 Found 1038 hits with Last Name = 'haughan' and Initial = 'af'
Found 1038 hits with Last Name = 'haughan' and Initial = 'af' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506948
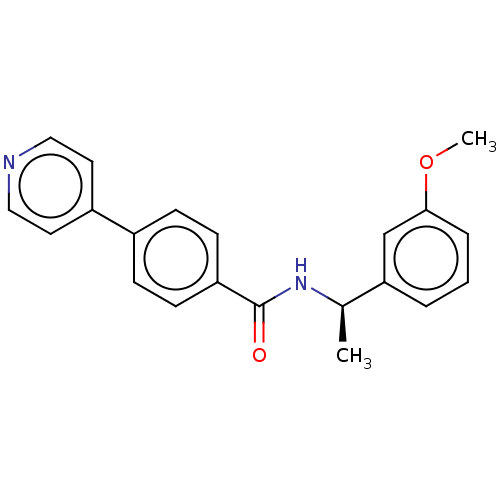
(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218255
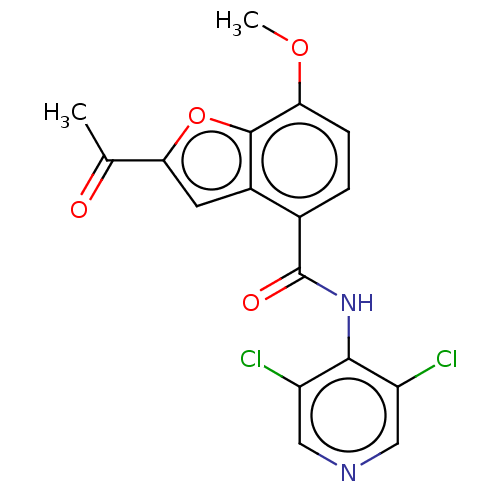
(CHEMBL290100)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)=O Show InChI InChI=1S/C17H12Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-7H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 (PDE-4) from human U937 cells |
Bioorg Med Chem Lett 12: 1613-5 (2002)
BindingDB Entry DOI: 10.7270/Q2D220S3 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218255

(CHEMBL290100)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)=O Show InChI InChI=1S/C17H12Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-7H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597493

(CHEMBL5192610)Show SMILES C[C@@H](NC(=O)N1CCN(C[C@H]1C)c1ccncc1F)c1cccn2nccc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50552912
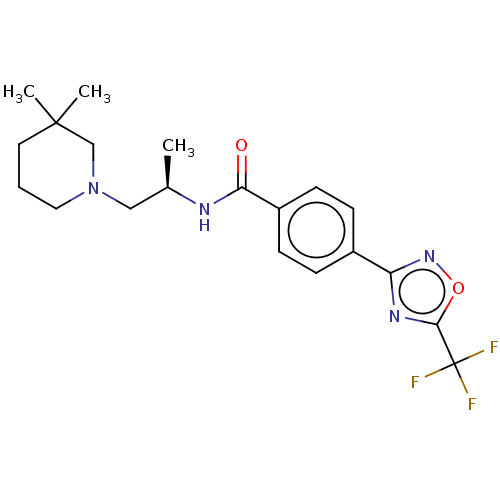
(CHEMBL4760047)Show SMILES C[C@H](CN1CCCC(C)(C)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218257
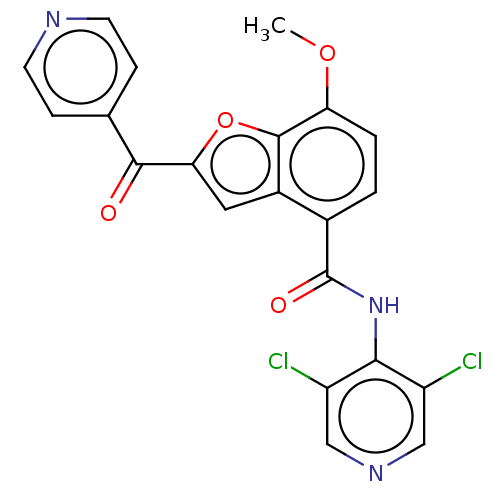
(CHEMBL69874)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(=O)c1ccncc1 Show InChI InChI=1S/C21H13Cl2N3O4/c1-29-16-3-2-12(21(28)26-18-14(22)9-25-10-15(18)23)13-8-17(30-20(13)16)19(27)11-4-6-24-7-5-11/h2-10H,1H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597488
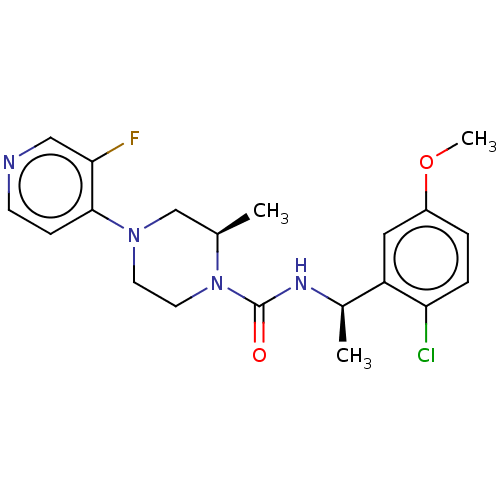
(CHEMBL5180112)Show SMILES COc1ccc(Cl)c(c1)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597489

(CHEMBL5208605)Show SMILES COc1cc(F)cc(c1)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597490

(CHEMBL5199904)Show SMILES COc1cc(ccc1F)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597496
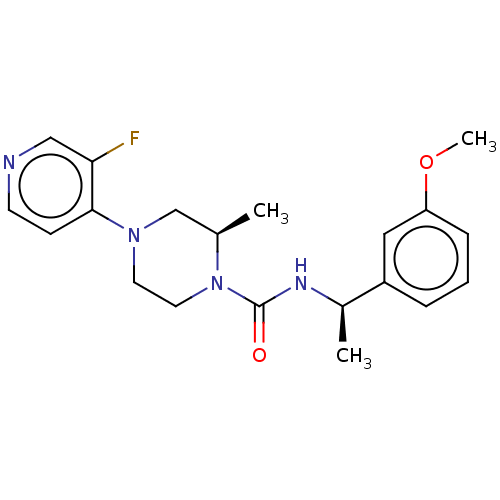
(CHEMBL5206259)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243152
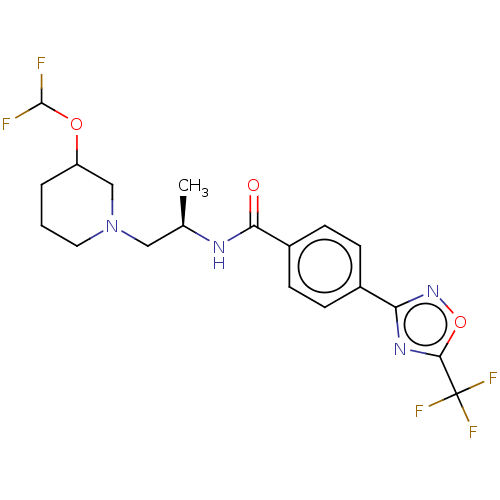
(D1: N-((R)-1-((abs)-3- (Difluoromethoxy)piperidin-...)Show SMILES C[C@H](CN1CCCC(C1)OC(F)F)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F5N4O3/c1-11(9-28-8-2-3-14(10-28)30-18(20)21)25-16(29)13-6-4-12(5-7-13)15-26-17(31-27-15)19(22,23)24/h4-7,11,14,18H,2-3,8-10H2,1H3,(H,25,29)/t11-,14?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50552909

(CHEMBL4754665)Show SMILES C[C@H](CN1CCC[C@@H](C1)OC(F)F)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162846

(US9056843, 137)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-26(11-5-2)12-13(3)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,4-5,10-12H2,1-3H3,(H,23,27)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM228164
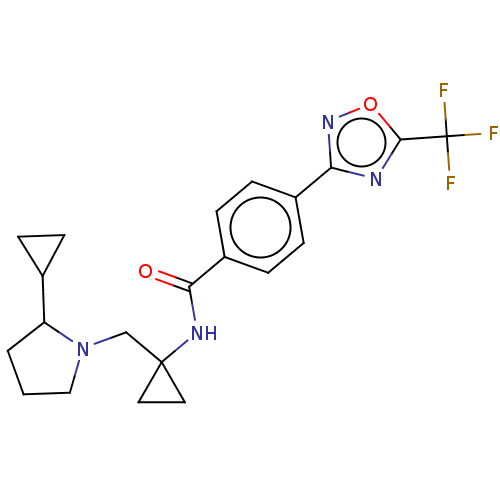
(US10047073, 6 | US10047073, 7)Show SMILES FC(F)(F)c1nc(no1)-c1ccc(cc1)C(=O)NC1(CN2CCCC2C2CC2)CC1 Show InChI InChI=1S/C21H23F3N4O2/c22-21(23,24)19-25-17(27-30-19)14-5-7-15(8-6-14)18(29)26-20(9-10-20)12-28-11-1-2-16(28)13-3-4-13/h5-8,13,16H,1-4,9-12H2,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC
US Patent
| Assay Description
The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a... |
US Patent US10047073 (2018)
BindingDB Entry DOI: 10.7270/Q2PG1TQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243173
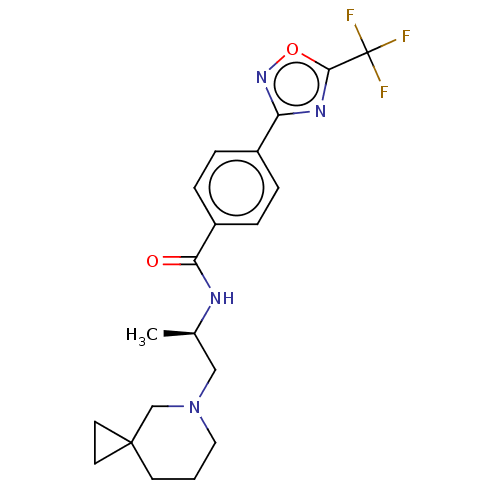
((R)-N-(1-(5-Azaspiro[2.5]octan-5- yl)propan-2-yl)-...)Show SMILES C[C@H](CN1CCCC2(CC2)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N4O2/c1-13(11-27-10-2-7-19(12-27)8-9-19)24-17(28)15-5-3-14(4-6-15)16-25-18(29-26-16)20(21,22)23/h3-6,13H,2,7-12H2,1H3,(H,24,28)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50219014
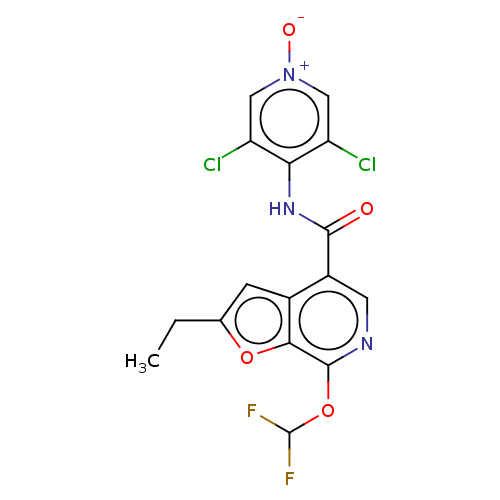
(CHEMBL149559)Show SMILES CCc1cc2c(cnc(OC(F)F)c2o1)C(=O)Nc1c(Cl)c[n+]([O-])cc1Cl Show InChI InChI=1S/C16H11Cl2F2N3O4/c1-2-7-3-8-9(4-21-15(13(8)26-7)27-16(19)20)14(24)22-12-10(17)5-23(25)6-11(12)18/h3-6,16H,2H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R& D
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 4 from U937 cells |
Bioorg Med Chem Lett 12: 509-12 (2002)
BindingDB Entry DOI: 10.7270/Q2T43W9C |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506921

(CHEMBL4459800)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccnc(F)c1 |r| Show InChI InChI=1S/C21H19FN2O2/c1-14(17-4-3-5-19(12-17)26-2)24-21(25)16-8-6-15(7-9-16)18-10-11-23-20(22)13-18/h3-14H,1-2H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243193
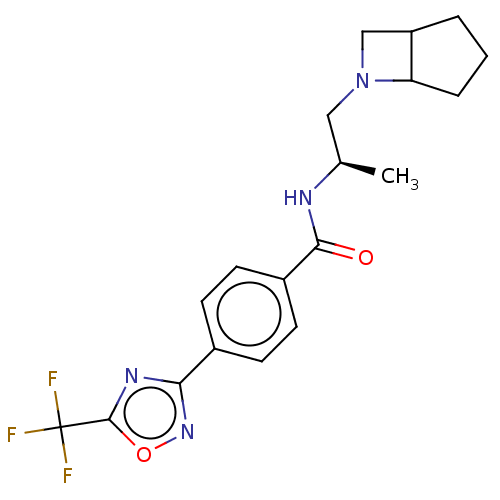
(D1: N-((R)-1-((abs-1,5-cis)-6- Azabicyclo[3.2.0]he...)Show SMILES C[C@H](CN1CC2CCCC12)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F3N4O2/c1-11(9-26-10-14-3-2-4-15(14)26)23-17(27)13-7-5-12(6-8-13)16-24-18(28-25-16)19(20,21)22/h5-8,11,14-15H,2-4,9-10H2,1H3,(H,23,27)/t11-,14?,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243192

(N-((2R)-1-(3-Azabicyclo[3.2.0]heptan- 3-yl)propan-...)Show SMILES C[C@H](CN1CC2CCC2C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F3N4O2/c1-11(8-26-9-14-6-7-15(14)10-26)23-17(27)13-4-2-12(3-5-13)16-24-18(28-25-16)19(20,21)22/h2-5,11,14-15H,6-10H2,1H3,(H,23,27)/t11-,14?,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597473

(CHEMBL5181094)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(nc1)-c1ccncc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597492

(CHEMBL5186165)Show SMILES C[C@@H](NC(=O)N1CCN(C[C@H]1C)c1ccncc1F)c1cccc2nccn12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211428
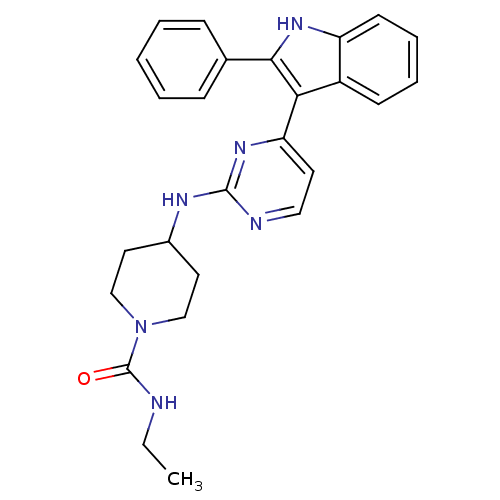
(CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1nccc(n1)-c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H28N6O/c1-2-27-26(33)32-16-13-19(14-17-32)29-25-28-15-12-22(31-25)23-20-10-6-7-11-21(20)30-24(23)18-8-4-3-5-9-18/h3-12,15,19,30H,2,13-14,16-17H2,1H3,(H,27,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50211428

(CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1nccc(n1)-c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H28N6O/c1-2-27-26(33)32-16-13-19(14-17-32)29-25-28-15-12-22(31-25)23-20-10-6-7-11-21(20)30-24(23)18-8-4-3-5-9-18/h3-12,15,19,30H,2,13-14,16-17H2,1H3,(H,27,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597494
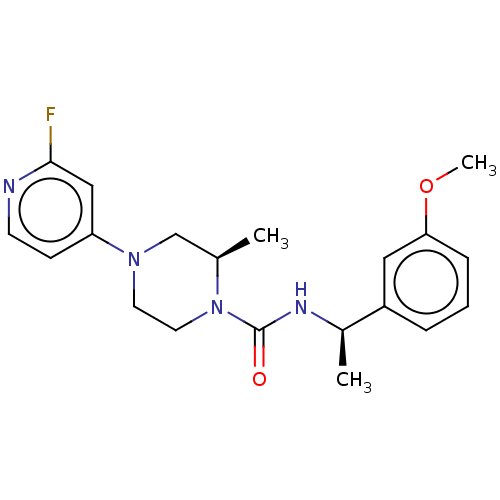
(CHEMBL5188032)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccnc(F)c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243189
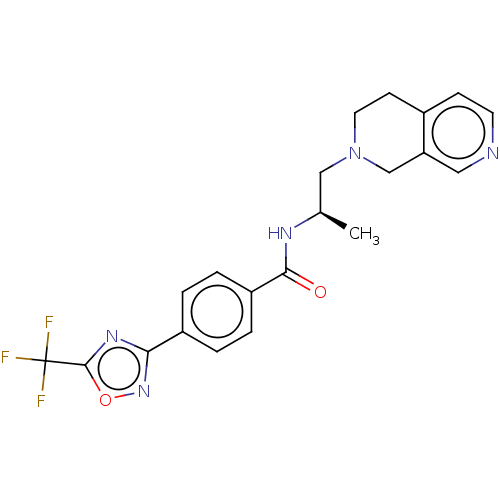
((R)-N-(1-(3,4-dihydro-2,7-naphthyridin- 2(1H)-yl)p...)Show SMILES C[C@H](CN1CCc2ccncc2C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N5O2/c1-13(11-29-9-7-14-6-8-25-10-17(14)12-29)26-19(30)16-4-2-15(3-5-16)18-27-20(31-28-18)21(22,23)24/h2-6,8,10,13H,7,9,11-12H2,1H3,(H,26,30)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218258
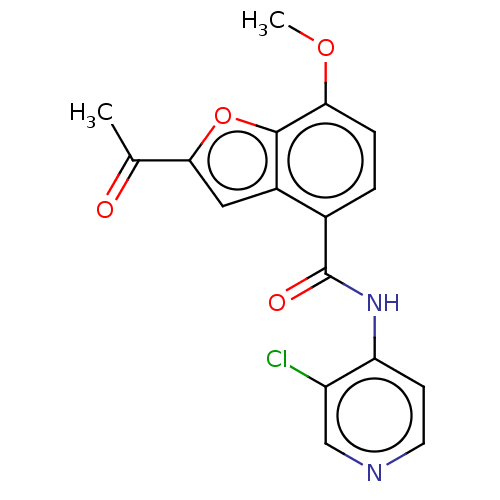
(CHEMBL70146)Show InChI InChI=1S/C17H13ClN2O4/c1-9(21)15-7-11-10(3-4-14(23-2)16(11)24-15)17(22)20-13-5-6-19-8-12(13)18/h3-8H,1-2H3,(H,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50218245
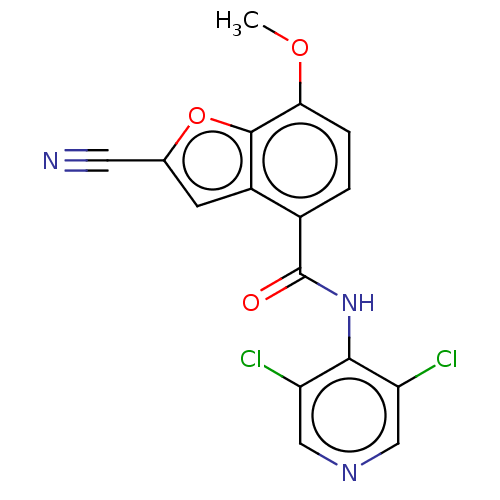
(CHEMBL65799)Show InChI InChI=1S/C16H9Cl2N3O3/c1-23-13-3-2-9(10-4-8(5-19)24-15(10)13)16(22)21-14-11(17)6-20-7-12(14)18/h2-4,6-7H,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to rat brain tissue at 20 uM |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218245

(CHEMBL65799)Show InChI InChI=1S/C16H9Cl2N3O3/c1-23-13-3-2-9(10-4-8(5-19)24-15(10)13)16(22)21-14-11(17)6-20-7-12(14)18/h2-4,6-7H,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597485

(CHEMBL5183751)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)N1CCN(CC1)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50114418
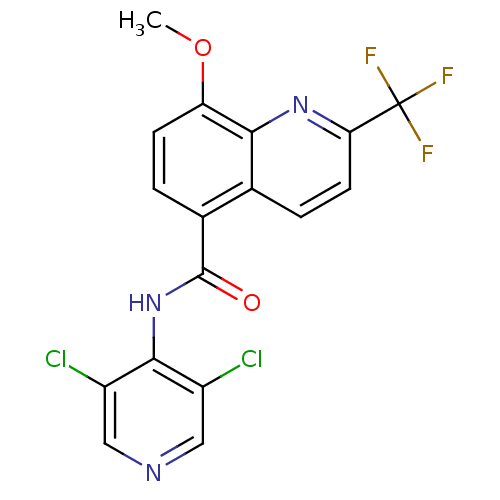
(8-Methoxy-2-trifluoromethyl-quinoline-5-carboxylic...)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H10Cl2F3N3O2/c1-27-12-4-2-9(8-3-5-13(17(20,21)22)24-14(8)12)16(26)25-15-10(18)6-23-7-11(15)19/h2-7H,1H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Corporation
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to PDE4 of rat brain |
Bioorg Med Chem Lett 12: 1621-3 (2002)
BindingDB Entry DOI: 10.7270/Q24J0HB2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243183
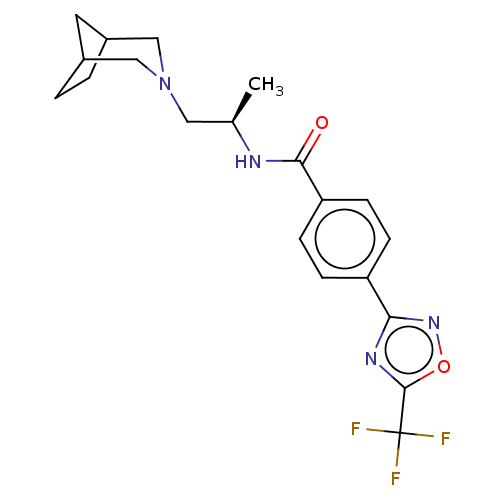
(N-((R)-1-(3-Azabicyclo[3.2.1]octan-3- yl)propan-2-...)Show SMILES C[C@H](CN1CC2CCC(C2)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N4O2/c1-12(9-27-10-13-2-3-14(8-13)11-27)24-18(28)16-6-4-15(5-7-16)17-25-19(29-26-17)20(21,22)23/h4-7,12-14H,2-3,8-11H2,1H3,(H,24,28)/t12-,13?,14?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597487

(CHEMBL5169406)Show SMILES COc1ccc(F)c(c1)[C@@H](C)NC(=O)N1CCN(C[C@H]1C)c1ccncc1F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243180
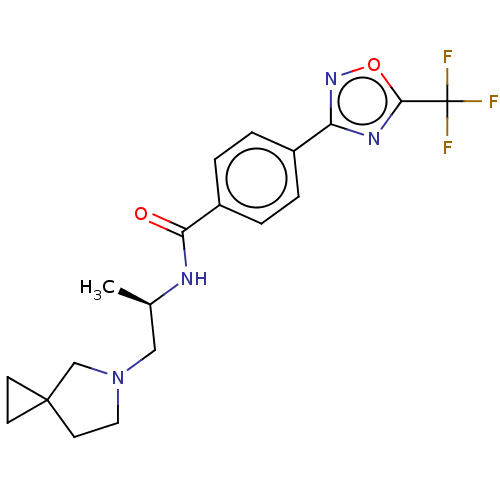
((R)-N-(1-(5-Azaspiro[2.4]heptan-5- yl)propan-2-yl)...)Show SMILES C[C@H](CN1CCC2(CC2)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F3N4O2/c1-12(10-26-9-8-18(11-26)6-7-18)23-16(27)14-4-2-13(3-5-14)15-24-17(28-25-15)19(20,21)22/h2-5,12H,6-11H2,1H3,(H,23,27)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM228164

(US10047073, 6 | US10047073, 7)Show SMILES FC(F)(F)c1nc(no1)-c1ccc(cc1)C(=O)NC1(CN2CCCC2C2CC2)CC1 Show InChI InChI=1S/C21H23F3N4O2/c22-21(23,24)19-25-17(27-30-19)14-5-7-15(8-6-14)18(29)26-20(9-10-20)12-28-11-1-2-16(28)13-3-4-13/h5-8,13,16H,1-4,9-12H2,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC
US Patent
| Assay Description
The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a... |
US Patent US10047073 (2018)
BindingDB Entry DOI: 10.7270/Q2PG1TQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243091
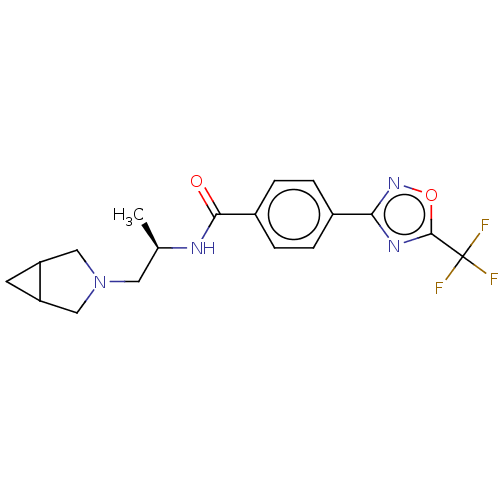
(N-((2R)-1-(3-Azabicyclo[3.1.0]hexan-3- yl)propan-2...)Show SMILES C[C@H](CN1CC2CC2C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C18H19F3N4O2/c1-10(7-25-8-13-6-14(13)9-25)22-16(26)12-4-2-11(3-5-12)15-23-17(27-24-15)18(19,20)21/h2-5,10,13-14H,6-9H2,1H3,(H,22,26)/t10-,13?,14?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50218257

(CHEMBL69874)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(=O)c1ccncc1 Show InChI InChI=1S/C21H13Cl2N3O4/c1-29-16-3-2-12(21(28)26-18-14(22)9-25-10-15(18)23)13-8-17(30-20(13)16)19(27)11-4-6-24-7-5-11/h2-10H,1H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of rolipram binding to rat brain tissue at 20 uM |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50218246
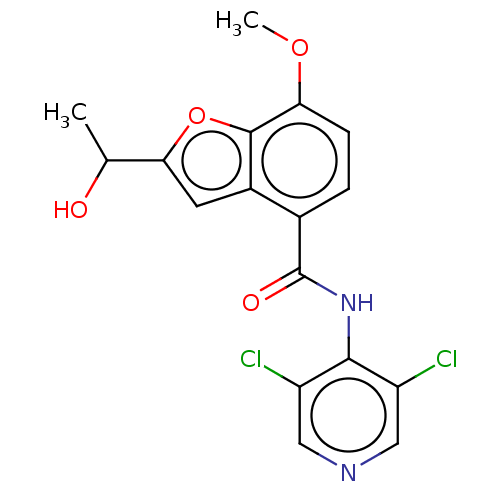
(CHEMBL68323)Show SMILES COc1ccc(C(=O)Nc2c(Cl)cncc2Cl)c2cc(oc12)C(C)O Show InChI InChI=1S/C17H14Cl2N2O4/c1-8(22)14-5-10-9(3-4-13(24-2)16(10)25-14)17(23)21-15-11(18)6-20-7-12(15)19/h3-8,22H,1-2H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech-Chiroscience Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Phosphodiesterase 4 from human U937 cells |
Bioorg Med Chem Lett 10: 2137-40 (2000)
BindingDB Entry DOI: 10.7270/Q2NP26MQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50597475

(CHEMBL5200987)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)N1CC[C@@H](C[C@H]1C)c1ccncc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00474
BindingDB Entry DOI: 10.7270/Q2P2735N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243121

((R)-N-(1-(3,4-Dihydroisoquinolin- 2(1H)-yl)propan-...)Show SMILES C[C@H](CN1CCc2ccccc2C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N4O2/c1-14(12-29-11-10-15-4-2-3-5-18(15)13-29)26-20(30)17-8-6-16(7-9-17)19-27-21(31-28-19)22(23,24)25/h2-9,14H,10-13H2,1H3,(H,26,30)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243202

((2S)-1-((R)-2-(3-Fluoro-4-(5- (trifluoromethyl)-1,...)Show SMILES C[C@H](C[NH+]1CCC[C@@H]1C)NC(=O)c1ccc(-c2noc(n2)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H20F4N4O2/c1-10(9-26-7-3-4-11(26)2)23-16(27)12-5-6-13(14(19)8-12)15-24-17(28-25-15)18(20,21)22/h5-6,8,10-11H,3-4,7,9H2,1-2H3,(H,23,27)/p+1/t10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM243178
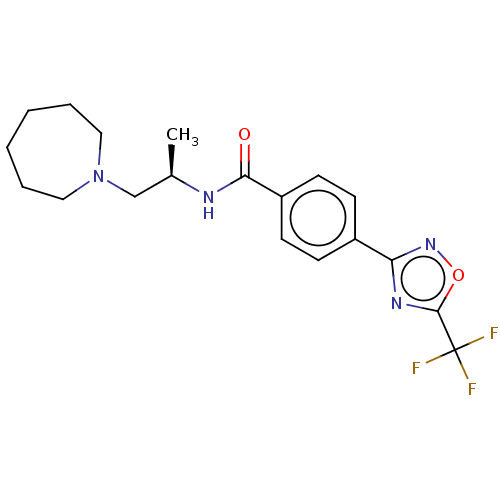
((R)-N-(1-(Azepan-1-yl)propan-2-yl)-4- (5-(trifluor...)Show SMILES C[C@H](CN1CCCCCC1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H23F3N4O2/c1-13(12-26-10-4-2-3-5-11-26)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,2-5,10-12H2,1H3,(H,23,27)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM293916
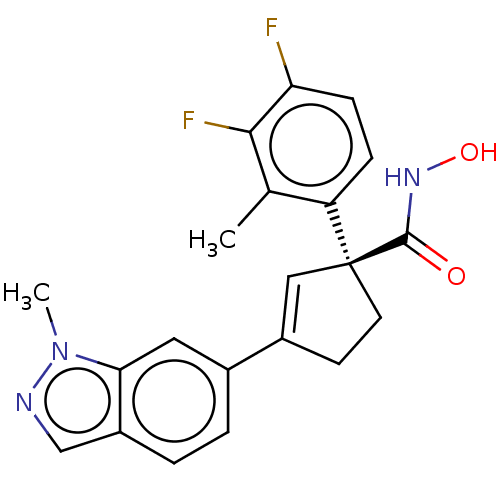
((S)-1-(3,4-Difluoro-2- methylphenyl)-N- hydroxy-3-...)Show SMILES Cc1c(F)c(F)ccc1[C@@]1(CCC(=C1)c1ccc2cnn(C)c2c1)C(=O)NO |r,c:13| Show InChI InChI=1S/C21H19F2N3O2/c1-12-16(5-6-17(22)19(12)23)21(20(27)25-28)8-7-14(10-21)13-3-4-15-11-24-26(2)18(15)9-13/h3-6,9-11,28H,7-8H2,1-2H3,(H,25,27)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... |
US Patent US10106535 (2018)
BindingDB Entry DOI: 10.7270/Q2F47R6G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50552915

(CHEMBL4798111)Show SMILES C[C@H](CN1CCC[C@@H]1C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM162839

(US9056843, 130)Show SMILES C[C@H](CN1CCCC1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C17H19F3N4O2/c1-11(10-24-8-2-3-9-24)21-15(25)13-6-4-12(5-7-13)14-22-16(26-23-14)17(18,19)20/h4-7,11H,2-3,8-10H2,1H3,(H,21,25)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC4 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM162846

(US9056843, 137)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-26(11-5-2)12-13(3)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,4-5,10-12H2,1-3H3,(H,23,27)/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC5 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM162846

(US9056843, 137)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-26(11-5-2)12-13(3)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,4-5,10-12H2,1-3H3,(H,23,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC7 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM162846

(US9056843, 137)Show SMILES CCCN(CCC)C[C@@H](C)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H25F3N4O2/c1-4-10-26(11-5-2)12-13(3)23-17(27)15-8-6-14(7-9-15)16-24-18(28-25-16)19(20,21)22/h6-9,13H,4-5,10-12H2,1-3H3,(H,23,27)/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC9 (unknown origin) using Boc Lys(TFA) as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50552909

(CHEMBL4754665)Show SMILES C[C@H](CN1CCC[C@@H](C1)OC(F)F)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Class 2A HDAC4 in human Jurkat E6.1 cells using Boc-Lys-(TFA)-AMC as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50552912

(CHEMBL4760047)Show SMILES C[C@H](CN1CCCC(C)(C)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Class 2A HDAC4 in human Jurkat E6.1 cells using Boc-Lys-(TFA)-AMC as substrate by fluorogenic assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00532
BindingDB Entry DOI: 10.7270/Q2474FHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM293880
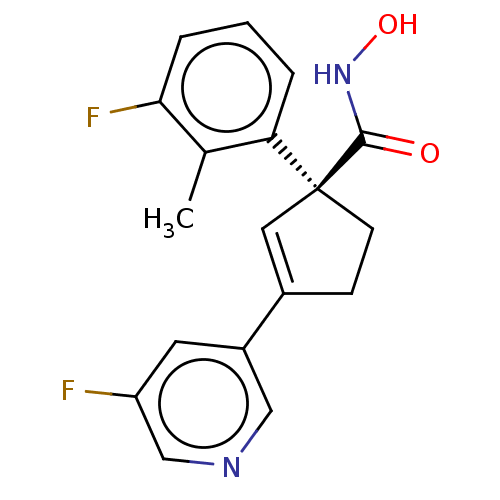
((S)-1-(3-Fluoro-2- methylphenyl)-3-(5- fluoropyrid...)Show SMILES Cc1c(F)cccc1[C@@]1(CCC(=C1)c1cncc(F)c1)C(=O)NO |r,c:12| Show InChI InChI=1S/C18H16F2N2O2/c1-11-15(3-2-4-16(11)20)18(17(23)22-24)6-5-12(8-18)13-7-14(19)10-21-9-13/h2-4,7-10,24H,5-6H2,1H3,(H,22,23)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc.
US Patent
| Assay Description
2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... |
US Patent US9617259 (2017)
BindingDB Entry DOI: 10.7270/Q2DN4743 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data