

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21279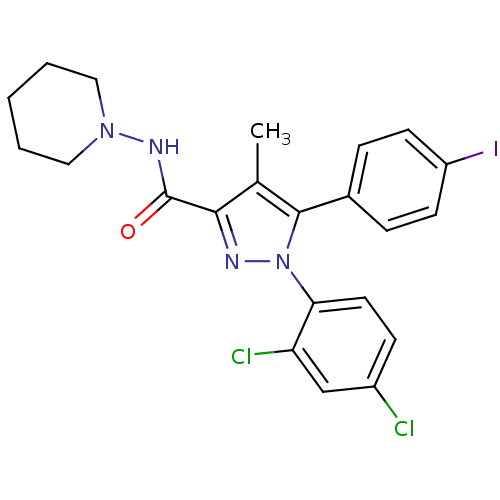 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123685 (4-Bromo-5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266832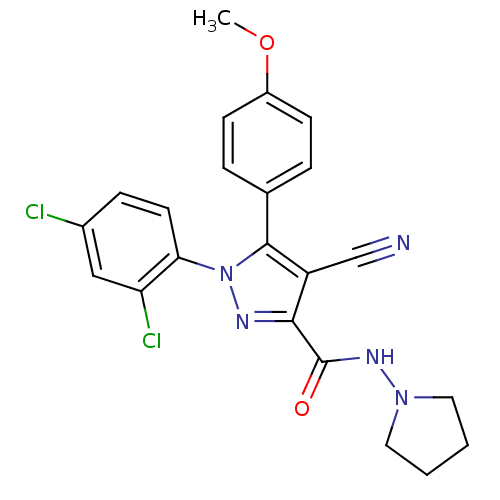 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004796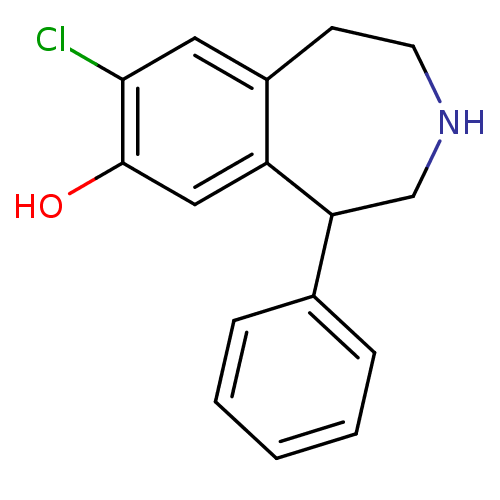 (8-Chloro-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from 5-HT1B receptor of rat frontal cortex homogenate | J Med Chem 31: 1406-12 (1988) BindingDB Entry DOI: 10.7270/Q2JD500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123690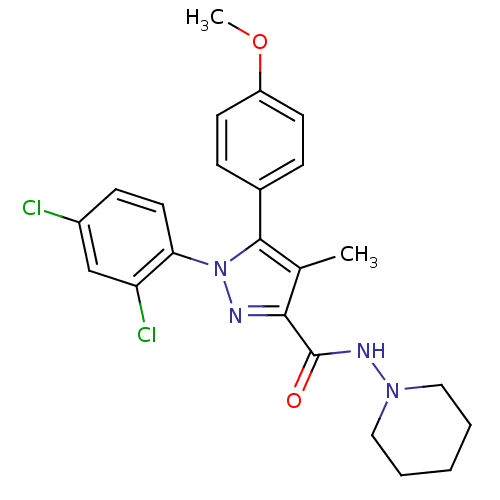 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267374 (1-(2-bromophenyl)-4-cyano-5-(4-methoxyphenyl)-N-(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123688 (4-Bromo-1-(2,4-dichloro-phenyl)-5-(4-methoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123693 (4-Bromo-1-(2-chloro-phenyl)-5-(4-methoxy-phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM82087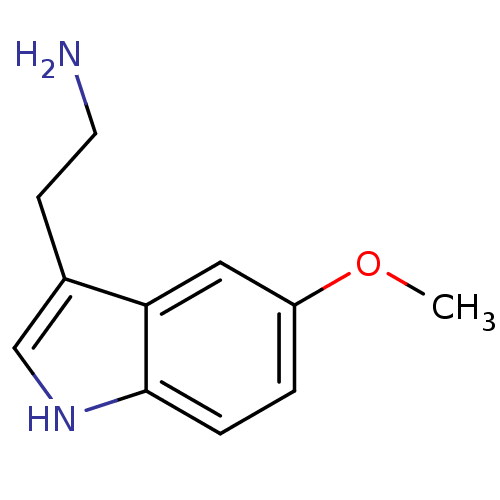 