 Found 432 hits with Last Name = 'geddes' and Initial = 'b'
Found 432 hits with Last Name = 'geddes' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183974
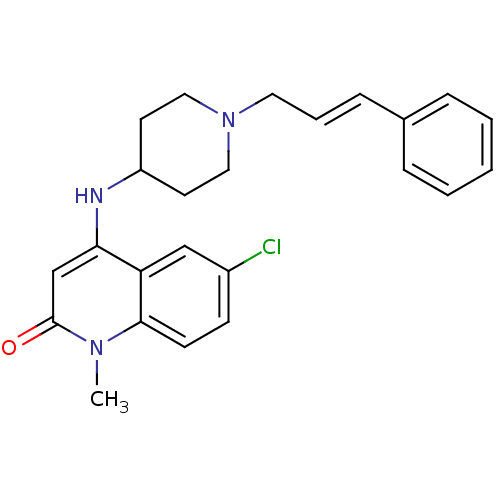
((E)-6-chloro-4-(1-cinnamylpiperidin-4-ylamino)-1-m...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(C\C=C\c3ccccc3)CC2)cc1=O Show InChI InChI=1S/C24H26ClN3O/c1-27-23-10-9-19(25)16-21(23)22(17-24(27)29)26-20-11-14-28(15-12-20)13-5-8-18-6-3-2-4-7-18/h2-10,16-17,20,26H,11-15H2,1H3/b8-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183965
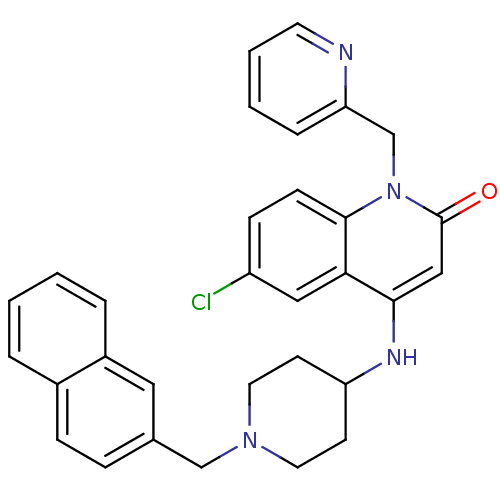
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183969
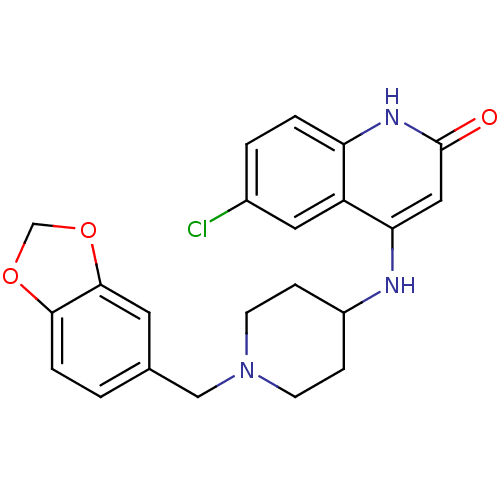
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H22ClN3O3/c23-15-2-3-18-17(10-15)19(11-22(27)25-18)24-16-5-7-26(8-6-16)12-14-1-4-20-21(9-14)29-13-28-20/h1-4,9-11,16H,5-8,12-13H2,(H2,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183966
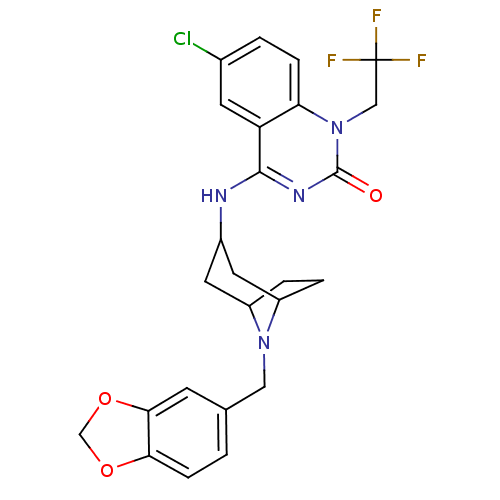
(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:14:15:22:18.19| Show InChI InChI=1S/C25H24ClF3N4O3/c26-15-2-5-20-19(8-15)23(31-24(34)33(20)12-25(27,28)29)30-16-9-17-3-4-18(10-16)32(17)11-14-1-6-21-22(7-14)36-13-35-21/h1-2,5-8,16-18H,3-4,9-13H2,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183960
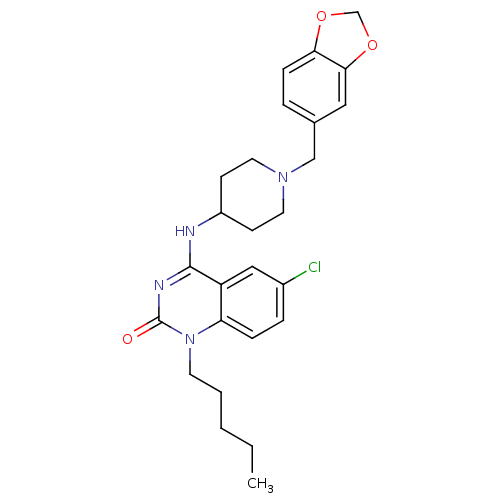
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES CCCCCn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C26H31ClN4O3/c1-2-3-4-11-31-22-7-6-19(27)15-21(22)25(29-26(31)32)28-20-9-12-30(13-10-20)16-18-5-8-23-24(14-18)34-17-33-23/h5-8,14-15,20H,2-4,9-13,16-17H2,1H3,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183972
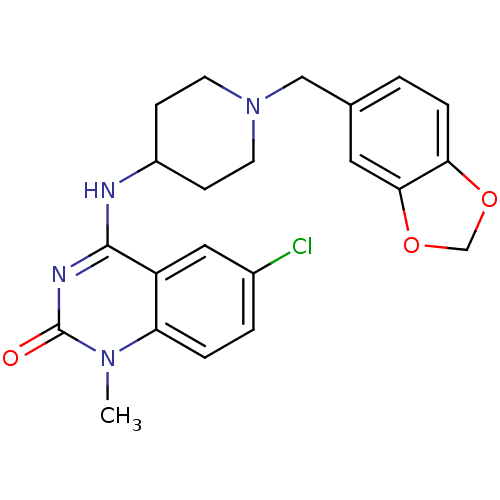
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C22H23ClN4O3/c1-26-18-4-3-15(23)11-17(18)21(25-22(26)28)24-16-6-8-27(9-7-16)12-14-2-5-19-20(10-14)30-13-29-19/h2-5,10-11,16H,6-9,12-13H2,1H3,(H,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183971
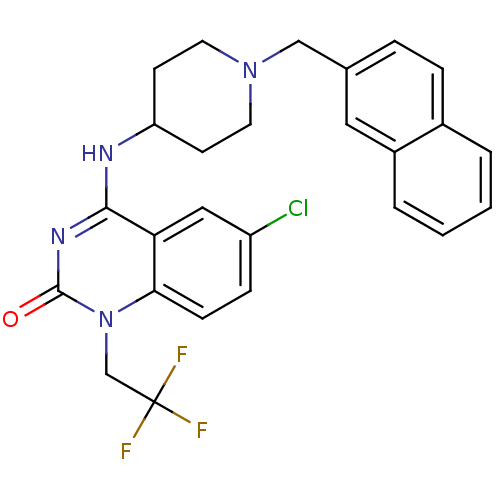
