

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061308 ((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061305 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Mus musculus) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B [I199F] (Homo sapiens (Human)) | BDBM10989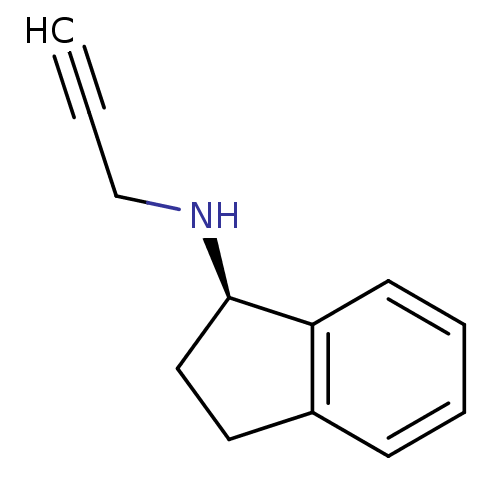 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B [I199F] (Homo sapiens (Human)) | BDBM11022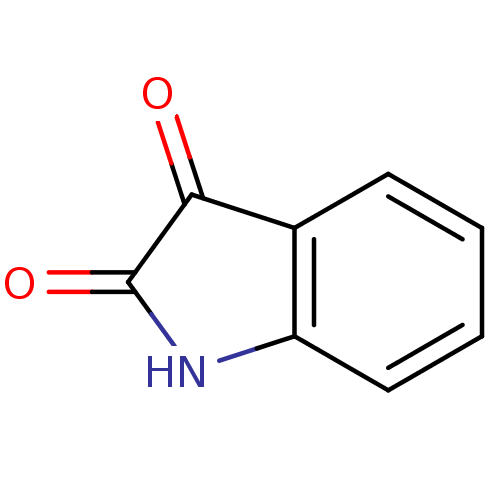 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50155318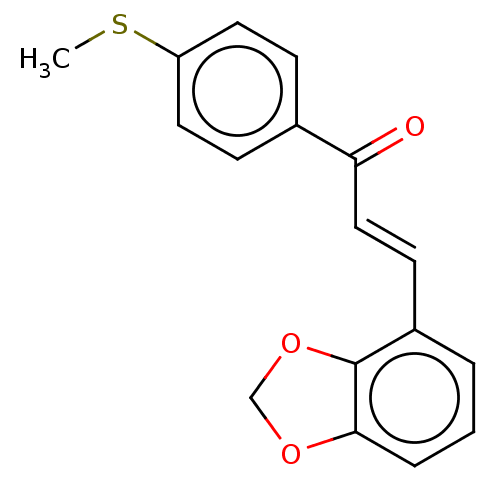 (CHEMBL3781063) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qatar University Curated by ChEMBL | Assay Description Competitive inhibition of human MAOB overexpressed in Pichia pastoris using MMTP as substrate preincubated for 5 mins followed by substrate addition ... | Eur J Med Chem 114: 162-9 (2016) Article DOI: 10.1016/j.ejmech.2016.02.038 BindingDB Entry DOI: 10.7270/Q2N018F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11020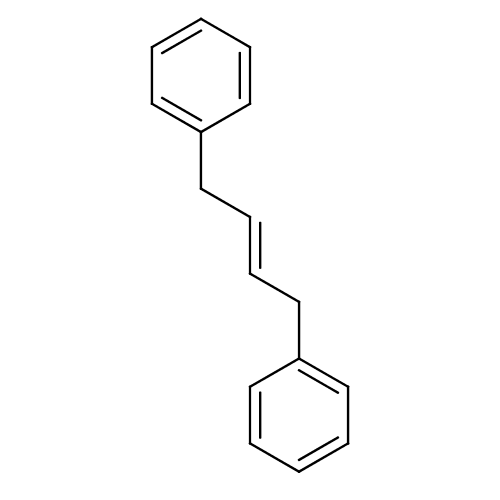 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 700 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50155319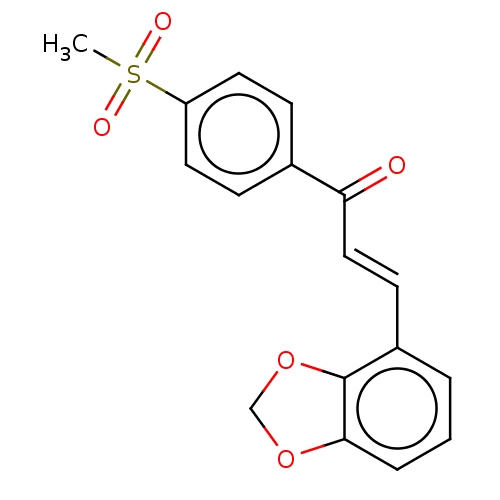 (CHEMBL3781774) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qatar University Curated by ChEMBL | Assay Description Competitive inhibition of human MAOB overexpressed in Pichia pastoris using MMTP as substrate preincubated for 5 mins followed by substrate addition ... | Eur J Med Chem 114: 162-9 (2016) Article DOI: 10.1016/j.ejmech.2016.02.038 BindingDB Entry DOI: 10.7270/Q2N018F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50155322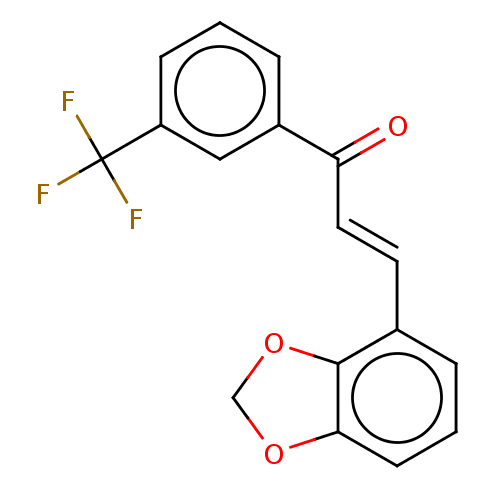 (CHEMBL3780640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qatar University Curated by ChEMBL | Assay Description Competitive inhibition of human MAOB overexpressed in Pichia pastoris using MMTP as substrate preincubated for 5 mins followed by substrate addition ... | Eur J Med Chem 114: 162-9 (2016) Article DOI: 10.1016/j.ejmech.2016.02.038 BindingDB Entry DOI: 10.7270/Q2N018F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11021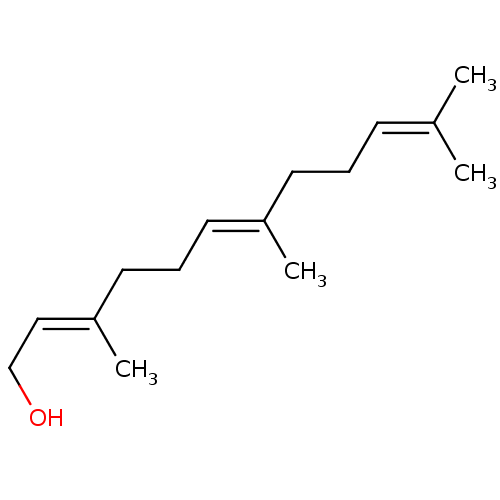 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50155321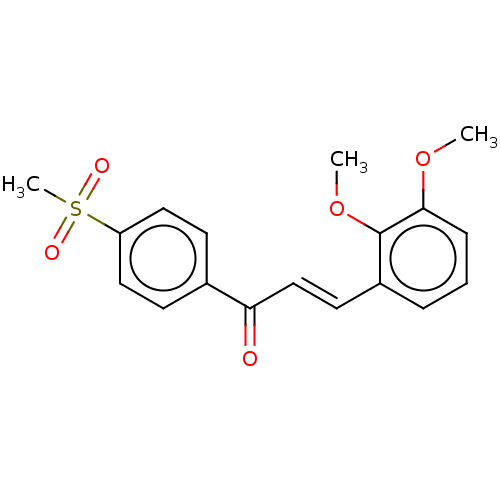 (CHEMBL3782038) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qatar University Curated by ChEMBL | Assay Description Competitive inhibition of human MAOB overexpressed in Pichia pastoris using MMTP as substrate preincubated for 5 mins followed by substrate addition ... | Eur J Med Chem 114: 162-9 (2016) Article DOI: 10.1016/j.ejmech.2016.02.038 BindingDB Entry DOI: 10.7270/Q2N018F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (Equus caballus (horse)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | -33.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50155312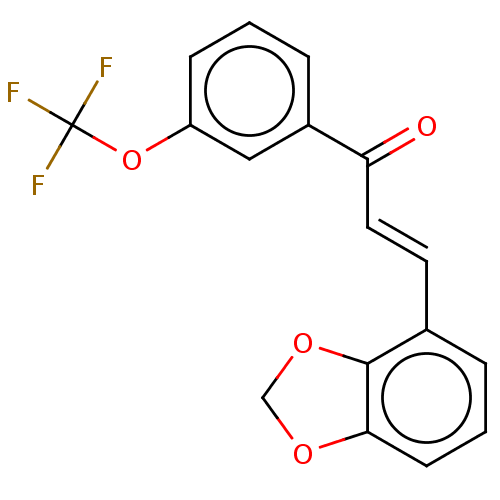 (CHEMBL3780160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qatar University Curated by ChEMBL | Assay Description Competitive inhibition of human MAOB overexpressed in Pichia pastoris using MMTP as substrate preincubated for 5 mins followed by substrate addition ... | Eur J Med Chem 114: 162-9 (2016) Article DOI: 10.1016/j.ejmech.2016.02.038 BindingDB Entry DOI: 10.7270/Q2N018F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Mus musculus) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.40E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11022 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.00E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5.00E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11022 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase (Equus caballus (horse)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 8.30E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11022 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+4 | -27.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B [I199F] (Homo sapiens (Human)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Bos taurus) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase (Ovis aries (sheep)) | BDBM11020 (1,4-diphenyl-2-butene (DPB) | [(2E)-4-phenylbut-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (Ovis aries (sheep)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B [I199F] (Homo sapiens (Human)) | BDBM11021 ((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B [I199F] (Homo sapiens (Human)) | BDBM11018 (2-[(E)-2-(3-chlorophenyl)ethenyl]-3,5,7-trimethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University | Assay Description MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ... | J Biol Chem 280: 15761-6 (2005) Article DOI: 10.1074/jbc.M500949200 BindingDB Entry DOI: 10.7270/Q2DR2SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123975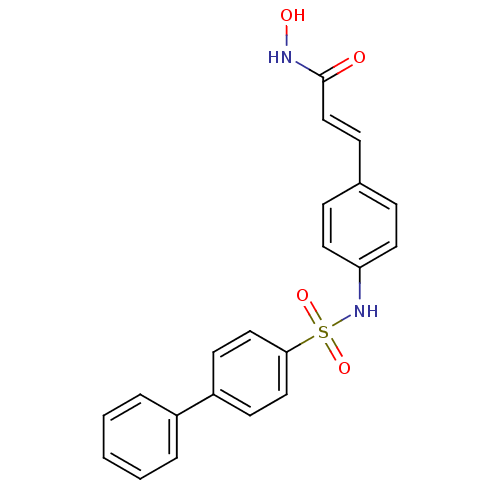 (3-[4-(biphenyl-4-sulfonylamino)-phenyl]-N-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123967 (3-[4-(Biphenyl-4-ylsulfamoyl)-phenyl]-N-hydroxy-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123955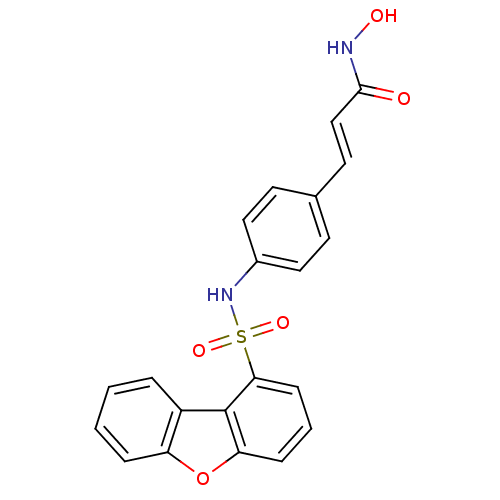 (3-[4-(Dibenzofuran-1-sulfonylamino)-phenyl]-N-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123970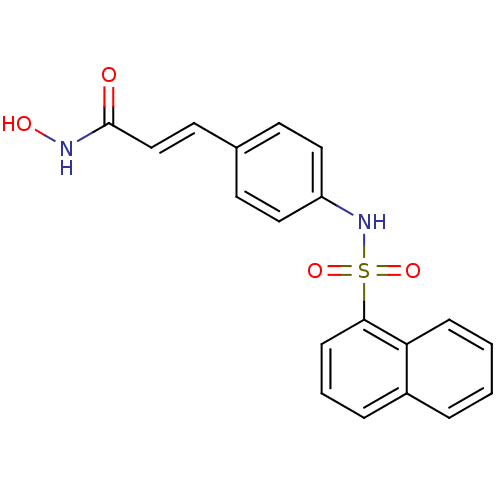 (CHEMBL168961 | N-Hydroxy-3-[4-(naphthalene-1-sulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123958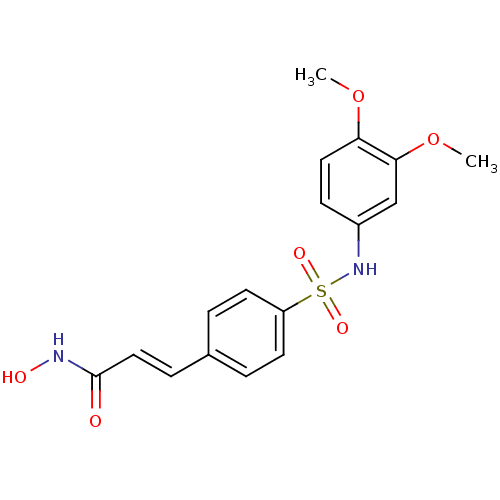 (3-[4-(3,4-Dimethoxy-phenylsulfamoyl)-phenyl]-N-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105678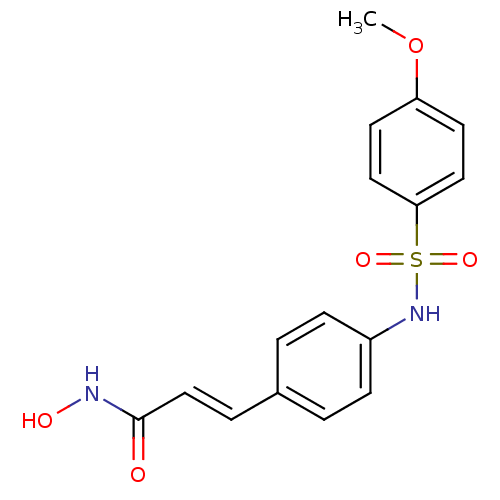 ((E)-N-Hydroxy-3-[4-(4-methoxy-benzenesulfonylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105689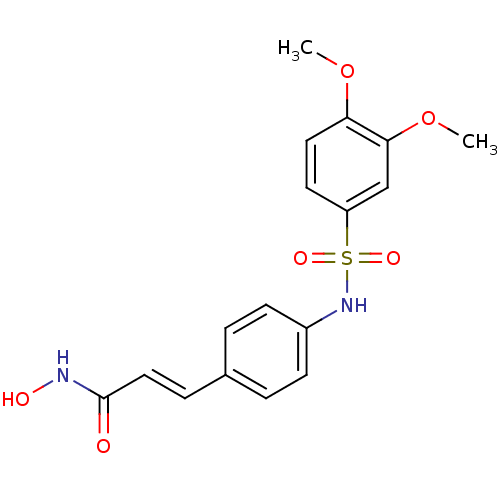 ((E)-3-[4-(3,4-Dimethoxy-benzenesulfonylamino)-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128308 BindingDB Entry DOI: 10.7270/Q23B63W5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50531285 (CHEMBL4581121) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of SIRT1 (unknown origin) | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111926 BindingDB Entry DOI: 10.7270/Q21R6V04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50531285 (CHEMBL4581121) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University Curated by ChEMBL | Assay Description Inhibition of SIRT1 (unknown origin) | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111926 BindingDB Entry DOI: 10.7270/Q21R6V04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50123957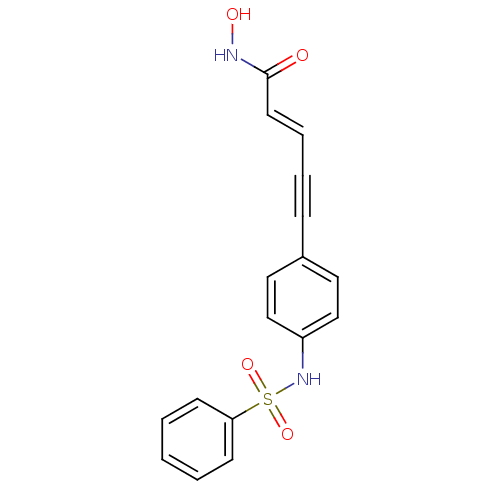 ((E)-5-(3-Benzenesulfonylamino-phenyl)-pent-2-en-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105692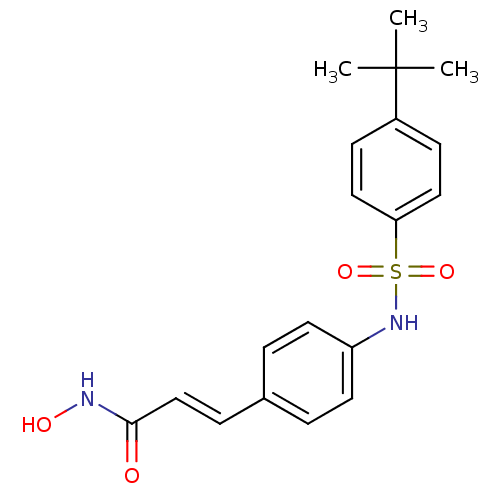 ((E)-3-[4-(4-tert-Butyl-benzenesulfonylamino)-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1) | J Med Chem 46: 820-30 (2003) Article DOI: 10.1021/jm020377a BindingDB Entry DOI: 10.7270/Q22V2FG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |