

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM50378784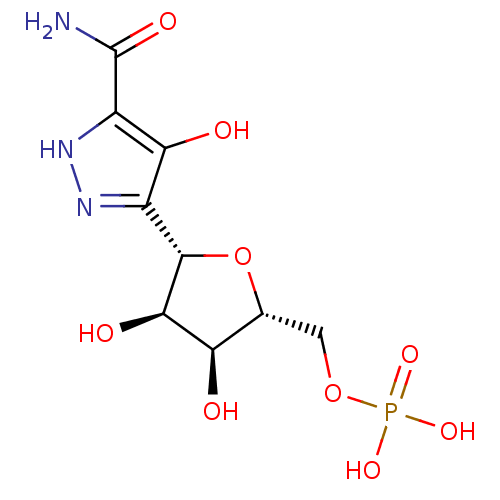 (CHEMBL1164953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 17 | -44.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of human uridine 5'-monophosphate synthase after overnight incubation at room temperature by UV spectroscopy | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM50459997 (47599 | CHEBI:90284 | Pirazofurin | Pyrazofurin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of human ODCase | Bioorg Med Chem 26: 551-565 (2018) Article DOI: 10.1016/j.bmc.2017.11.037 BindingDB Entry DOI: 10.7270/Q2639SBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214779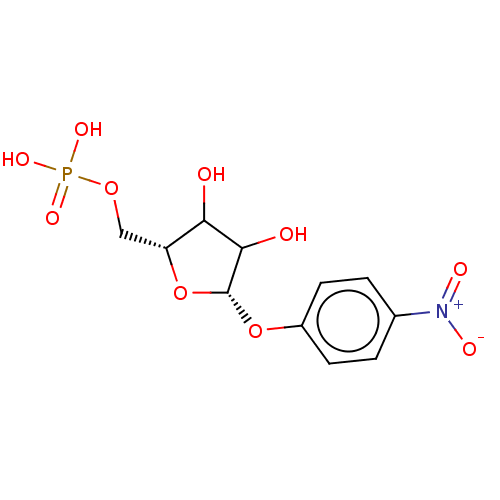 (OPRT inhibitor, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | -42.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214786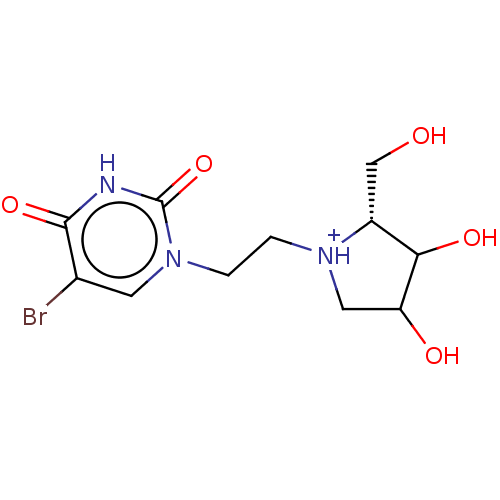 (OPRT inhibitor, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214791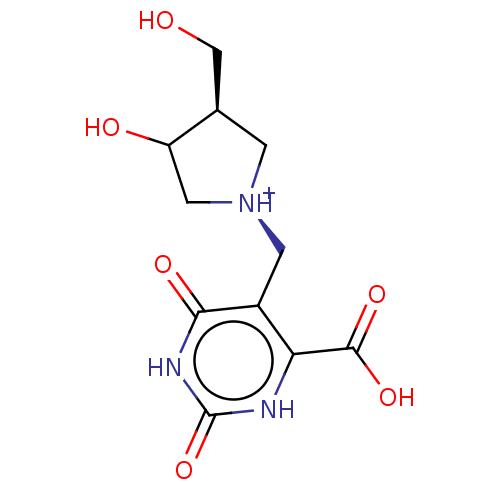 (OPRT inhibitor, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 83 | -40.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214785 (OPRT inhibitor, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | -40.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214784 (OPRT inhibitor, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214797 (OPRT inhibitor, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214778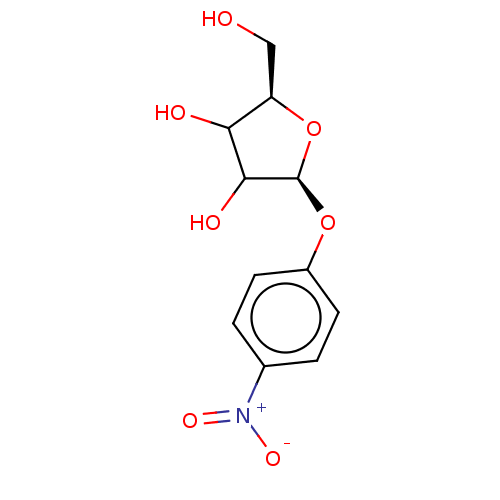 (OPRT inhibitor, 1 ) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214783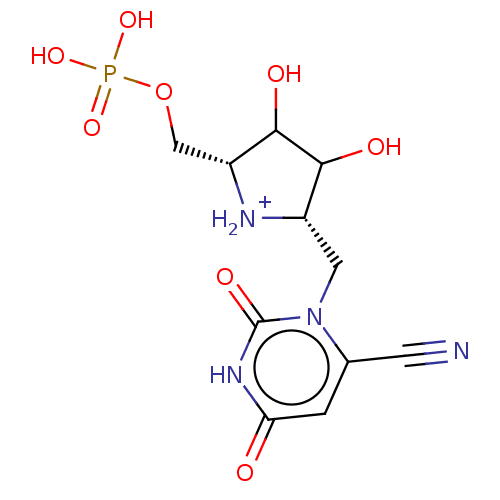 (OPRT inhibitor, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | -39.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214796 (OPRT inhibitor, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | -38.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21337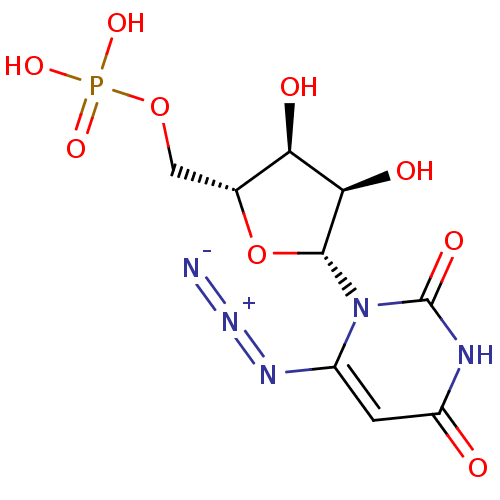 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21337 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214781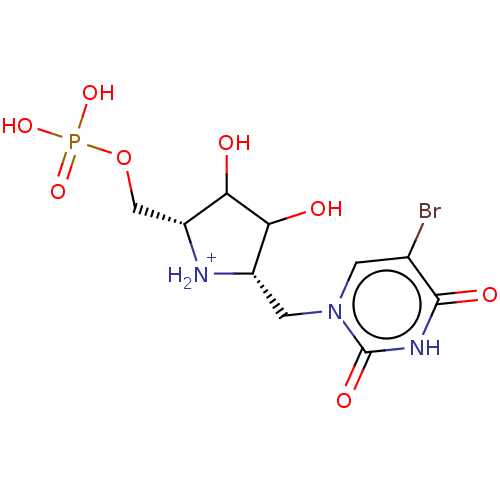 (OPRT inhibitor, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214794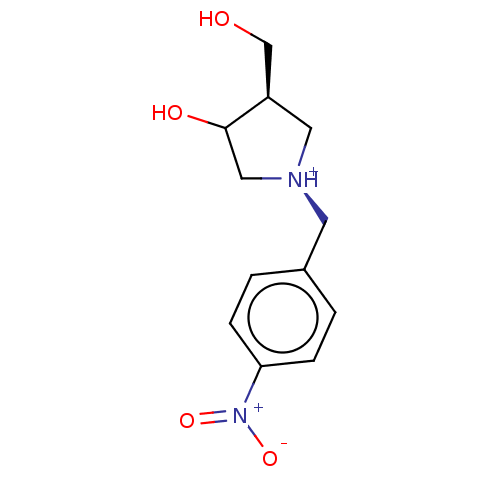 (OPRT inhibitor, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214793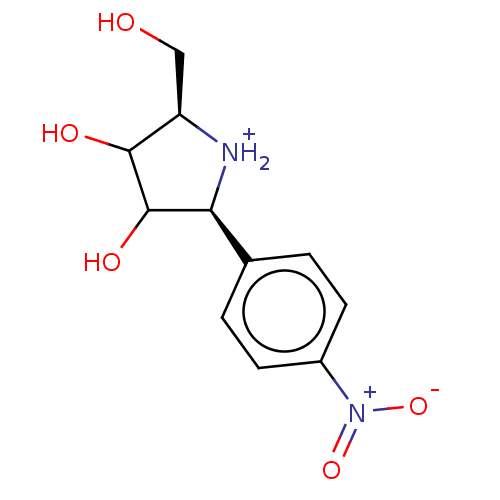 (OPRT inhibitor, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 230 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214795 (OPRT inhibitor, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 230 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214792 (OPRT inhibitor, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | -37.