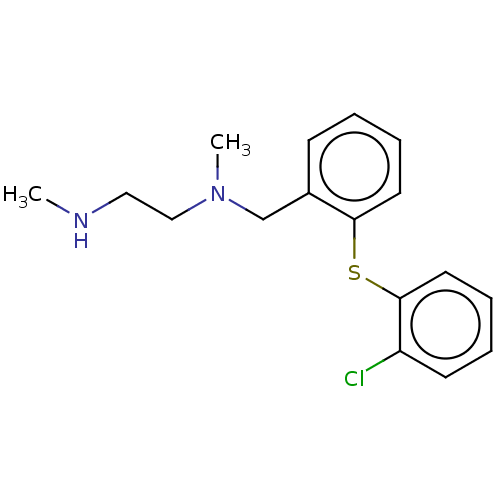 (CHEMBL4084293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University Curated by ChEMBL | Assay Description Inhibition of rat His-tagged PRMT1 (11 to 353 residues) expressed in Escherichia coli BL21(DE3) using biotinylated H4 peptide (1 to 21 residues) as s... | J Med Chem 60: 8888-8905 (2017) Article DOI: 10.1021/acs.jmedchem.7b01134 BindingDB Entry DOI: 10.7270/Q27D2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM496954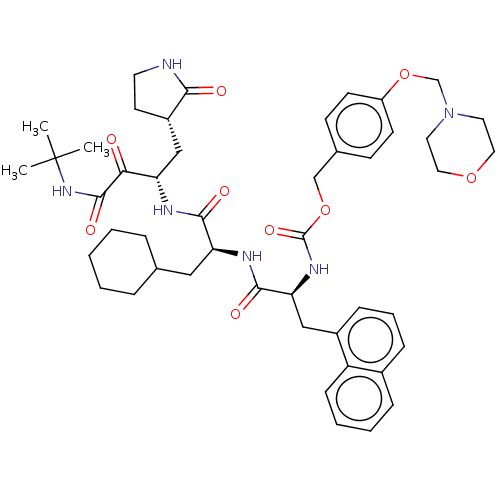 (cmdc.202100576, 22b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283173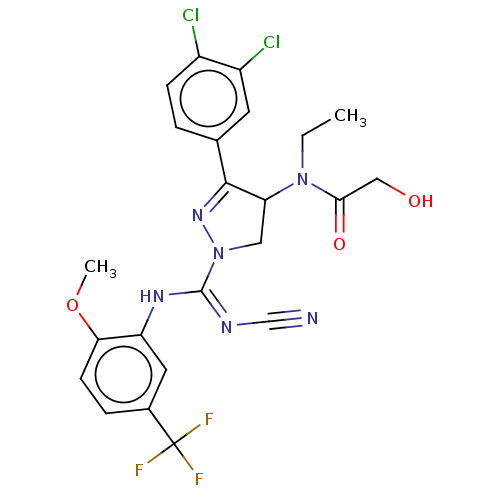 (N-[1-{N′-cyano-N-[2-methoxy-5-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM496955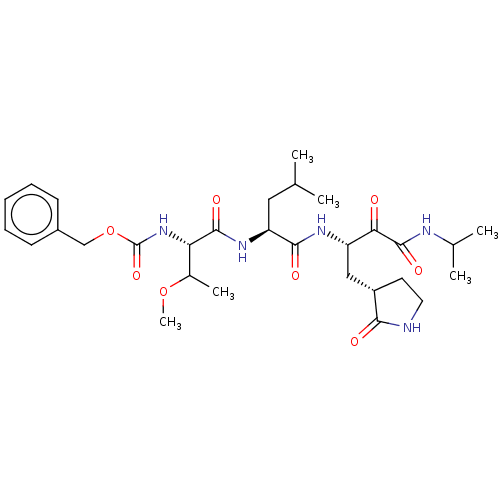 (cmdc.202100576, 22c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM496956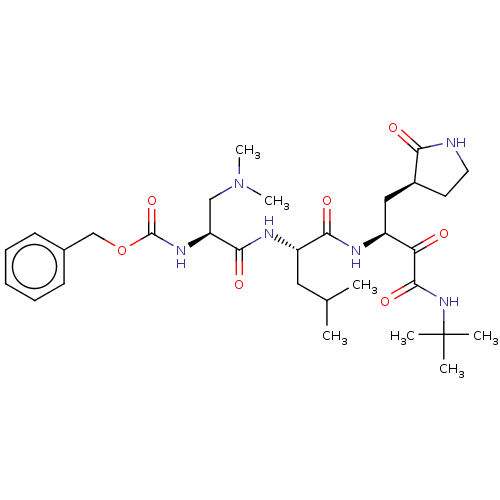 (cmdc.202100576, 22d) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM496957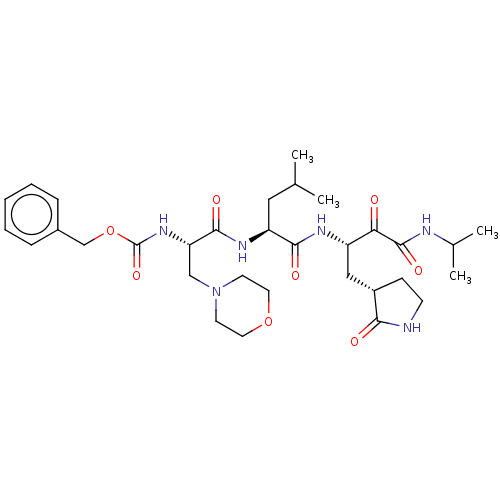 (cmdc.202100576, 22e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM496958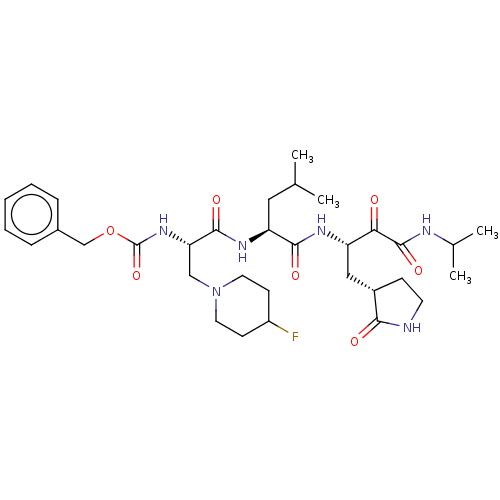 (cmdc.202100576, 22f) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre | Assay Description Please point to the patents. | ChemMedChem (2021) Article DOI: 10.1002/cmdc.202100576 BindingDB Entry DOI: 10.7270/Q27M0C3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50095354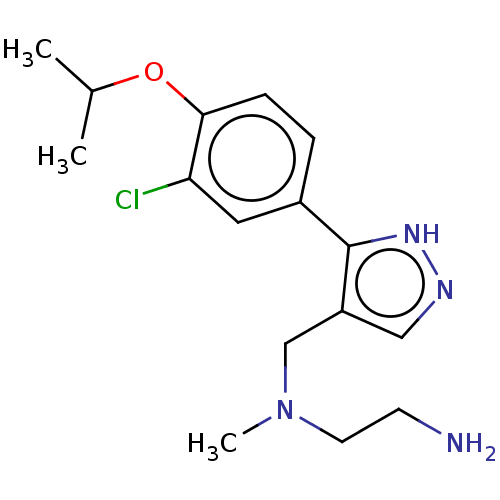 (CHEMBL3589030) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged full-length human PRMT8 expressed in Escherichia coli (BL21(DE3) pre-incubated for 30 mins before addition of a [... | ACS Med Chem Lett 6: 655-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00071 BindingDB Entry DOI: 10.7270/Q20003V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM476979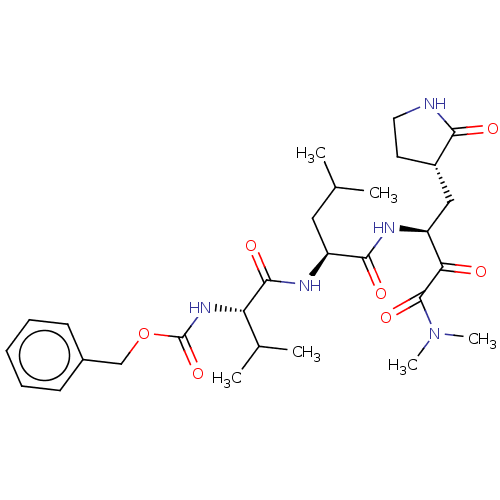 (peptidomimetic coronavirus 3CLpro inhibitors 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
A*STAR | Assay Description The assays are from references cited in this article. | Bioorg Med Chem Lett 48: 128263 (2021) Article DOI: 10.1016/j.bmcl.2021.128263 BindingDB Entry DOI: 10.7270/Q27084H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM512408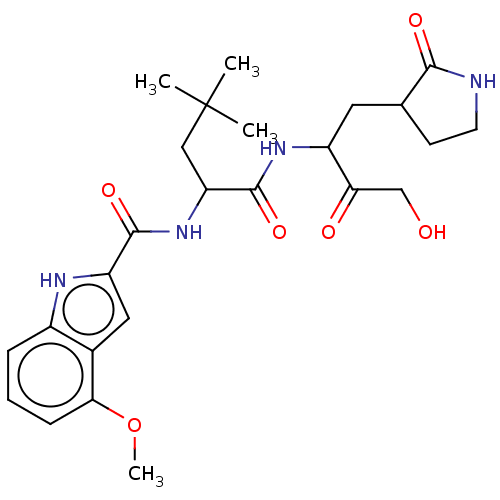 (acs.jmedchem.1c00409_ST.5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This is a review article. Please point to the original journal. | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00409 BindingDB Entry DOI: 10.7270/Q2J1069F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378750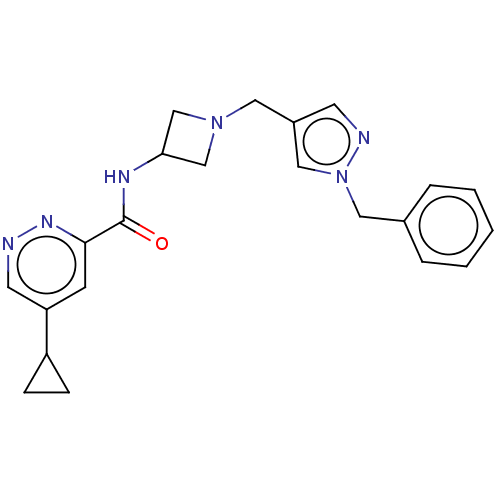 (N-(1-((1-benzyl-1H-pyrazol-4-yl)methyl)azetidin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
A*STAR | Assay Description SARS-CoV-2 3CLpro expression and purification is based on a published procedure and our modified protocol is found in the supplementary file. A highl... | Bioorg Med Chem Lett 48: 128263 (2021) Article DOI: 10.1016/j.bmcl.2021.128263 BindingDB Entry DOI: 10.7270/Q27084H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50519300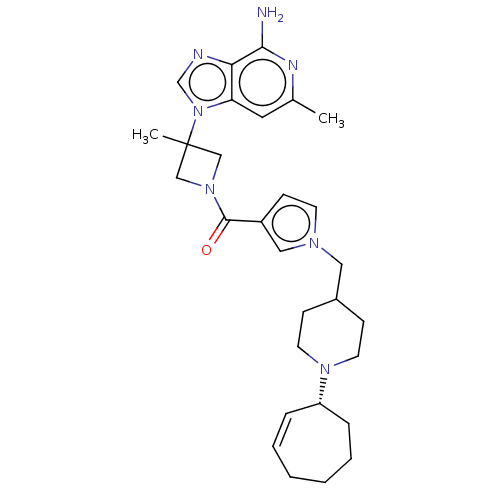 (CHEMBL4520134) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human SMYD2 (1 to 433 residues) expressed in baculovirus infected Sf9 insect cells using biotinylated-p53 peptide (361 to 3... | J Med Chem 62: 7669-7683 (2019) Article DOI: 10.1021/acs.jmedchem.9b00112 BindingDB Entry DOI: 10.7270/Q2R78JK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM476989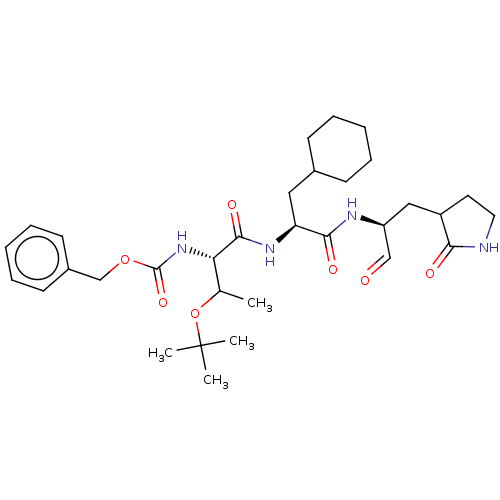 (TG-0205221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
A*STAR | Assay Description SARS-CoV-2 3CLpro expression and purification is based on a published procedure and our modified protocol is found in the supplementary file. A highl... | Bioorg Med Chem Lett 48: 128263 (2021) Article DOI: 10.1016/j.bmcl.2021.128263 BindingDB Entry DOI: 10.7270/Q27084H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283196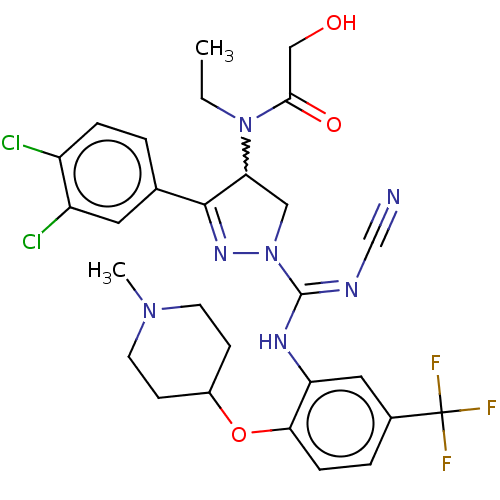 (Rac-N-[1-(N′-cyano-N-{2-[(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.09 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283172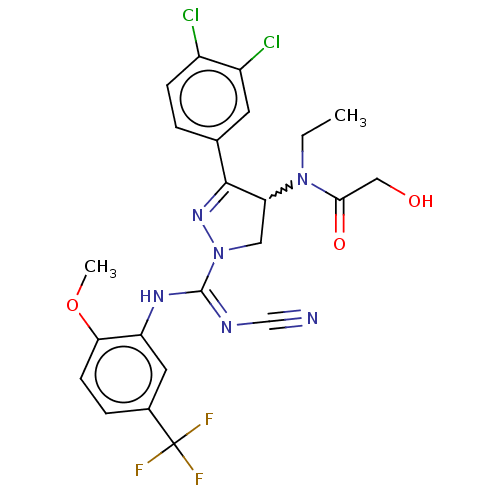 (Rac-N-[1-{N′-cyano-N-[2-methoxy-5-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.98 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50095353 (CHEMBL3589031) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged full-length human PRMT8 expressed in Escherichia coli (BL21(DE3) pre-incubated for 30 mins before addition of a [... | ACS Med Chem Lett 6: 655-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00071 BindingDB Entry DOI: 10.7270/Q20003V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50095432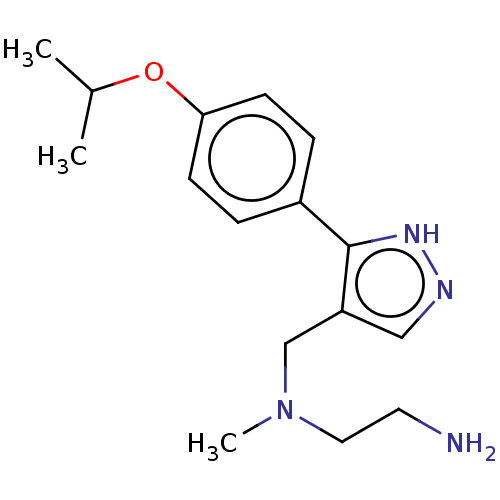 (CHEMBL3589026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged full-length human PRMT8 expressed in Escherichia coli (BL21(DE3) pre-incubated for 30 mins before addition of a [... | ACS Med Chem Lett 6: 655-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00071 BindingDB Entry DOI: 10.7270/Q20003V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50095356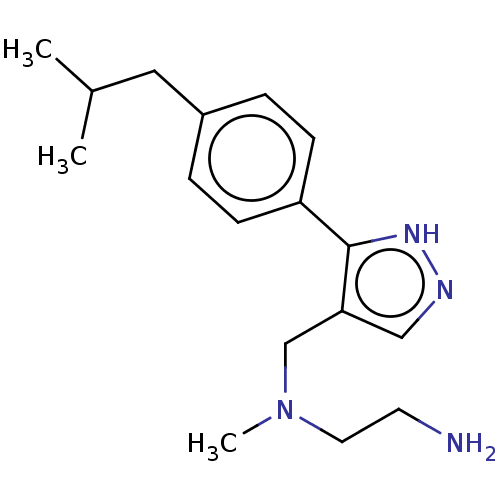 (CHEMBL3589028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST tagged full-length human PRMT8 expressed in Escherichia coli (BL21(DE3) pre-incubated for 30 mins before addition of a [... | ACS Med Chem Lett 6: 655-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00071 BindingDB Entry DOI: 10.7270/Q20003V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378744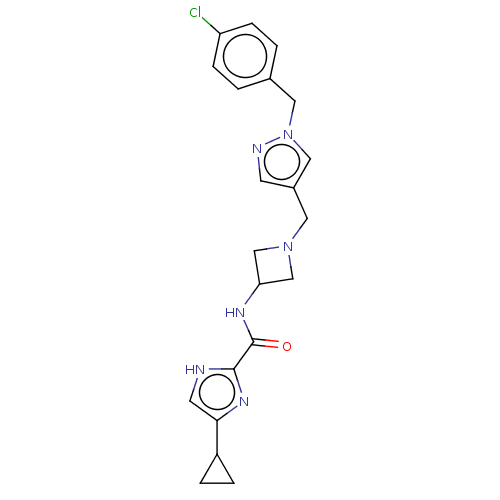 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50550528 (CHEMBL4756148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SMYD2 (unknown