

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM132084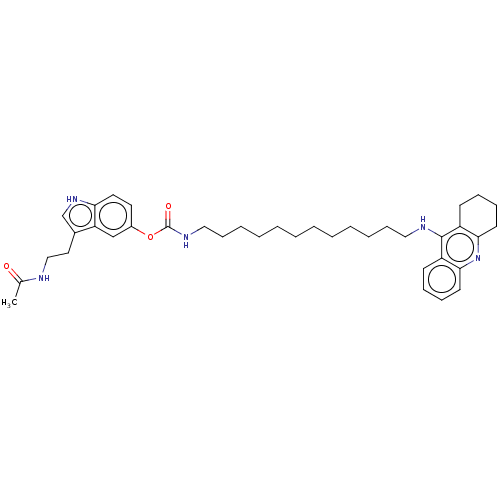 (US8841453, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132080 (US8841453, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132071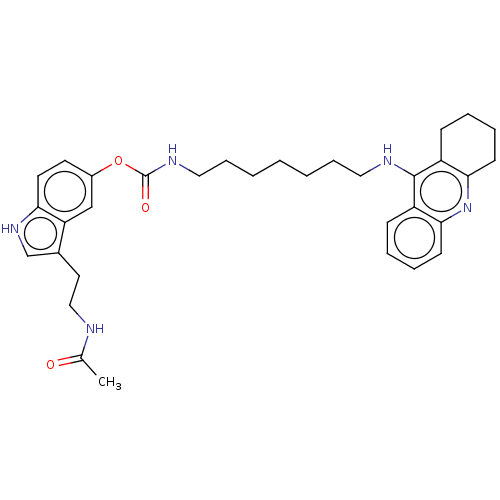 (US8841453, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132083 (US8841453, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132074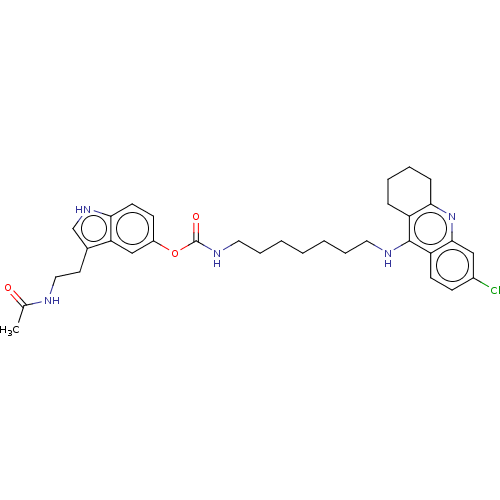 (US8841453, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132088 (US8841453, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132081 (US8841453, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132083 (US8841453, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132086 (US8841453, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132087 (US8841453, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132085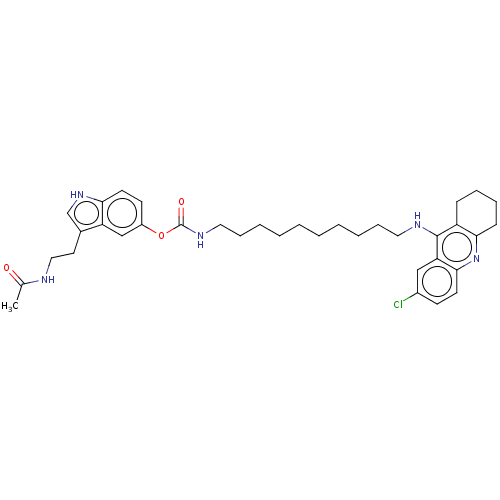 (US8841453, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132085 (US8841453, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132073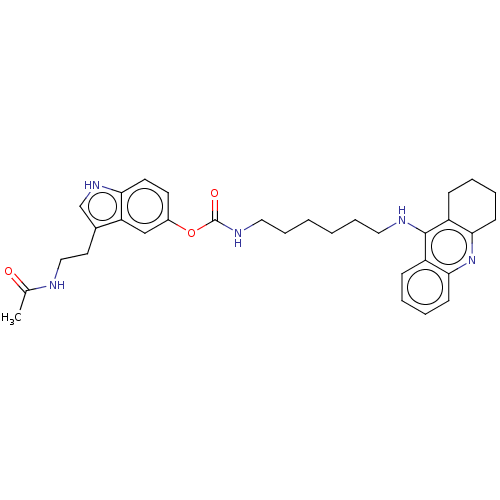 (US8841453, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132079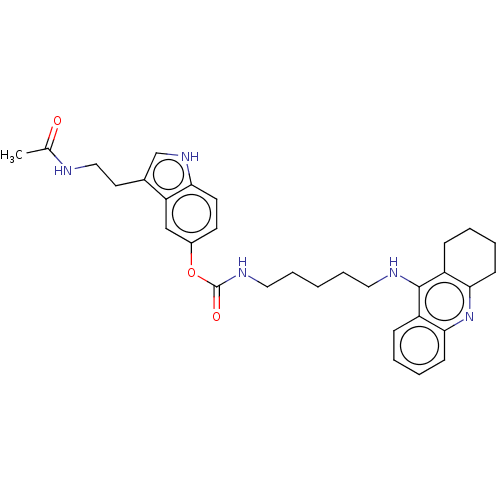 (US8841453, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132076 (US8841453, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132084 (US8841453, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132072 (US8841453, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132080 (US8841453, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132088 (US8841453, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132074 (US8841453, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132086 (US8841453, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132082 (US8841453, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132078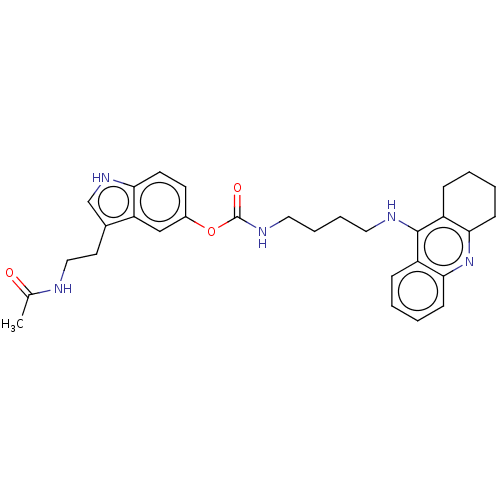 (US8841453, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132075 (US8841453, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132087 (US8841453, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132071 (US8841453, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132075 (US8841453, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132076 (US8841453, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132081 (US8841453, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM132077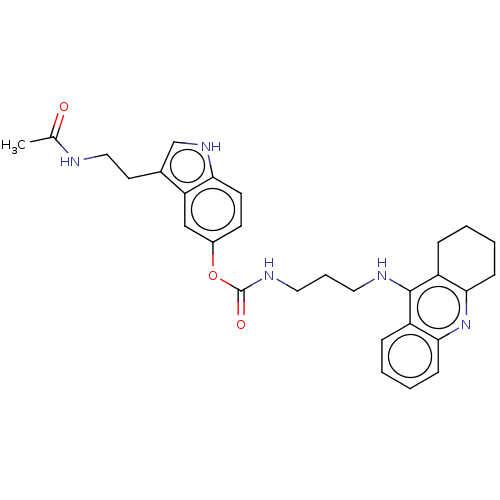 (US8841453, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132073 (US8841453, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132079 (US8841453, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132077 (US8841453, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132078 (US8841453, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132072 (US8841453, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM132082 (US8841453, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego US Patent | Assay Description The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... | US Patent US8841453 (2014) BindingDB Entry DOI: 10.7270/Q2W957VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||