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214790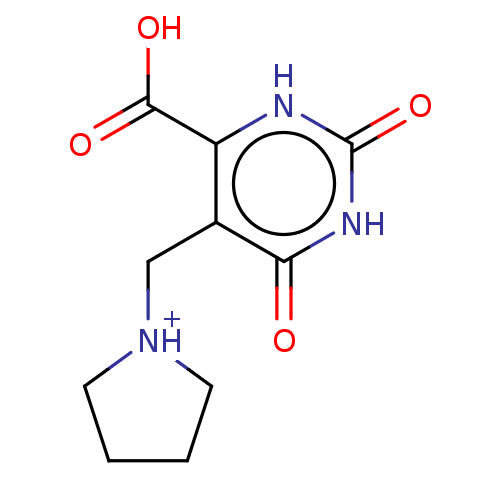 (OPRT inhibitor, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 250 | -37.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214787 (OPRT inhibitor, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | -37.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27946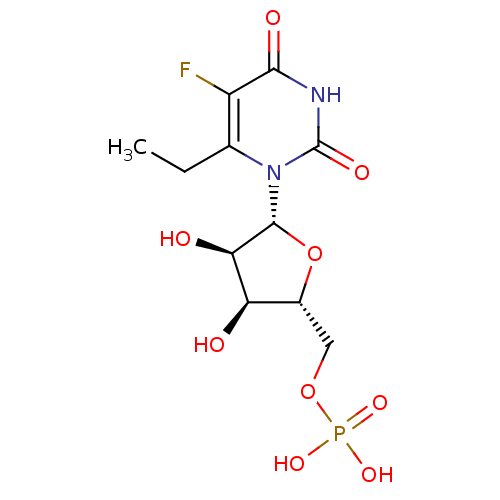 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214782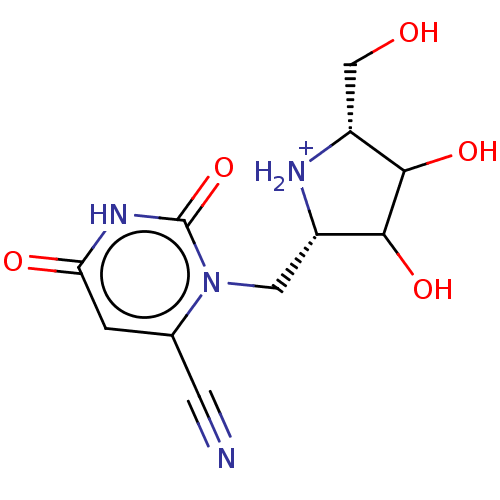 (OPRT inhibitor, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27944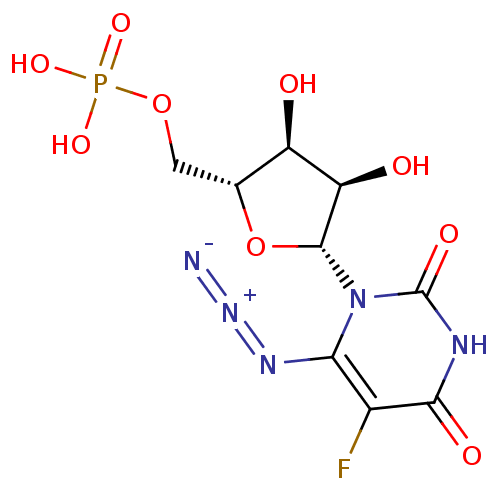 (uridine derivative, 41 | {[(2R,3S,4R,5R)-5-(6-azid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214788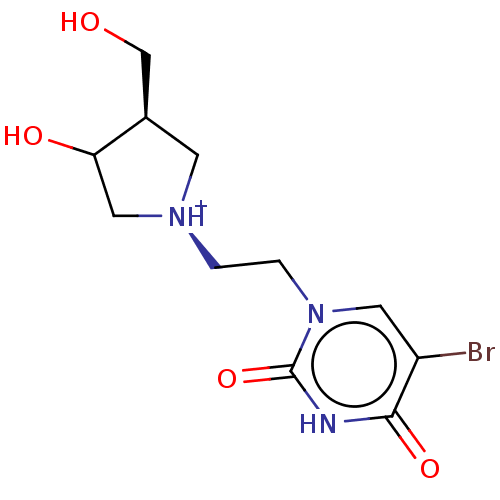 (OPRT inhibitor, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 520 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214780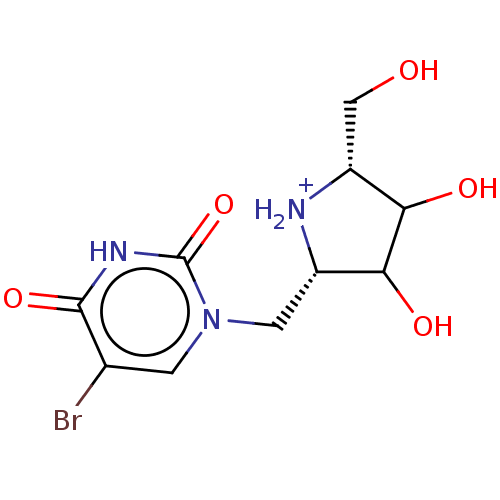 (OPRT inhibitor, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | -35.