(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C26H24ClF3N4O/c27-20-7-8-23-22(14-20)24(32-25(35)34(23)16-26(28,29)30)31-21-9-11-33(12-10-21)15-17-5-6-18-3-1-2-4-19(18)13-17/h1-8,13-14,21H,9-12,15-16H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183968
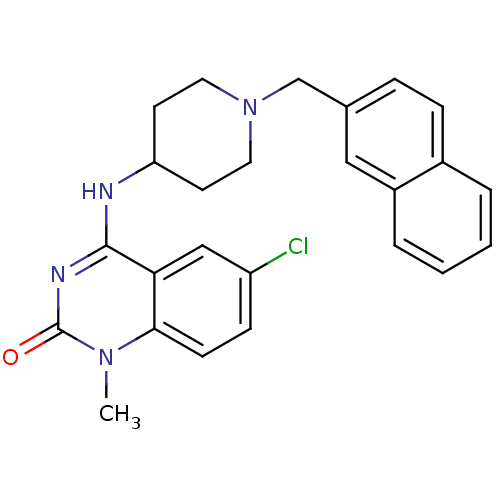
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)nc1=O Show InChI InChI=1S/C25H25ClN4O/c1-29-23-9-8-20(26)15-22(23)24(28-25(29)31)27-21-10-12-30(13-11-21)16-17-6-7-18-4-2-3-5-19(18)14-17/h2-9,14-15,21H,10-13,16H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183975
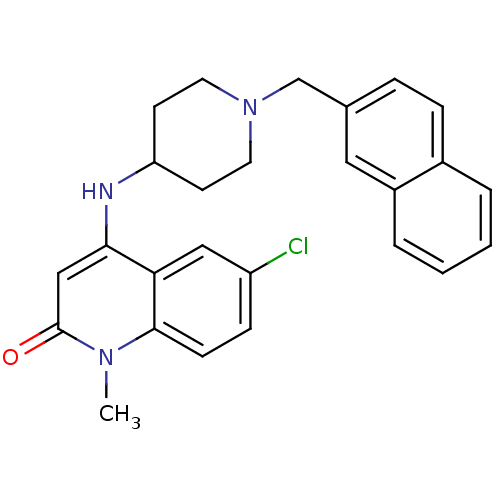
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)cc1=O Show InChI InChI=1S/C26H26ClN3O/c1-29-25-9-8-21(27)15-23(25)24(16-26(29)31)28-22-10-12-30(13-11-22)17-18-6-7-19-4-2-3-5-20(19)14-18/h2-9,14-16,22,28H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183964
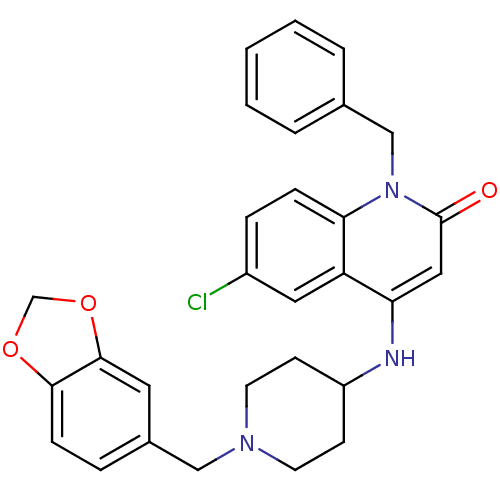
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C29H28ClN3O3/c30-22-7-8-26-24(15-22)25(16-29(34)33(26)18-20-4-2-1-3-5-20)31-23-10-12-32(13-11-23)17-21-6-9-27-28(14-21)36-19-35-27/h1-9,14-16,23,31H,10-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183958
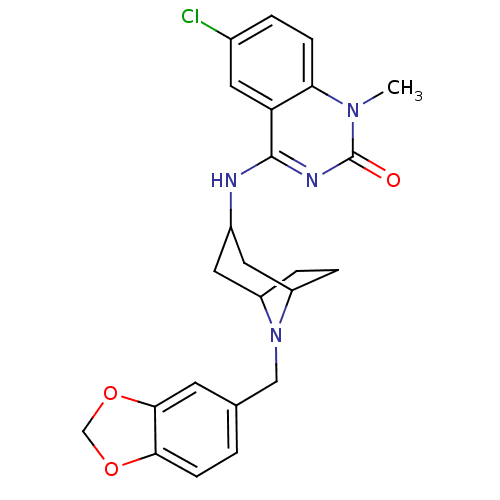
(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:10:11:18:14.15| Show InChI InChI=1S/C24H25ClN4O3/c1-28-20-6-3-15(25)9-19(20)23(27-24(28)30)26-16-10-17-4-5-18(11-16)29(17)12-14-2-7-21-22(8-14)32-13-31-21/h2-3,6-9,16-18H,4-5,10-13H2,1H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183958

(4-(8-(benzo[d][1,3]dioxol-5-ylmethyl)-8-aza-bicycl...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CC3CCC(C2)N3Cc2ccc3OCOc3c2)nc1=O |TLB:10:11:18:14.15| Show InChI InChI=1S/C24H25ClN4O3/c1-28-20-6-3-15(25)9-19(20)23(27-24(28)30)26-16-10-17-4-5-18(11-16)29(17)12-14-2-7-21-22(8-14)32-13-31-21/h2-3,6-9,16-18H,4-5,10-13H2,1H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183954
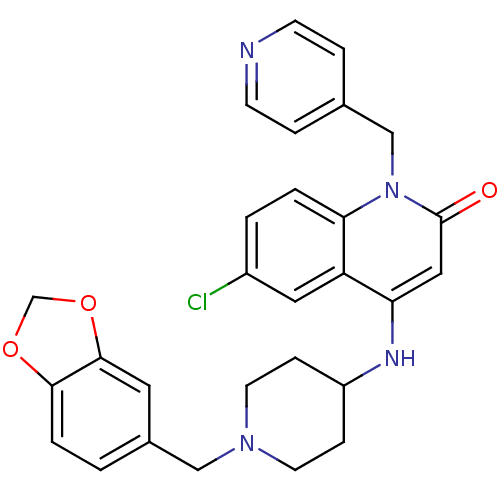
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccncc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-21-2-3-25-23(14-21)24(15-28(34)33(25)17-19-5-9-30-10-6-19)31-22-7-11-32(12-8-22)16-20-1-4-26-27(13-20)36-18-35-26/h1-6,9-10,13-15,22,31H,7-8,11-12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183973
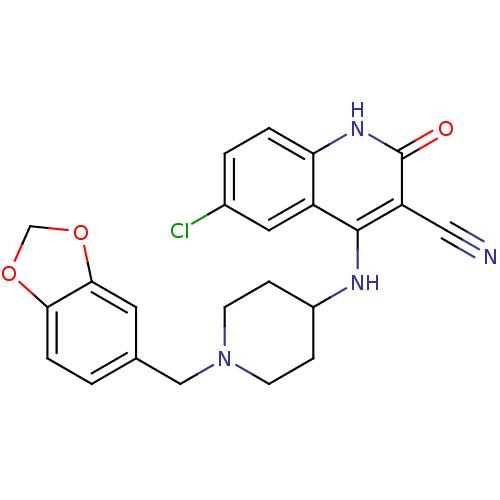
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2[nH]c(=O)c(C#N)c(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C23H21ClN4O3/c24-15-2-3-19-17(10-15)22(18(11-25)23(29)27-19)26-16-5-7-28(8-6-16)12-14-1-4-20-21(9-14)31-13-30-20/h1-4,9-10,16H,5-8,12-13H2,(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183967
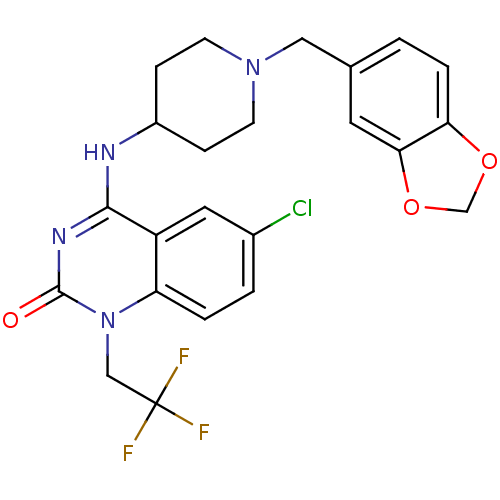
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C23H22ClF3N4O3/c24-15-2-3-18-17(10-15)21(29-22(32)31(18)12-23(25,26)27)28-16-5-7-30(8-6-16)11-14-1-4-19-20(9-14)34-13-33-19/h1-4,9-10,16H,5-8,11-13H2,(H,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183953
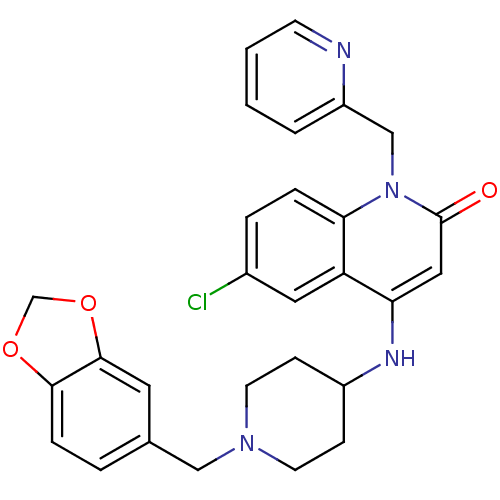
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-20-5-6-25-23(14-20)24(15-28(34)33(25)17-22-3-1-2-10-30-22)31-21-8-11-32(12-9-21)16-19-4-7-26-27(13-19)36-18-35-26/h1-7,10,13-15,21,31H,8-9,11-12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183957
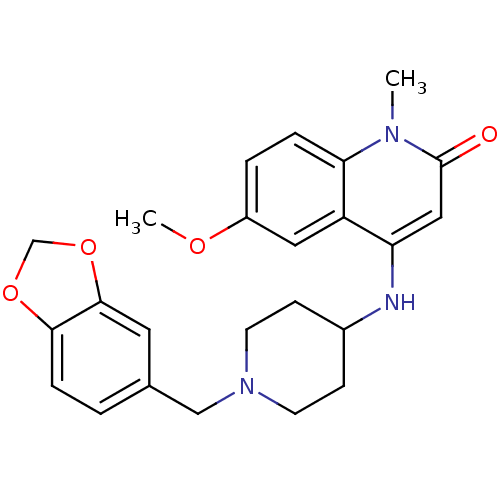
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES COc1ccc2n(C)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C24H27N3O4/c1-26-21-5-4-18(29-2)12-19(21)20(13-24(26)28)25-17-7-9-27(10-8-17)14-16-3-6-22-23(11-16)31-15-30-22/h3-6,11-13,17,25H,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183961
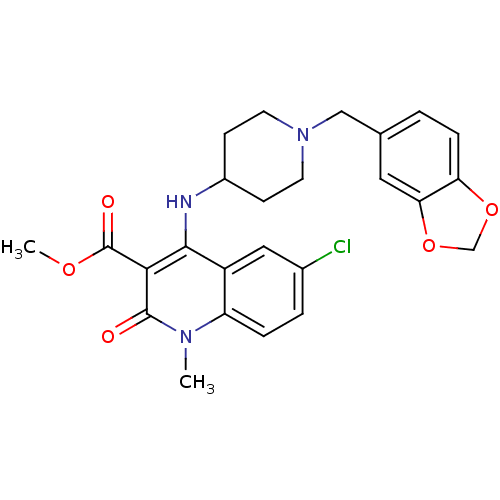
(CHEMBL205741 | methyl 4-(1-(benzo[d][1,3]dioxol-5-...)Show SMILES COC(=O)c1c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c2cc(Cl)ccc2n(C)c1=O Show InChI InChI=1S/C25H26ClN3O5/c1-28-19-5-4-16(26)12-18(19)23(22(24(28)30)25(31)32-2)27-17-7-9-29(10-8-17)13-15-3-6-20-21(11-15)34-14-33-20/h3-6,11-12,17,27H,7-10,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183970
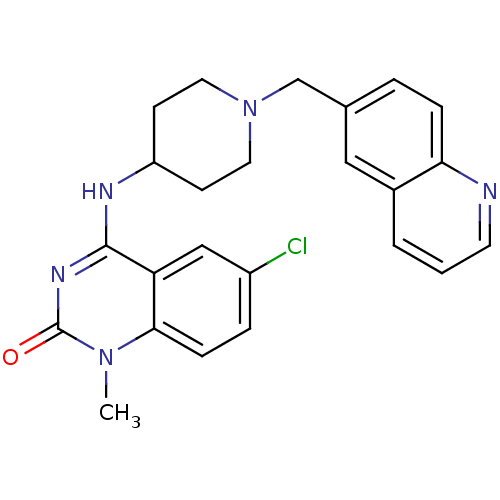
(6-chloro-1-methyl-4-(1-(quinolin-6-ylmethyl)piperi...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ncccc4c3)CC2)nc1=O Show InChI InChI=1S/C24H24ClN5O/c1-29-22-7-5-18(25)14-20(22)23(28-24(29)31)27-19-8-11-30(12-9-19)15-16-4-6-21-17(13-16)3-2-10-26-21/h2-7,10,13-14,19H,8-9,11-12,15H2,1H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183963

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(OC(F)(F)F)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)cc1=O Show InChI InChI=1S/C24H24F3N3O4/c1-29-20-4-3-17(34-24(25,26)27)11-18(20)19(12-23(29)31)28-16-6-8-30(9-7-16)13-15-2-5-21-22(10-15)33-14-32-21/h2-5,10-12,16,28H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183959
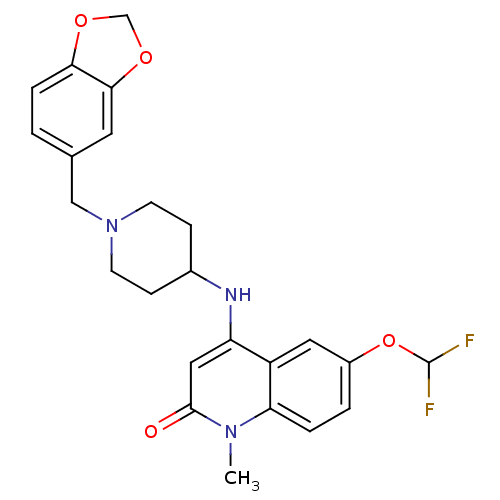
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(OC(F)F)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)cc1=O Show InChI InChI=1S/C24H25F2N3O4/c1-28-20-4-3-17(33-24(25)26)11-18(20)19(12-23(28)30)27-16-6-8-29(9-7-16)13-15-2-5-21-22(10-15)32-14-31-21/h2-5,10-12,16,24,27H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183962
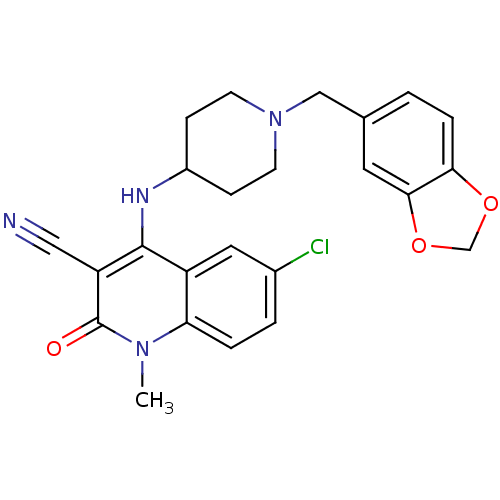
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c(C#N)c1=O Show InChI InChI=1S/C24H23ClN4O3/c1-28-20-4-3-16(25)11-18(20)23(19(12-26)24(28)30)27-17-6-8-29(9-7-17)13-15-2-5-21-22(10-15)32-14-31-21/h2-5,10-11,17,27H,6-9,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183955
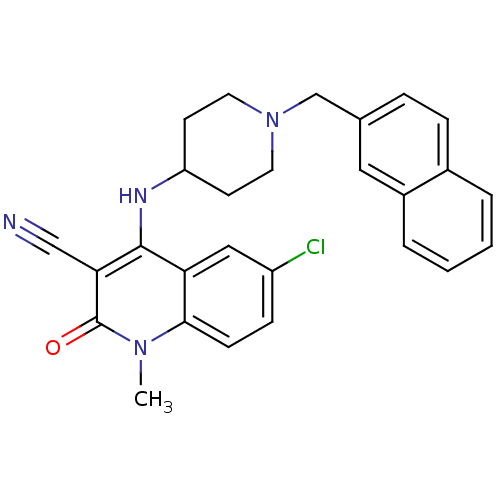
(6-chloro-1-methyl-4-(1-(naphthalen-2-ylmethyl)pipe...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4ccccc4c3)CC2)c(C#N)c1=O Show InChI InChI=1S/C27H25ClN4O/c1-31-25-9-8-21(28)15-23(25)26(24(16-29)27(31)33)30-22-10-12-32(13-11-22)17-18-6-7-19-4-2-3-5-20(19)14-18/h2-9,14-15,22,30H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50183956
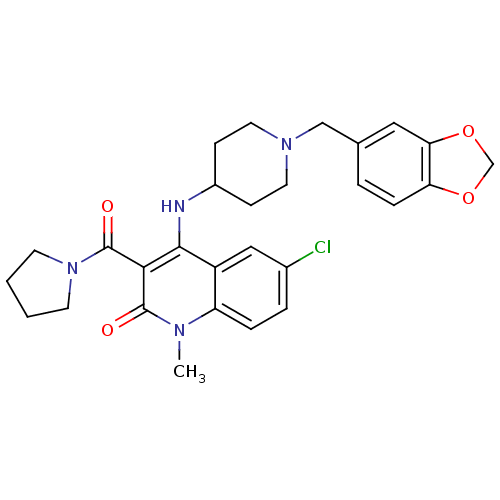
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)c(C(=O)N2CCCC2)c1=O Show InChI InChI=1S/C28H31ClN4O4/c1-31-22-6-5-19(29)15-21(22)26(25(27(31)34)28(35)33-10-2-3-11-33)30-20-8-12-32(13-9-20)16-18-4-7-23-24(14-18)37-17-36-23/h4-7,14-15,20,30H,2-3,8-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG stably transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513015
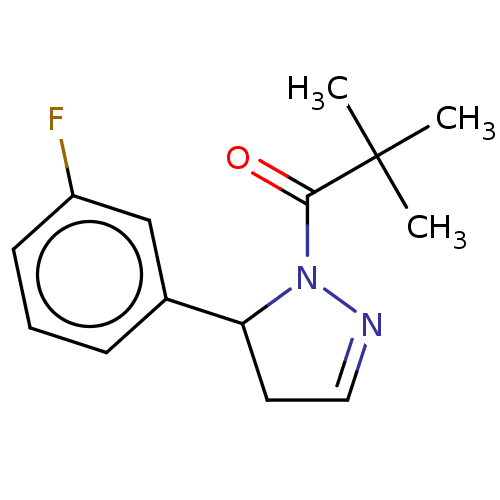
(CHEMBL4537171)Show InChI InChI=1S/C14H17FN2O/c1-14(2,3)13(18)17-12(7-8-16-17)10-5-4-6-11(15)9-10/h4-6,8-9,12H,7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183964

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C29H28ClN3O3/c30-22-7-8-26-24(15-22)25(16-29(34)33(26)18-20-4-2-1-3-5-20)31-23-10-12-32(13-11-23)17-21-6-9-27-28(14-21)36-19-35-27/h1-9,14-16,23,31H,10-13,17-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183965

(6-chloro-4-(1-(naphthalen-2-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2c1 Show InChI InChI=1S/C31H29ClN4O/c32-25-10-11-30-28(18-25)29(19-31(37)36(30)21-27-7-3-4-14-33-27)34-26-12-15-35(16-13-26)20-22-8-9-23-5-1-2-6-24(23)17-22/h1-11,14,17-19,26,34H,12-13,15-16,20-21H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50507336
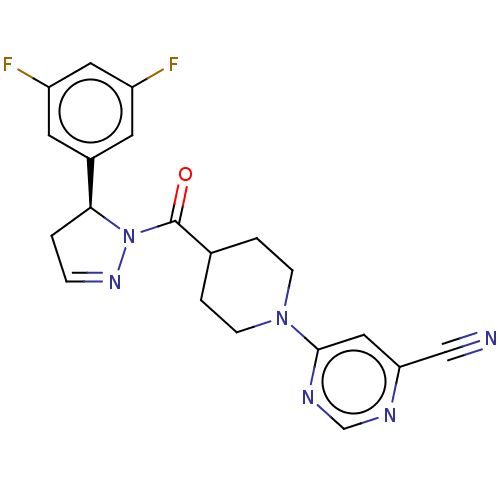
(CHEMBL4514271)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1cc(ncn1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-7-14(8-16(22)9-15)18-1-4-26-28(18)20(29)13-2-5-27(6-3-13)19-10-17(11-23)24-12-25-19/h4,7-10,12-13,18H,1-3,5-6H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183954

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccncc3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-21-2-3-25-23(14-21)24(15-28(34)33(25)17-19-5-9-30-10-6-19)31-22-7-11-32(12-8-22)16-20-1-4-26-27(13-20)36-18-35-26/h1-6,9-10,13-15,22,31H,7-8,11-12,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183953

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES Clc1ccc2n(Cc3ccccn3)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C28H27ClN4O3/c29-20-5-6-25-23(14-20)24(15-28(34)33(25)17-22-3-1-2-10-30-22)31-21-8-11-32(12-9-21)16-19-4-7-26-27(13-19)36-18-35-26/h1-7,10,13-15,21,31H,8-9,11-12,16-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513040

(CHEMBL4593226)Show InChI InChI=1S/C14H16F2N2O/c1-14(2,3)13(19)18-12(4-5-17-18)9-6-10(15)8-11(16)7-9/h5-8,12H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513011
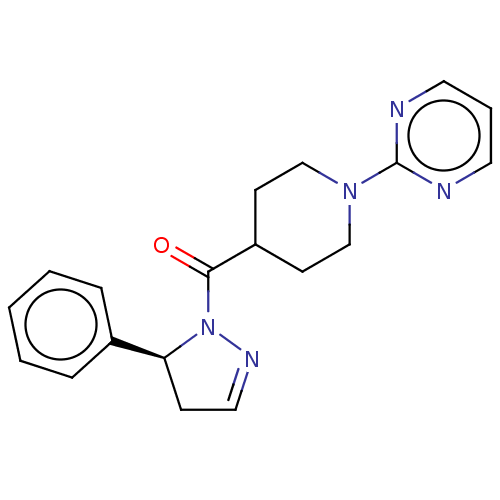
(CHEMBL4455042)Show SMILES O=C(C1CCN(CC1)c1ncccn1)N1N=CC[C@H]1c1ccccc1 |r,c:17| Show InChI InChI=1S/C19H21N5O/c25-18(24-17(7-12-22-24)15-5-2-1-3-6-15)16-8-13-23(14-9-16)19-20-10-4-11-21-19/h1-6,10-12,16-17H,7-9,13-14H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513004
