

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139046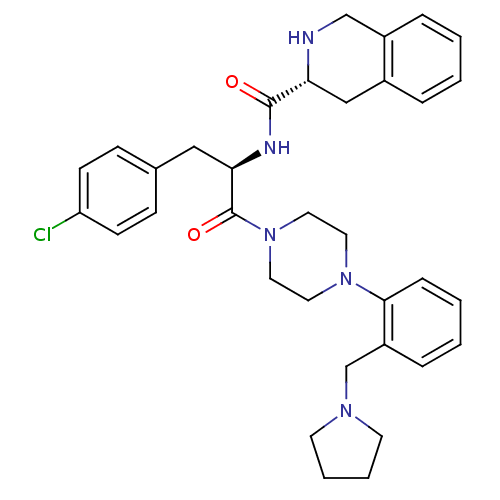 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139032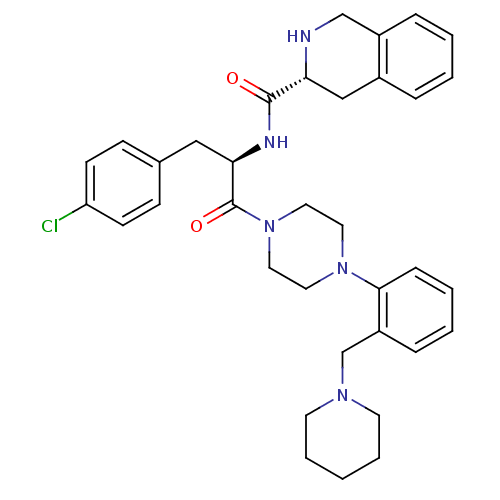 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139027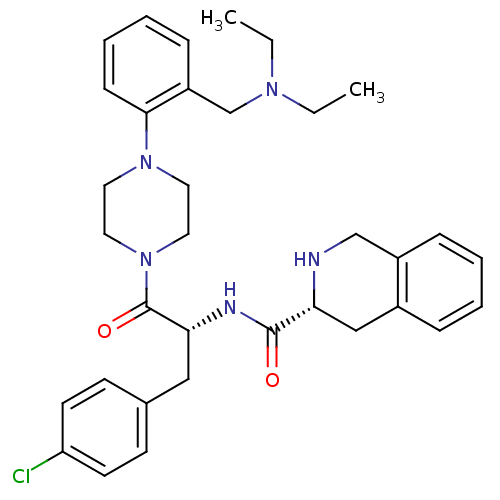 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139043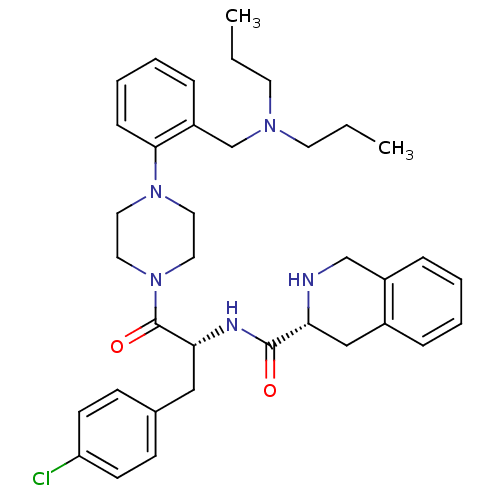 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139028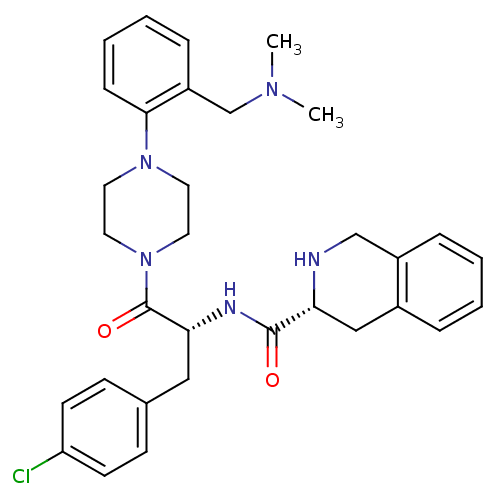 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139023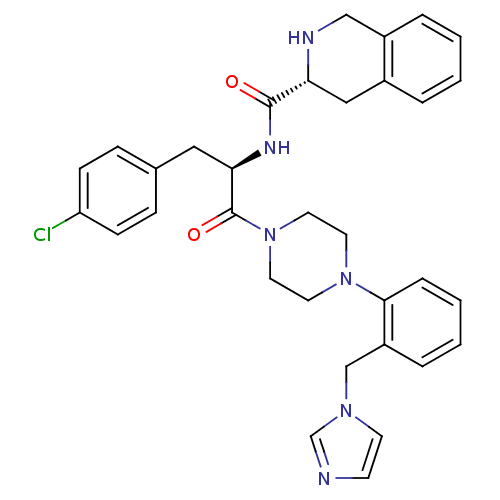 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139029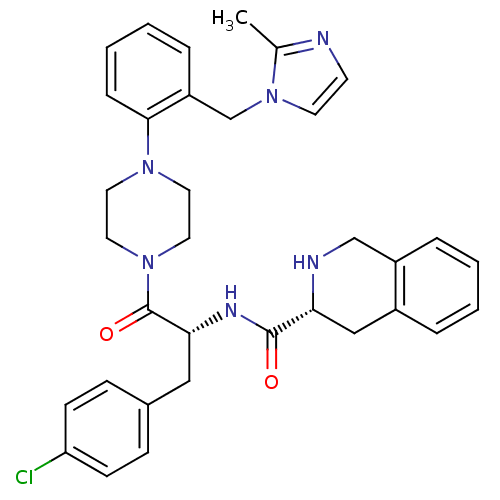 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139036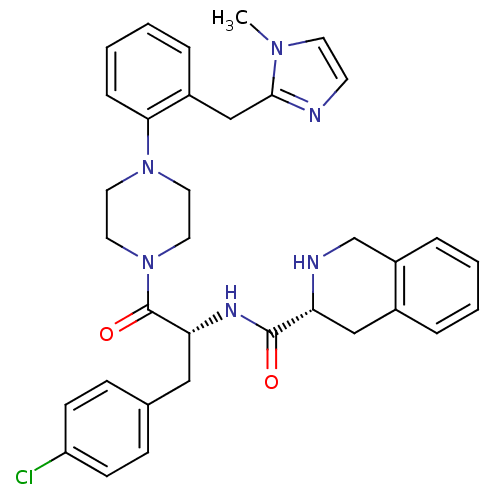 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139045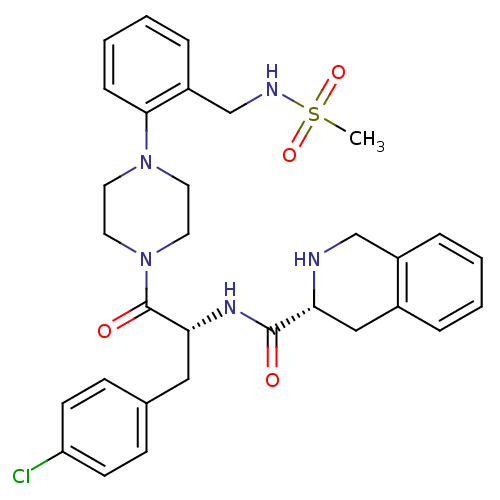 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50134496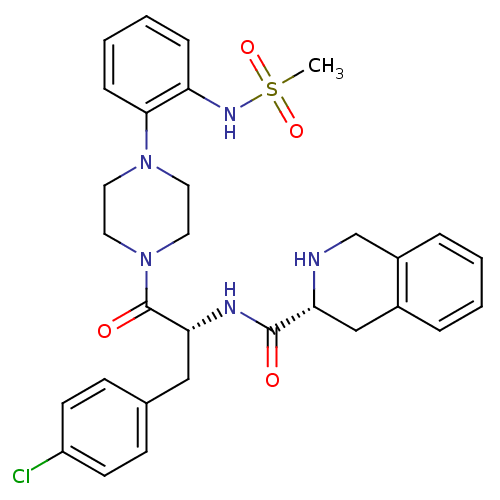 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139047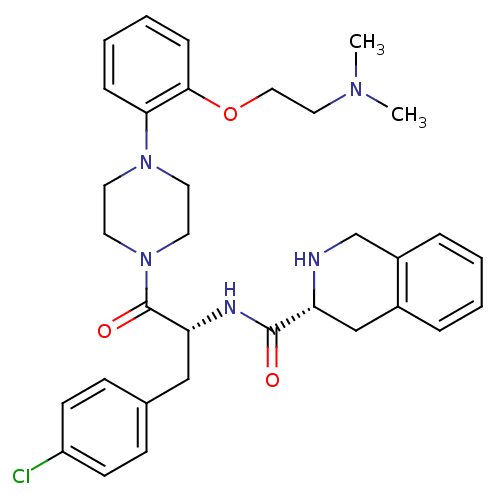 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139048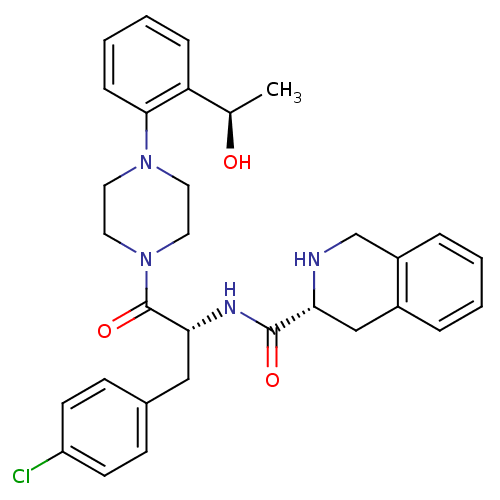 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139044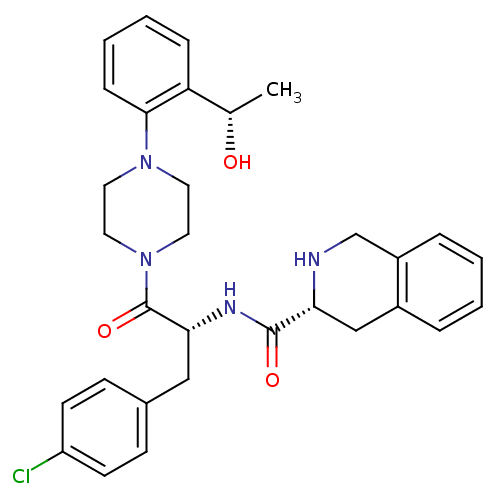 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139040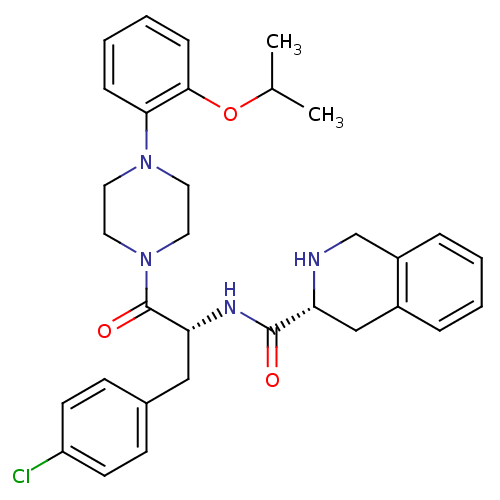 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139025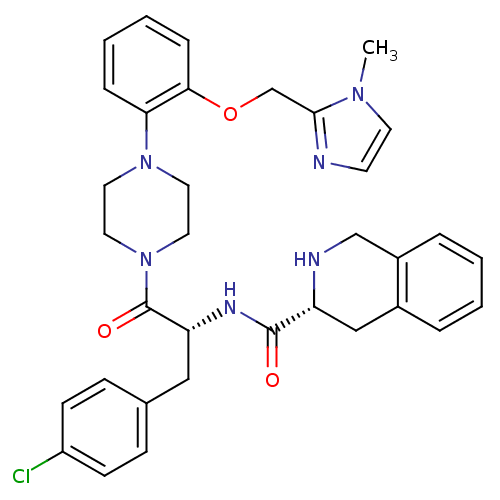 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139022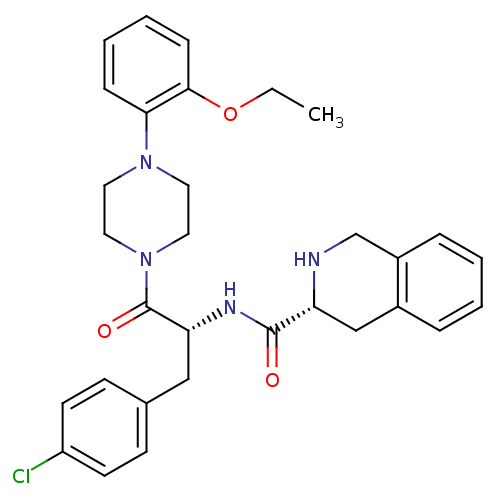 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139038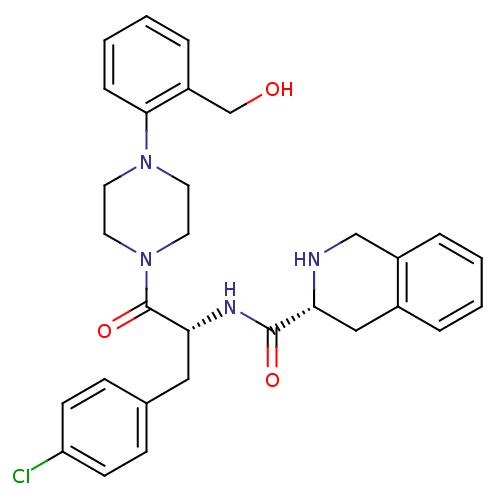 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139030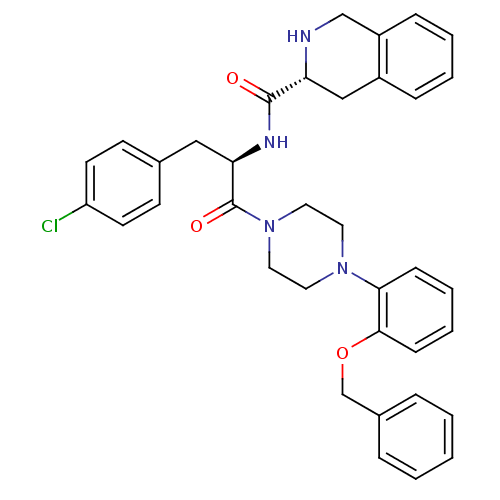 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139031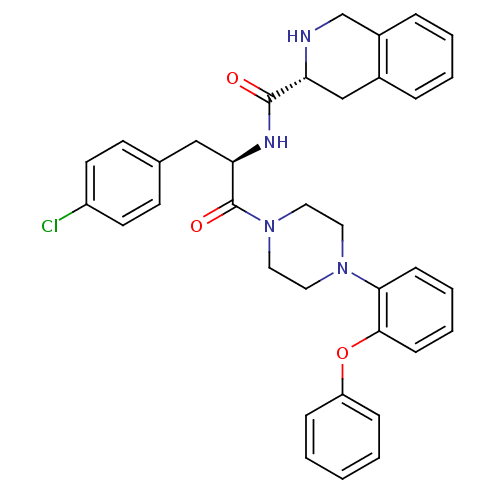 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139039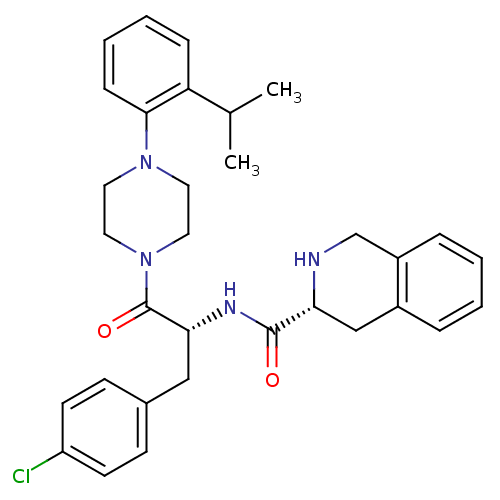 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139042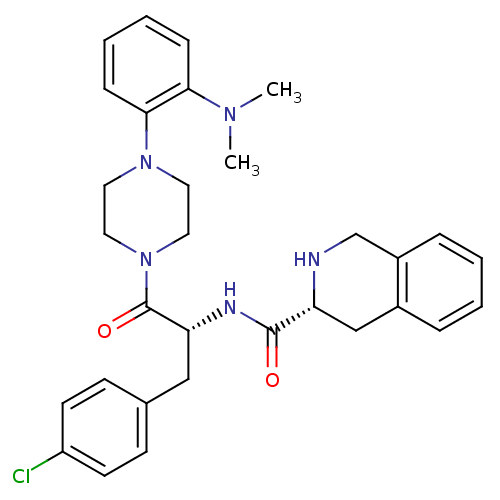 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139041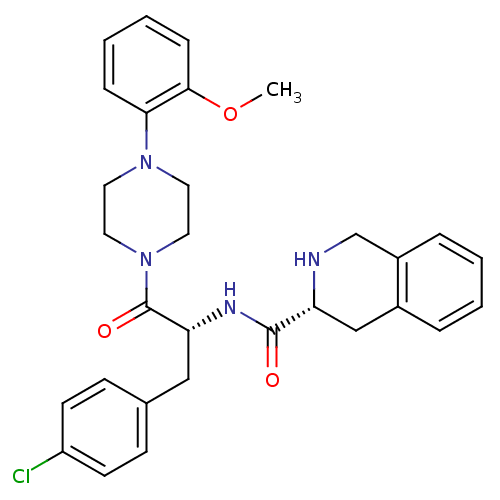 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139034 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139033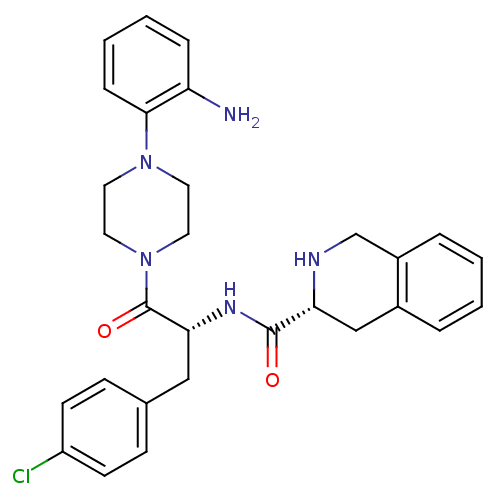 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139035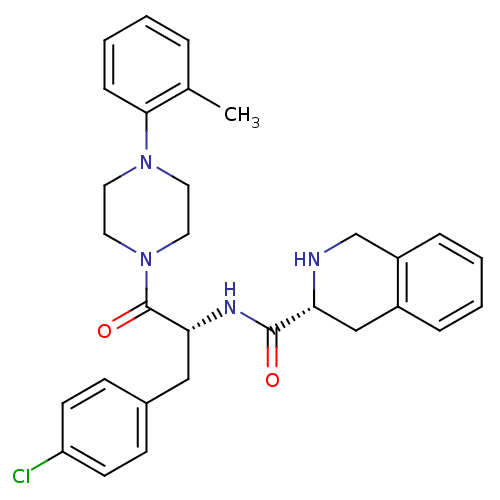 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139026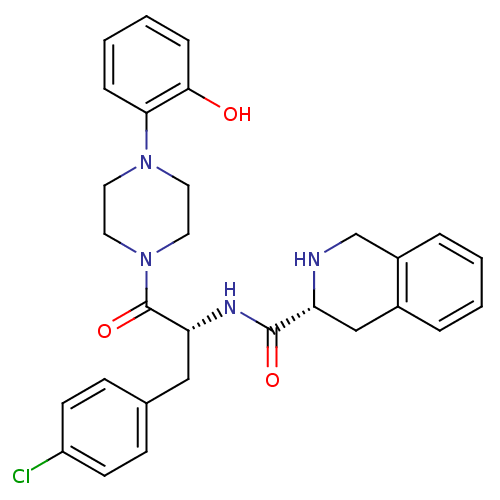 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139037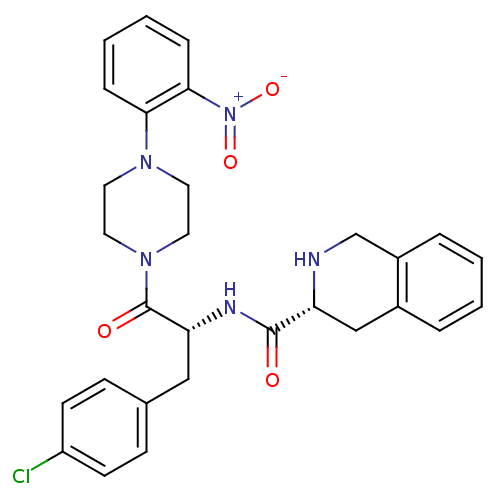 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139024 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139048 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human Melanocortin 3 receptor (MC3R) was determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139039 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139045 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139044 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139030 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139029 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139026 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139036 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human Melanocortin 3 receptor (MC3R) was determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139041 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139035 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139022 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139047 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139040 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139024 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139043 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human Melanocortin 3 receptor (MC3R) was determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139033 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139025 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139034 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139046 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139032 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139042 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139027 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139037 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139031 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139038 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||