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM50341908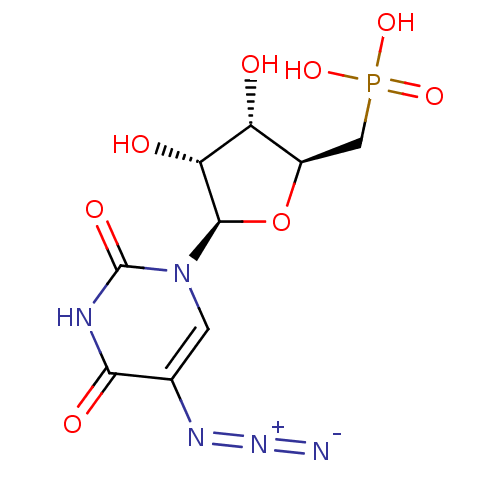 (((2S,3S,4R,5R)-5-(5-azido-2,4-dioxo-3,4-dihydropyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Irreversible inhibition of Methanobacterium thermoautotrophicum 5'-monophosphate decarboxylase | J Med Chem 54: 2891-901 (2011) Article DOI: 10.1021/jm101642g BindingDB Entry DOI: 10.7270/Q2V98924 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338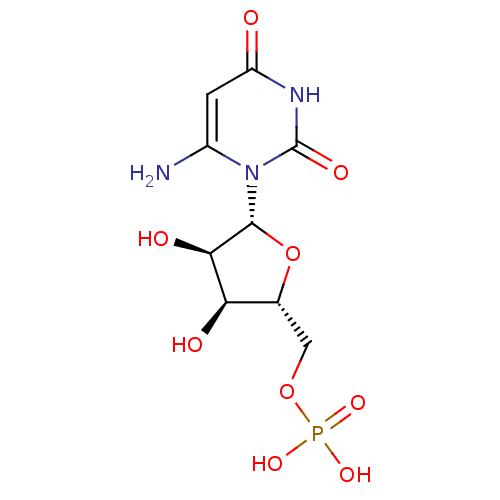 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21340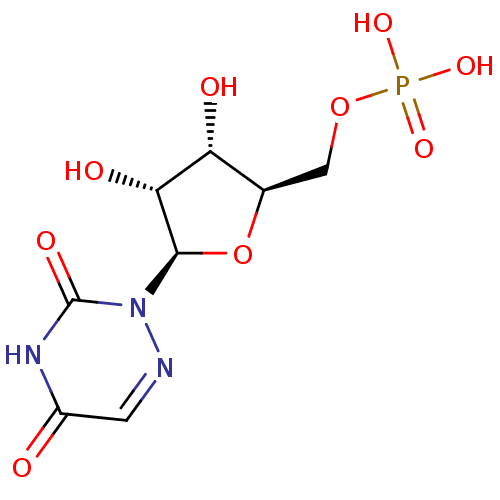 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ODCase at 25 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+3 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21337 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 2.00E+3 | -33.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.10E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214789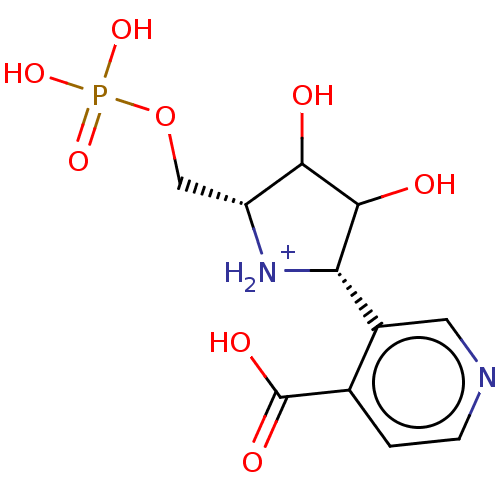 (OPRT inhibitor, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM50459997 (47599 | CHEBI:90284 | Pirazofurin | Pyrazofurin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase | Bioorg Med Chem 26: 551-565 (2018) Article DOI: 10.1016/j.bmc.2017.11.037 BindingDB