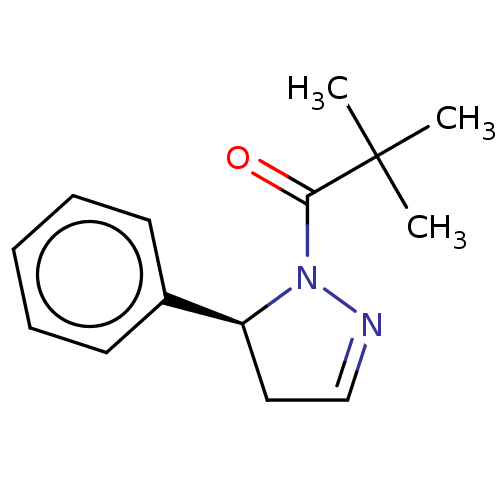
(CHEMBL4521353)Show InChI InChI=1S/C14H18N2O/c1-14(2,3)13(17)16-12(9-10-15-16)11-7-5-4-6-8-11/h4-8,10,12H,9H2,1-3H3/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513024
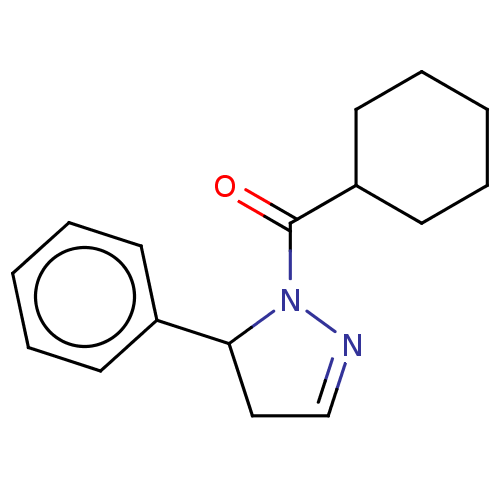
(CHEMBL4471642)Show InChI InChI=1S/C16H20N2O/c19-16(14-9-5-2-6-10-14)18-15(11-12-17-18)13-7-3-1-4-8-13/h1,3-4,7-8,12,14-15H,2,5-6,9-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50171590
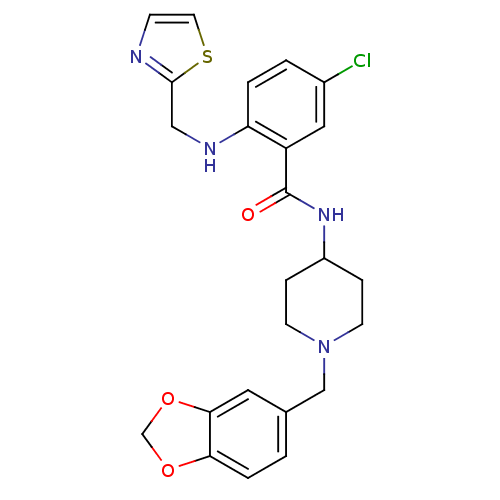
(CHEMBL194697 | N-(1-Benzo[1,3]dioxol-5-ylmethyl-pi...)Show SMILES Clc1ccc(NCc2nccs2)c(c1)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O3S/c25-17-2-3-20(27-13-23-26-7-10-33-23)19(12-17)24(30)28-18-5-8-29(9-6-18)14-16-1-4-21-22(11-16)32-15-31-21/h1-4,7,10-12,18,27H,5-6,8-9,13-15H2,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity towards melanin-concentrating hormone receptor 1 in IMR32 cells |
Bioorg Med Chem Lett 15: 4174-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.089
BindingDB Entry DOI: 10.7270/Q2N29WGN |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513034
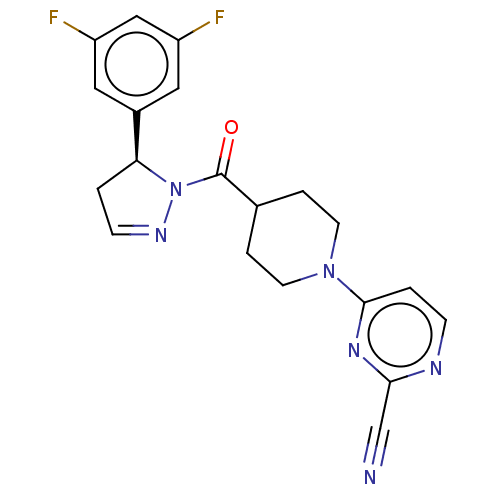
(CHEMBL4541955)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1ccnc(n1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-9-14(10-16(22)11-15)17-1-6-25-28(17)20(29)13-3-7-27(8-4-13)19-2-5-24-18(12-23)26-19/h2,5-6,9-11,13,17H,1,3-4,7-8H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513016
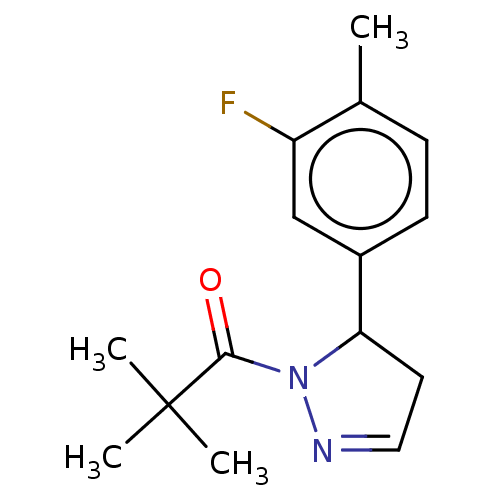
(CHEMBL4514028)Show InChI InChI=1S/C15H19FN2O/c1-10-5-6-11(9-12(10)16)13-7-8-17-18(13)14(19)15(2,3)4/h5-6,8-9,13H,7H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513013
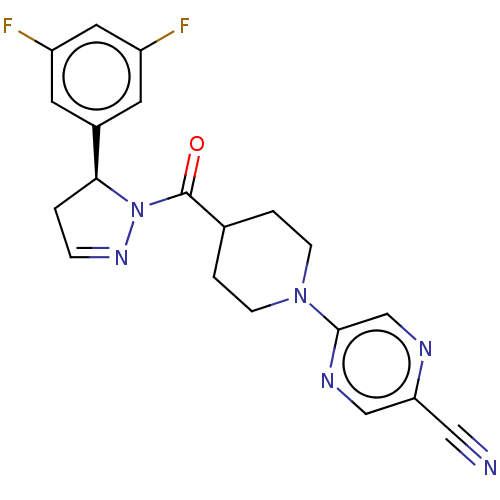
(CHEMBL4450890)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1cnc(cn1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-7-14(8-16(22)9-15)18-1-4-26-28(18)20(29)13-2-5-27(6-3-13)19-12-24-17(10-23)11-25-19/h4,7-9,11-13,18H,1-3,5-6H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183967

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES FC(F)(F)Cn1c2ccc(Cl)cc2c(NC2CCN(Cc3ccc4OCOc4c3)CC2)nc1=O Show InChI InChI=1S/C23H22ClF3N4O3/c24-15-2-3-18-17(10-15)21(29-22(32)31(18)12-23(25,26)27)28-16-5-7-30(8-6-16)11-14-1-4-19-20(9-14)34-13-33-19/h1-4,9-10,16H,5-8,11-13H2,(H,28,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173461

(4-(1-Benzo[1,3]dioxol-5-ylmethyl-piperidin-4-ylami...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H21ClN2O4/c23-15-2-4-19-17(10-15)18(11-22(26)29-19)24-16-5-7-25(8-6-16)12-14-1-3-20-21(9-14)28-13-27-20/h1-4,9-11,16,24H,5-8,12-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513016

(CHEMBL4514028)Show InChI InChI=1S/C15H19FN2O/c1-10-5-6-11(9-12(10)16)13-7-8-17-18(13)14(19)15(2,3)4/h5-6,8-9,13H,7H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513034

(CHEMBL4541955)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CC=NN1C(=O)C1CCN(CC1)c1ccnc(n1)C#N |r,c:11| Show InChI InChI=1S/C20H18F2N6O/c21-15-9-14(10-16(22)11-15)17-1-6-25-28(17)20(29)13-3-7-27(8-4-13)19-2-5-24-18(12-23)26-19/h2,5-6,9-11,13,17H,1,3-4,7-8H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50512996
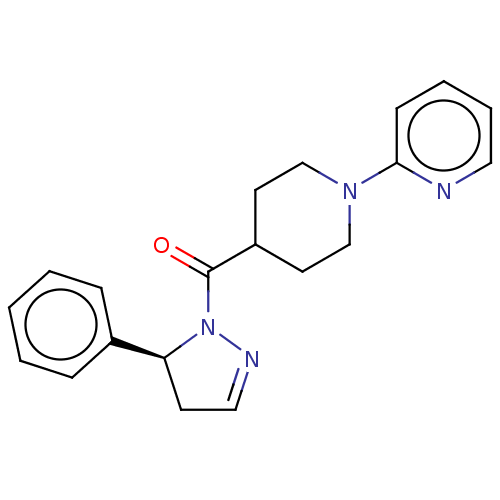
(CHEMBL4454462)Show SMILES O=C(C1CCN(CC1)c1ccccn1)N1N=CC[C@H]1c1ccccc1 |r,c:17| Show InChI InChI=1S/C20H22N4O/c25-20(24-18(9-13-22-24)16-6-2-1-3-7-16)17-10-14-23(15-11-17)19-8-4-5-12-21-19/h1-8,12-13,17-18H,9-11,14-15H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50512998
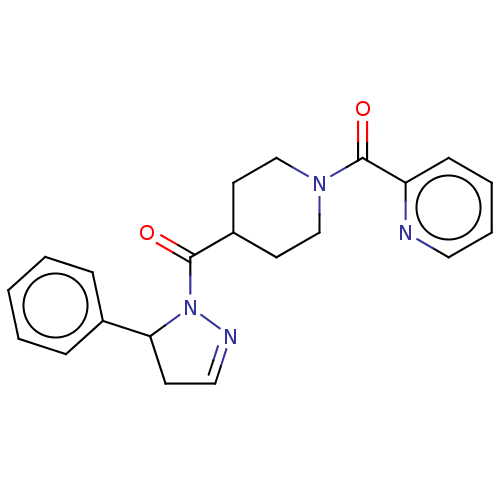
(CHEMBL4575848)Show SMILES O=C(C1CCN(CC1)C(=O)c1ccccn1)N1N=CCC1c1ccccc1 |c:19| Show InChI InChI=1S/C21H22N4O2/c26-20(25-19(9-13-23-25)16-6-2-1-3-7-16)17-10-14-24(15-11-17)21(27)18-8-4-5-12-22-18/h1-8,12-13,17,19H,9-11,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513030
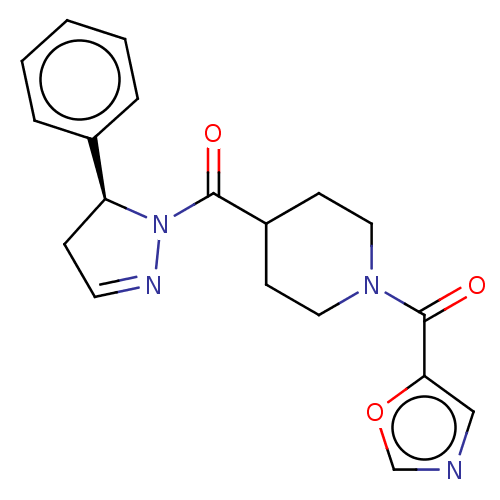
(CHEMBL4548784)Show SMILES O=C(C1CCN(CC1)C(=O)c1cnco1)N1N=CC[C@H]1c1ccccc1 |r,c:18| Show InChI InChI=1S/C19H20N4O3/c24-18(23-16(6-9-21-23)14-4-2-1-3-5-14)15-7-10-22(11-8-15)19(25)17-12-20-13-26-17/h1-5,9,12-13,15-16H,6-8,10-11H2/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173454

(4-(1-Benzooxazol-5-ylmethyl-piperidin-4-ylamino)-6...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5ocnc5c4)CC3)c2c1 Show InChI InChI=1S/C22H20ClN3O3/c23-15-2-4-20-17(10-15)18(11-22(27)29-20)25-16-5-7-26(8-6-16)12-14-1-3-21-19(9-14)24-13-28-21/h1-4,9-11,13,16,25H,5-8,12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50168509
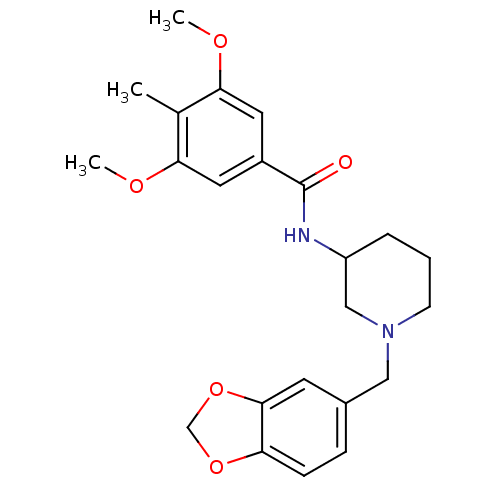
(CHEMBL188937 | N-(1-Benzo[1,3]dioxol-5-ylmethyl-pi...)Show SMILES COc1cc(cc(OC)c1C)C(=O)NC1CCCN(Cc2ccc3OCOc3c2)C1 Show InChI InChI=1S/C23H28N2O5/c1-15-20(27-2)10-17(11-21(15)28-3)23(26)24-18-5-4-8-25(13-18)12-16-6-7-19-22(9-16)30-14-29-19/h6-7,9-11,18H,4-5,8,12-14H2,1-3H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration required for binding affinity against mouse MCHr1 competing human neuronal IMR32 cells receptor in a radiometric binding ass... |
Bioorg Med Chem Lett 15: 3412-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.023
BindingDB Entry DOI: 10.7270/Q26D5SHR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50183957

(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES COc1ccc2n(C)c(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C24H27N3O4/c1-26-21-5-4-18(29-2)12-19(21)20(13-24(26)28)25-17-7-9-27(10-8-17)14-16-3-6-22-23(11-16)31-15-30-22/h3-6,11-13,17,25H,7-10,14-15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
Bioorg Med Chem Lett 16: 2621-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.044
BindingDB Entry DOI: 10.7270/Q2SB45CQ |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Mus musculus) | BDBM50512997
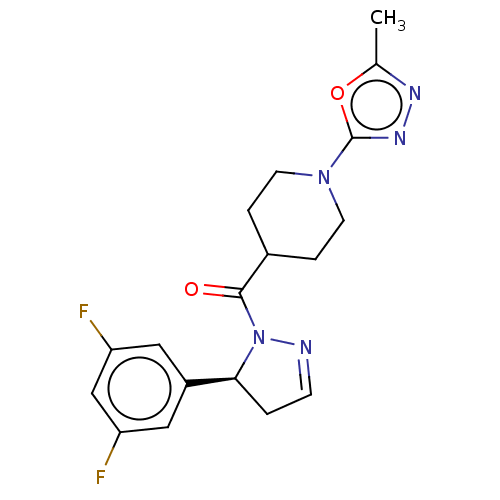
(CHEMBL4545939)Show SMILES Cc1nnc(o1)N1CCC(CC1)C(=O)N1N=CC[C@H]1c1cc(F)cc(F)c1 |r,c:17| Show InChI InChI=1S/C18H19F2N5O2/c1-11-22-23-18(27-11)24-6-3-12(4-7-24)17(26)25-16(2-5-21-25)13-8-14(19)10-15(20)9-13/h5,8-10,12,16H,2-4,6-7H2,1H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in mouse L929 cells assessed as reduction in TNF/zVAD.fmk-induced necrotic death measured after 24 hrs by cell titer-glo luminesce... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513007
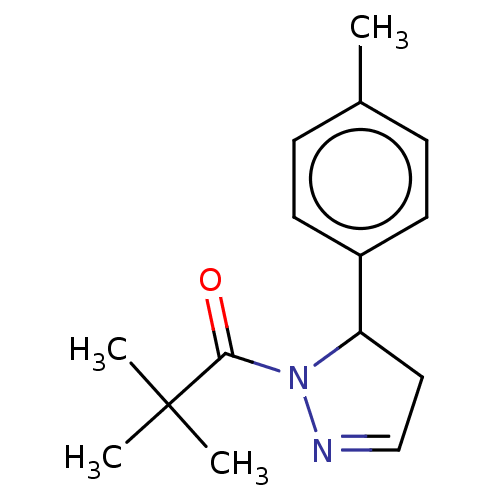
(CHEMBL4436215)Show InChI InChI=1S/C15H20N2O/c1-11-5-7-12(8-6-11)13-9-10-16-17(13)14(18)15(2,3)4/h5-8,10,13H,9H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data