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from 5-HT1B receptor of rat frontal cortex homogenate | J Med Chem 31: 1406-12 (1988) BindingDB Entry DOI: 10.7270/Q2JD500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50038349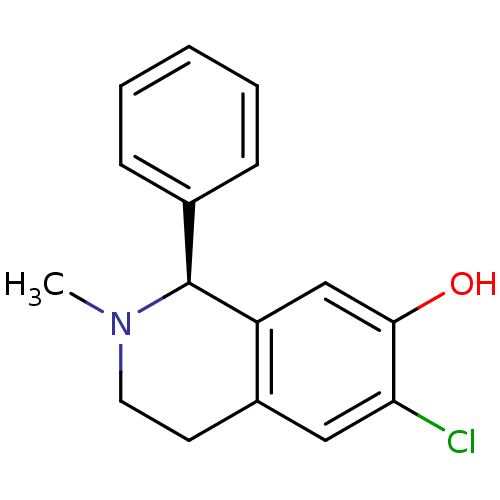 ((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50038349 ((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123689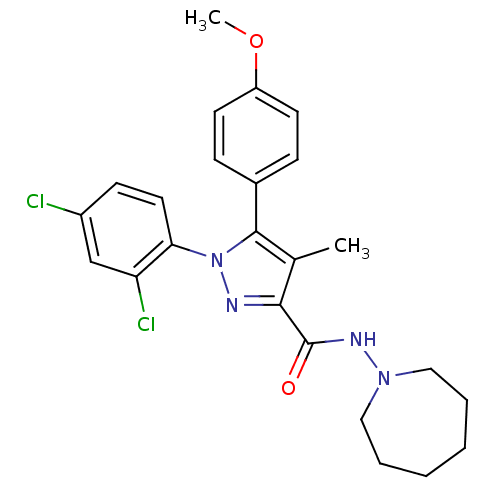 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123691 (5-Benzo[1,3]dioxol-5-yl-1-(2,4-dichloro-phenyl)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123684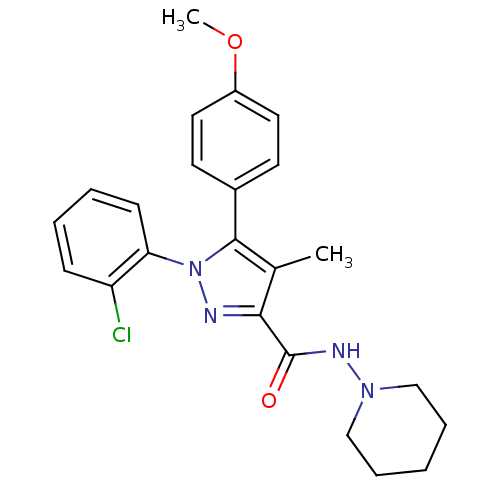 (1-(2-Chloro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123692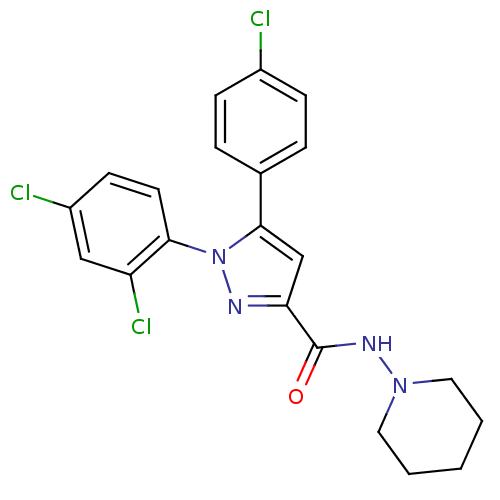 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266833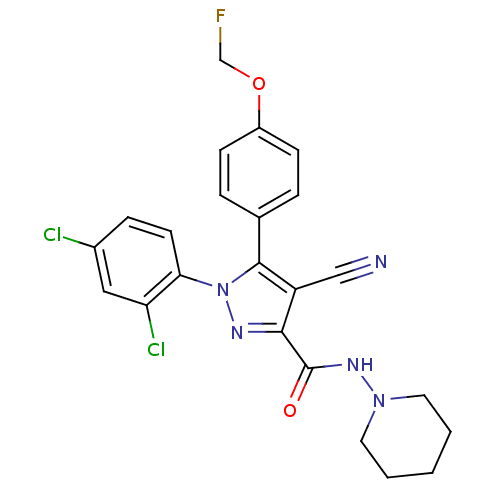 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-(fluoromethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267373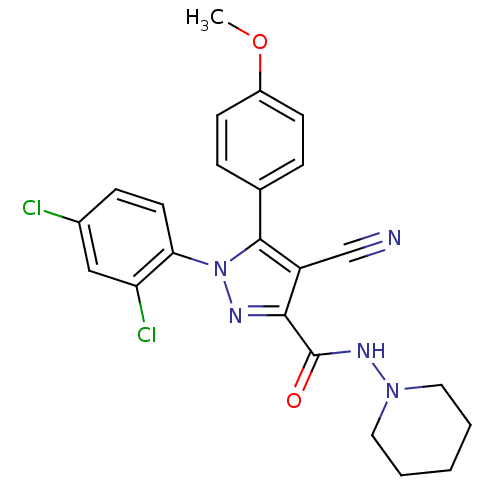 (4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50022051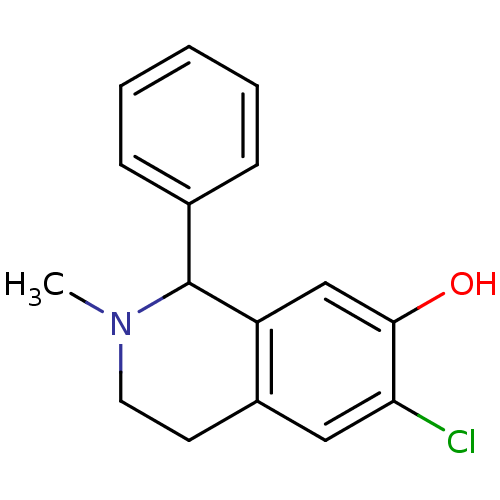 (1-Phenyl-6-chloro-7-hydroxy-N-methyl-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267375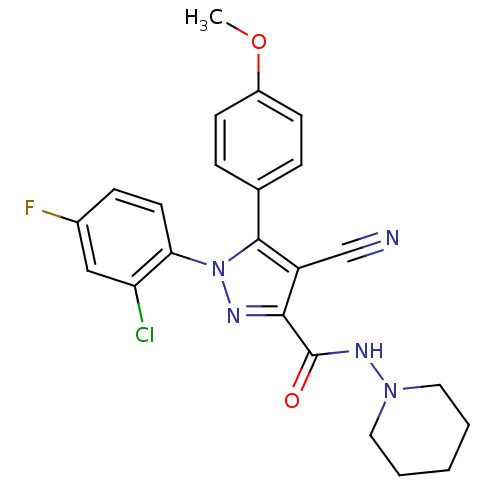 (1-(2-Chloro-4-fluorophenyl)-4-cyano-5-(4-methoxyph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266809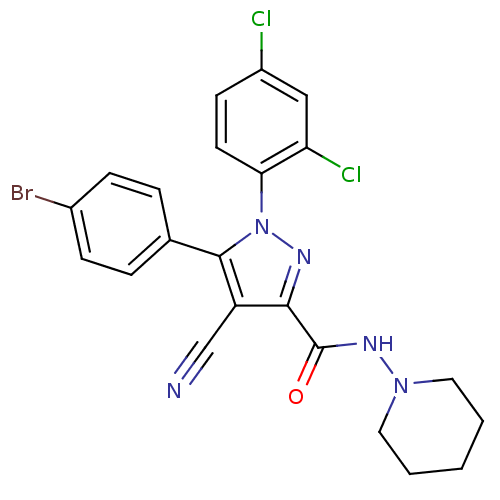 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123683 (1-(2-Chloro-phenyl)-4-fluoro-5-(4-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266864 (5-(4-chlorophenyl)-4-cyano-1-(2,4-dichlorophenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50228322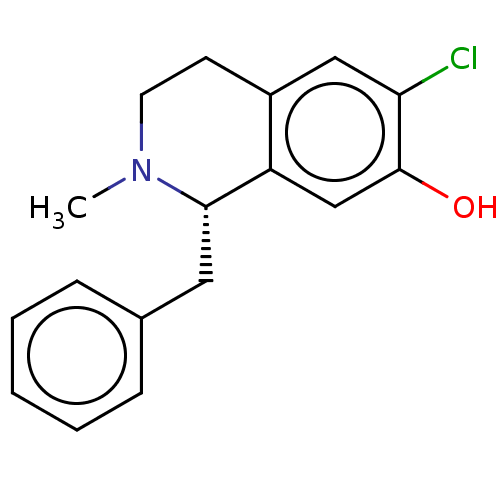 (CHEMBL64117) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266807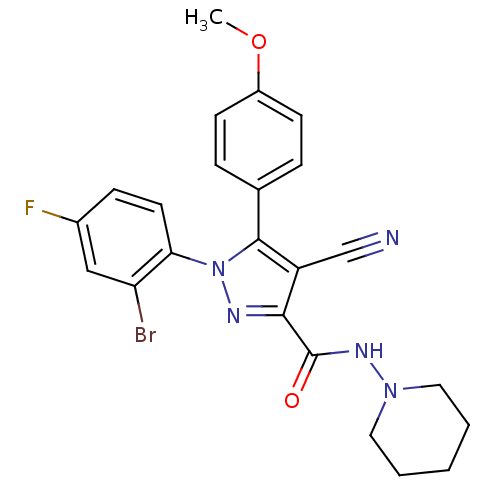 (1-(2-Bromo-4-fluorophenyl)-4-cyano-5-(4-methoxyphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266808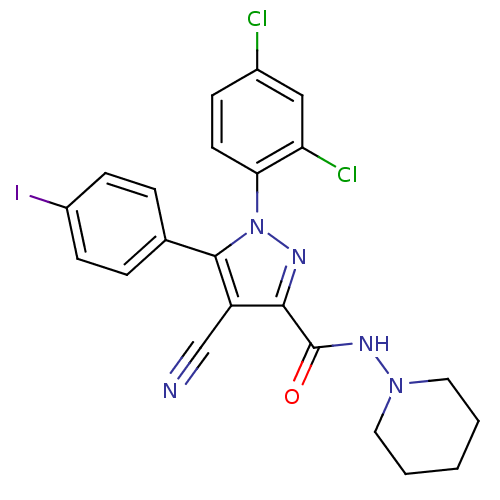 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123687 (1-(2-Fluoro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50227463 (CHEBI:48297 | CHEMBL1416204) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from 5-HT1B receptor of rat frontal cortex homogenate | J Med Chem 31: 1406-12 (1988) BindingDB Entry DOI: 10.7270/Q2JD500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50022052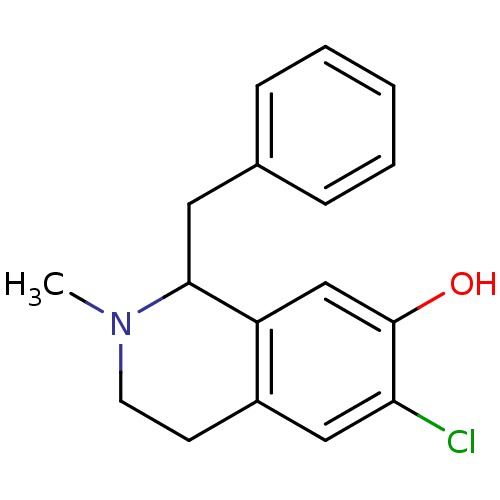 (1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50228321 (CHEMBL302393) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123694 (1-(2,4-Dichloro-phenyl)-5-(4-hydroxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50227465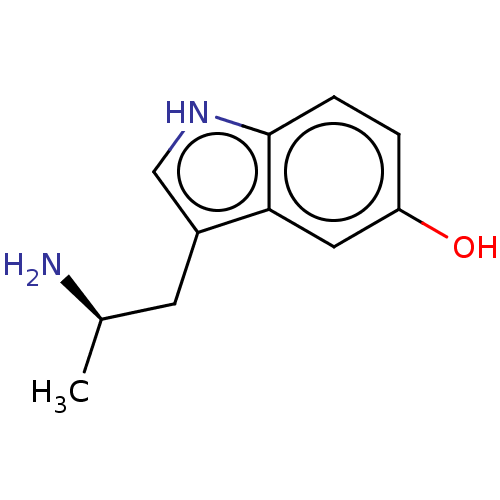 (CHEBI:48298 | CHEMBL1788239) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from 5-HT1B receptor of rat frontal cortex homogenate | J Med Chem 31: 1406-12 (1988) BindingDB Entry DOI: 10.7270/Q2JD500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266830 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50367602 (CHEMBL65397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50367602 (CHEMBL65397) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50228322 (CHEMBL64117) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50022053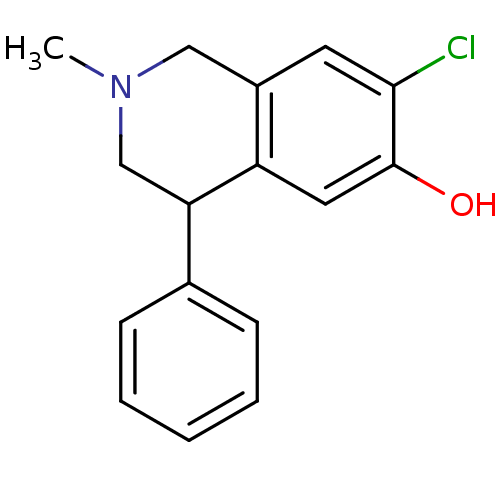 (1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50367601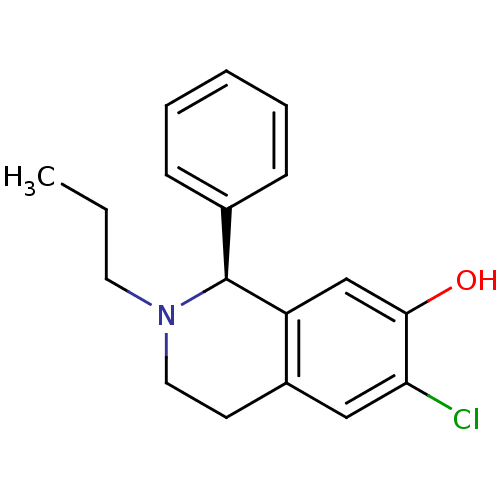 (CHEMBL293828) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Antibacterial activity against Escherichia coli | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50367601 (CHEMBL293828) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50228321 (CHEMBL302393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50228323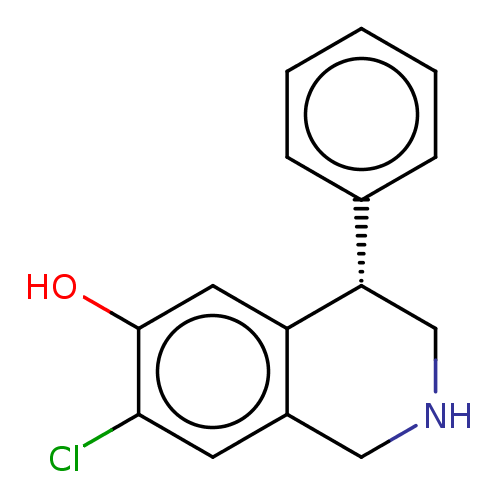 (CHEMBL59603) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 32: 2050-8 (1989) BindingDB Entry DOI: 10.7270/Q2RV0QXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50022052 (1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM | J Med Chem 31: 1941-6 (1988) BindingDB Entry DOI: 10.7270/Q20002PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123682 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 510 total ) | Next | Last >> |