origin) | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2SKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283169 (Rac-N-[1-{N′-cyano-N-[5-(difluoromethoxy)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378783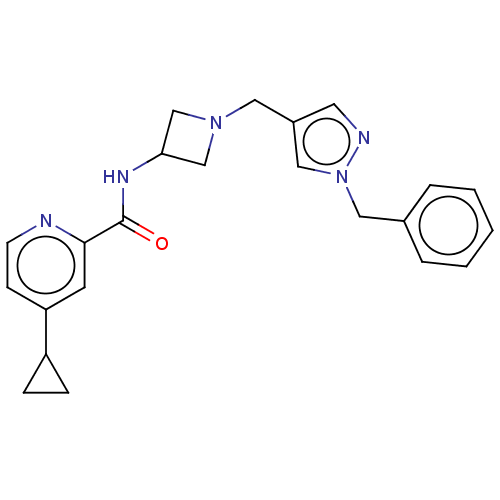 (N-(1-((1-benzyl-1H-pyrazol-4-yl)methyl)azetidin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283143 ((2R)—N-[1-{N′-cyano-N-[3-(difluorometho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139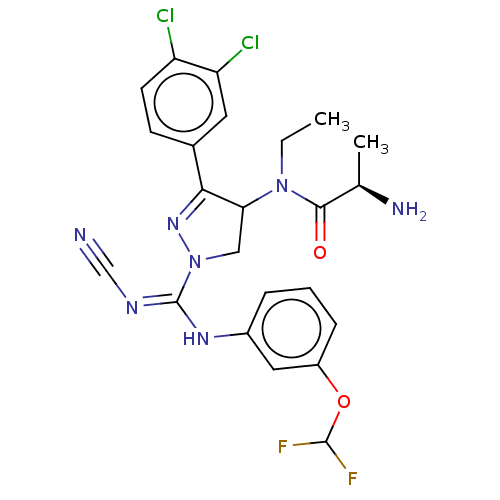 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the detection of SMYD2 cellular methylation activity an In Cell Western (ICW) assay was established. This assay allows rapid processing of multip... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM283139 (N-[1-{N′-cyano-N-[3-(difluoromethoxy)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 9.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description SMYD2 inhibitory activities of the compounds described in the present invention were quantified using a scintillation proximity assay (SPA) which mea... | US Patent US10023539 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM429298 (jm5b01461, Compound 118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn | Assay Description This is a review article. | J Med Chem 59: 6595-628 (2016) Article DOI: 10.1021/acs.jmedchem.5b01461 BindingDB Entry DOI: 10.7270/Q2PK0JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1936 total ) | Next | Last >> |