Entry DOI: 10.7270/Q2639SBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM47402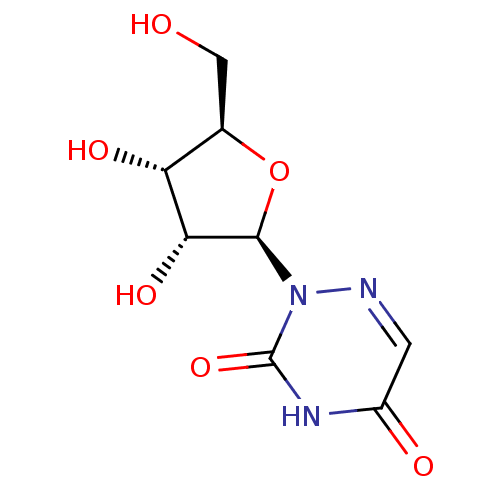 (2-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase | Bioorg Med Chem 26: 551-565 (2018) Article DOI: 10.1016/j.bmc.2017.11.037 BindingDB Entry DOI: 10.7270/Q2639SBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM50398698 (CHEMBL2178721) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.14E+4 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.24E+4 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.66E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM50398698 (CHEMBL2178721) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21335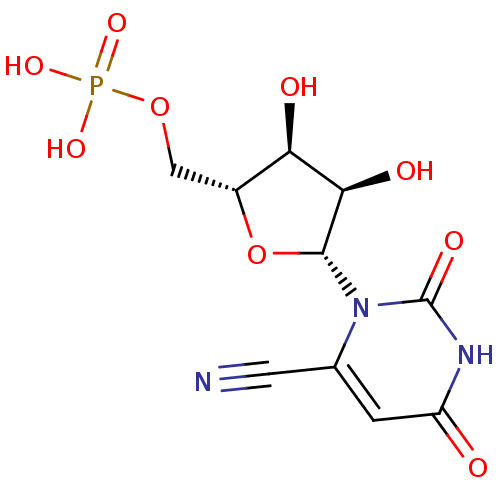 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+4 | -27.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM50398698 (CHEMBL2178721) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of human ODCase by isothermal titration calorimetry | J Med Chem 55: 9988-97 (2012) Article DOI: 10.1021/jm301176r BindingDB Entry DOI: 10.7270/Q2DR2WPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27946 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.41E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |