 Found 171 hits with Last Name = 'xiang' and Initial = 'b'
Found 171 hits with Last Name = 'xiang' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286261
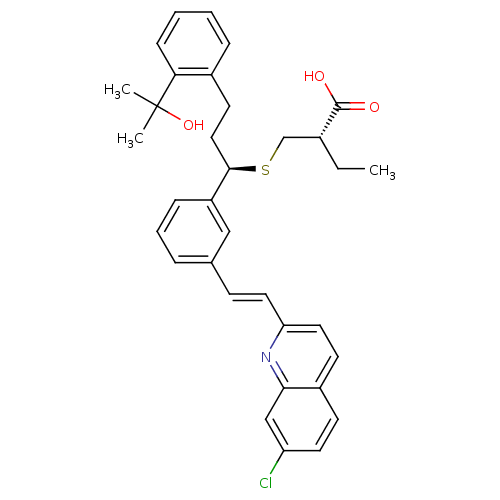
((S)-2-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...)Show SMILES CC[C@H](CS[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)C(O)=O Show InChI InChI=1S/C34H36ClNO3S/c1-4-24(33(37)38)22-40-32(19-15-25-9-5-6-11-30(25)34(2,3)39)27-10-7-8-23(20-27)12-17-29-18-14-26-13-16-28(35)21-31(26)36-29/h5-14,16-18,20-21,24,32,39H,4,15,19,22H2,1-3H3,(H,37,38)/b17-12+/t24-,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286257

((2R,3R)-3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl...)Show SMILES C[C@@H](S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)[C@H](C)C(O)=O Show InChI InChI=1S/C34H36ClNO3S/c1-22(33(37)38)23(2)40-32(19-15-25-9-5-6-11-30(25)34(3,4)39)27-10-7-8-24(20-27)12-17-29-18-14-26-13-16-28(35)21-31(26)36-29/h5-14,16-18,20-23,32,39H,15,19H2,1-4H3,(H,37,38)/b17-12+/t22-,23+,32+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286260

(4-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vinyl]...)Show SMILES CC(C)(CS[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)CC(O)=O Show InChI InChI=1S/C35H38ClNO3S/c1-34(2,22-33(38)39)23-41-32(19-15-25-9-5-6-11-30(25)35(3,4)40)27-10-7-8-24(20-27)12-17-29-18-14-26-13-16-28(36)21-31(26)37-29/h5-14,16-18,20-21,32,40H,15,19,22-23H2,1-4H3,(H,38,39)/b17-12+/t32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286253
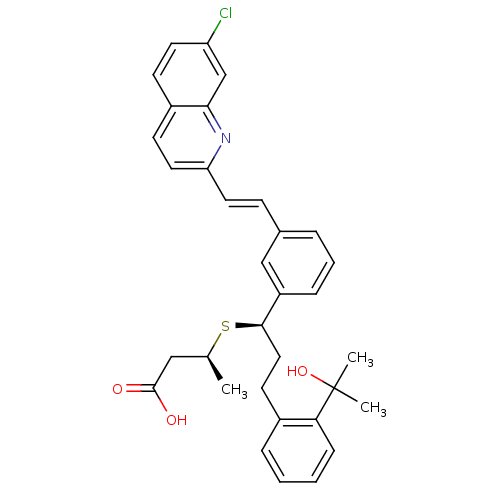
((S)-3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...)Show SMILES C[C@@H](CC(O)=O)S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C33H34ClNO3S/c1-22(19-32(36)37)39-31(18-14-24-8-4-5-10-29(24)33(2,3)38)26-9-6-7-23(20-26)11-16-28-17-13-25-12-15-27(34)21-30(25)35-28/h4-13,15-17,20-22,31,38H,14,18-19H2,1-3H3,(H,36,37)/b16-11+/t22-,31+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285678
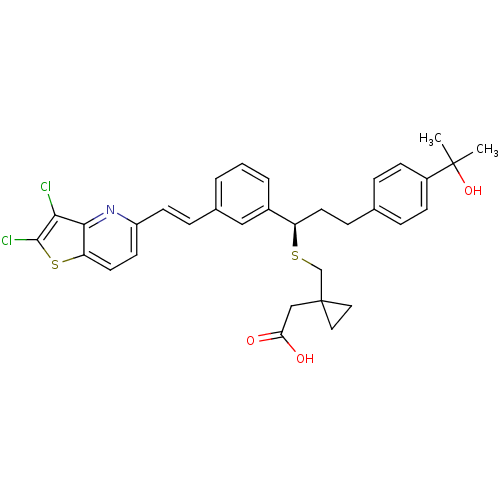
((1-{(R)-1-{3-[(E)-2-(2,3-Dichloro-thieno[3,2-b]pyr...)Show SMILES CC(C)(O)c1ccc(CC[C@@H](SCC2(CC(O)=O)CC2)c2cccc(\C=C\c3ccc4sc(Cl)c(Cl)c4n3)c2)cc1 Show InChI InChI=1S/C33H33Cl2NO3S2/c1-32(2,39)24-10-6-21(7-11-24)9-14-26(40-20-33(16-17-33)19-28(37)38)23-5-3-4-22(18-23)8-12-25-13-15-27-30(36-25)29(34)31(35)41-27/h3-8,10-13,15,18,26,39H,9,14,16-17,19-20H2,1-2H3,(H,37,38)/b12-8+/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286262
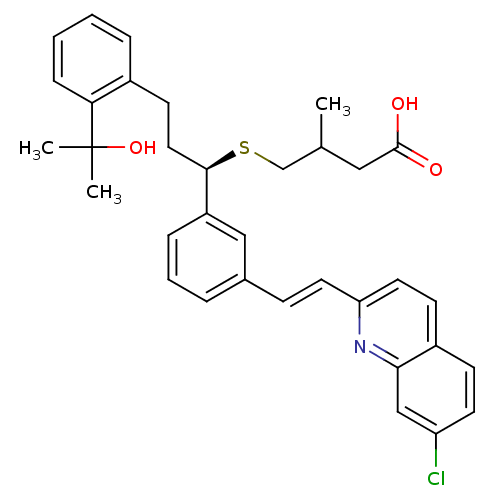
(4-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vinyl]...)Show SMILES CC(CS[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)CC(O)=O Show InChI InChI=1S/C34H36ClNO3S/c1-23(19-33(37)38)22-40-32(18-14-25-8-4-5-10-30(25)34(2,3)39)27-9-6-7-24(20-27)11-16-29-17-13-26-12-15-28(35)21-31(26)36-29/h4-13,15-17,20-21,23,32,39H,14,18-19,22H2,1-3H3,(H,37,38)/b16-11+/t23?,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286255

(3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vinyl]...)Show SMILES CC(S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)C(C)C(O)=O Show InChI InChI=1S/C34H36ClNO3S/c1-22(33(37)38)23(2)40-32(19-15-25-9-5-6-11-30(25)34(3,4)39)27-10-7-8-24(20-27)12-17-29-18-14-26-13-16-28(35)21-31(26)36-29/h5-14,16-18,20-23,32,39H,15,19H2,1-4H3,(H,37,38)/b17-12+/t22?,23?,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285677
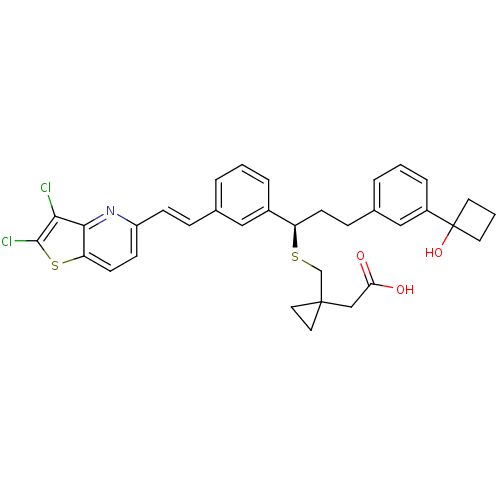
((1-{(R)-1-{3-[(E)-2-(2,3-Dichloro-thieno[3,2-b]pyr...)Show SMILES OC(=O)CC1(CS[C@H](CCc2cccc(c2)C2(O)CCC2)c2cccc(\C=C\c3ccc4sc(Cl)c(Cl)c4n3)c2)CC1 Show InChI InChI=1S/C34H33Cl2NO3S2/c35-30-31-28(42-32(30)36)13-11-26(37-31)10-8-22-4-1-6-24(18-22)27(41-21-33(16-17-33)20-29(38)39)12-9-23-5-2-7-25(19-23)34(40)14-3-15-34/h1-2,4-8,10-11,13,18-19,27,40H,3,9,12,14-17,20-21H2,(H,38,39)/b10-8+/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185884
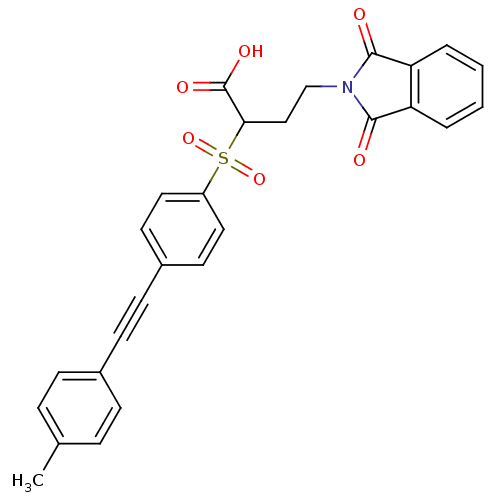
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50052024

(CHEMBL787 | montelukast)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| Show InChI InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10+/t32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285683

((1-{(R)-1-{3-[(E)-2-(2,3-Dichloro-thieno[3,2-b]pyr...)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3sc(Cl)c(Cl)c3n2)c1 Show InChI InChI=1S/C33H33Cl2NO3S2/c1-32(2,39)25-9-4-3-7-22(25)11-14-26(40-20-33(16-17-33)19-28(37)38)23-8-5-6-21(18-23)10-12-24-13-15-27-30(36-24)29(34)31(35)41-27/h3-10,12-13,15,18,26,39H,11,14,16-17,19-20H2,1-2H3,(H,37,38)/b12-10+/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286254

(3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vinyl]...)Show SMILES CCC(CC(O)=O)S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C34H36ClNO3S/c1-4-29(22-33(37)38)40-32(19-15-24-9-5-6-11-30(24)34(2,3)39)26-10-7-8-23(20-26)12-17-28-18-14-25-13-16-27(35)21-31(25)36-28/h5-14,16-18,20-21,29,32,39H,4,15,19,22H2,1-3H3,(H,37,38)/b17-12+/t29?,32-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286256
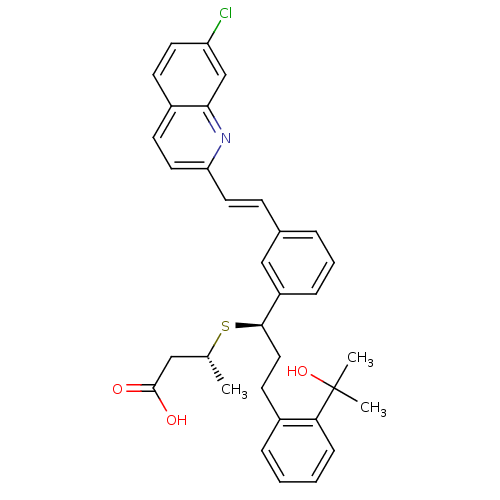
((R)-3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...)Show SMILES C[C@H](CC(O)=O)S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C33H34ClNO3S/c1-22(19-32(36)37)39-31(18-14-24-8-4-5-10-29(24)33(2,3)38)26-9-6-7-23(20-26)11-16-28-17-13-25-12-15-27(34)21-30(25)35-28/h4-13,15-17,20-22,31,38H,14,18-19H2,1-3H3,(H,36,37)/b16-11+/t22-,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185883
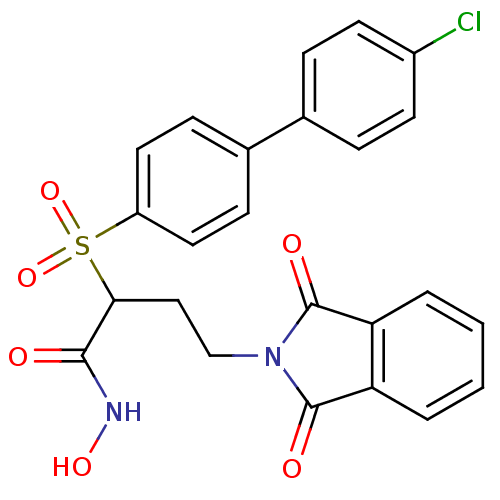
(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES ONC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN2O6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)34(32,33)21(22(28)26-31)13-14-27-23(29)19-3-1-2-4-20(19)24(27)30/h1-12,21,31H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285675
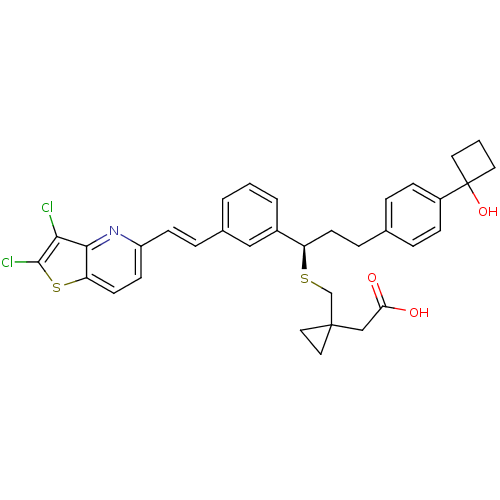
((1-{(R)-1-{3-[(E)-2-(2,3-Dichloro-thieno[3,2-b]pyr...)Show SMILES OC(=O)CC1(CS[C@H](CCc2ccc(cc2)C2(O)CCC2)c2cccc(\C=C\c3ccc4sc(Cl)c(Cl)c4n3)c2)CC1 Show InChI InChI=1S/C34H33Cl2NO3S2/c35-30-31-28(42-32(30)36)14-12-26(37-31)11-7-23-3-1-4-24(19-23)27(41-21-33(17-18-33)20-29(38)39)13-8-22-5-9-25(10-6-22)34(40)15-2-16-34/h1,3-7,9-12,14,19,27,40H,2,8,13,15-18,20-21H2,(H,38,39)/b11-7+/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286259

(3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vinyl]...)Show SMILES CC(C)(CC(O)=O)S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C34H36ClNO3S/c1-33(2,22-32(37)38)40-31(19-15-24-9-5-6-11-29(24)34(3,4)39)26-10-7-8-23(20-26)12-17-28-18-14-25-13-16-27(35)21-30(25)36-28/h5-14,16-18,20-21,31,39H,15,19,22H2,1-4H3,(H,37,38)/b17-12+/t31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185884

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285671

(CHEMBL86063 | {1-[(R)-1-{3-[(E)-2-(2,3-Dichloro-th...)Show SMILES OCc1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3sc(Cl)c(Cl)c3n2)c1 Show InChI InChI=1S/C31H29Cl2NO3S2/c32-28-29-26(39-30(28)33)13-11-24(34-29)10-8-20-4-3-7-22(16-20)25(38-19-31(14-15-31)17-27(36)37)12-9-21-5-1-2-6-23(21)18-35/h1-8,10-11,13,16,25,35H,9,12,14-15,17-19H2,(H,36,37)/b10-8+/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285674
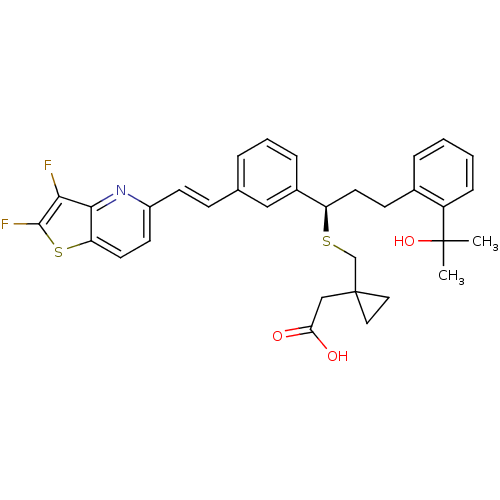
((1-{(R)-1-{3-[(E)-2-(2,3-Difluoro-thieno[3,2-b]pyr...)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3sc(F)c(F)c3n2)c1 Show InChI InChI=1S/C33H33F2NO3S2/c1-32(2,39)25-9-4-3-7-22(25)11-14-26(40-20-33(16-17-33)19-28(37)38)23-8-5-6-21(18-23)10-12-24-13-15-27-30(36-24)29(34)31(35)41-27/h3-10,12-13,15,18,26,39H,11,14,16-17,19-20H2,1-2H3,(H,37,38)/b12-10+/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50286253

((S)-3-{(R)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...)Show SMILES C[C@@H](CC(O)=O)S[C@H](CCc1ccccc1C(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C33H34ClNO3S/c1-22(19-32(36)37)39-31(18-14-24-8-4-5-10-29(24)33(2,3)38)26-9-6-7-23(20-26)11-16-28-17-13-25-12-15-27(34)21-30(25)35-28/h4-13,15-17,20-22,31,38H,14,18-19H2,1-3H3,(H,36,37)/b16-11+/t22-,31+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes(different expt) |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185888
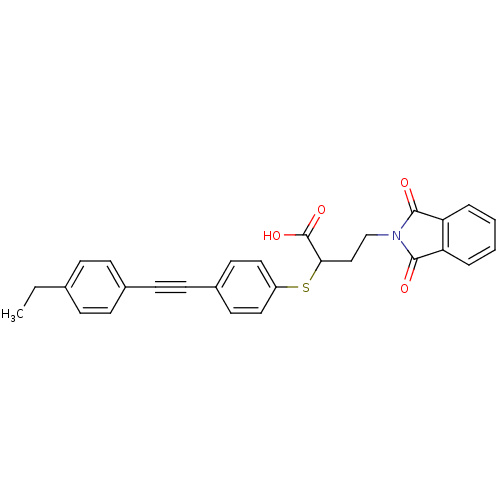
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-et...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C28H23NO4S/c1-2-19-7-9-20(10-8-19)11-12-21-13-15-22(16-14-21)34-25(28(32)33)17-18-29-26(30)23-5-3-4-6-24(23)27(29)31/h3-10,13-16,25H,2,17-18H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185875

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO7S/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374486
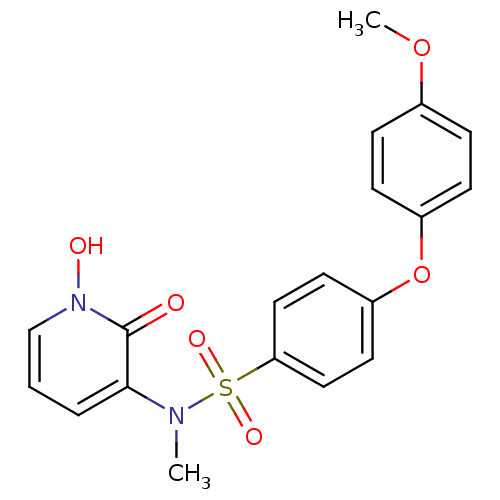
(CHEMBL408366)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)N(C)c2cccn(O)c2=O)cc1 Show InChI InChI=1S/C19H18N2O6S/c1-20(18-4-3-13-21(23)19(18)22)28(24,25)17-11-9-16(10-12-17)27-15-7-5-14(26-2)6-8-15/h3-13,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185900

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S2/c1-33-18-10-6-16(7-11-18)17-8-12-19(13-9-17)34(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185880

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-isop...)Show SMILES CC(C)c1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H25NO6S/c1-17(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)35(33,34)24(27(31)32)15-16-28-25(29)22-5-3-4-6-23(22)26(28)30/h3-14,17,24H,15-16H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50283939
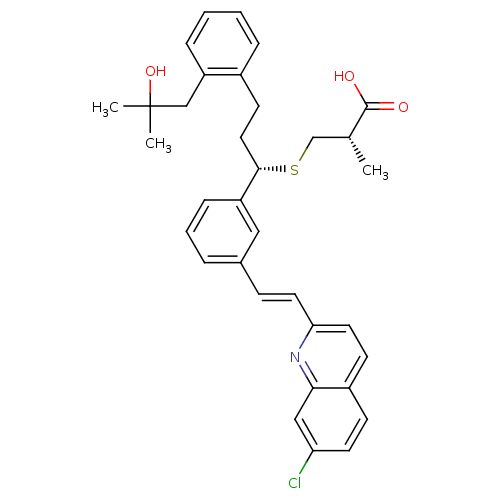
((S)-3-{(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...)Show SMILES C[C@H](CS[C@@H](CCc1ccccc1CC(C)(C)O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1)C(O)=O Show InChI InChI=1S/C34H36ClNO3S/c1-23(33(37)38)22-40-32(18-14-25-8-4-5-9-28(25)21-34(2,3)39)27-10-6-7-24(19-27)11-16-30-17-13-26-12-15-29(35)20-31(26)36-30/h4-13,15-17,19-20,23,32,39H,14,18,21-22H2,1-3H3,(H,37,38)/b16-11+/t23-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes |
Bioorg Med Chem Lett 4: 463-468 (1994)
Article DOI: 10.1016/0960-894X(94)80017-0
BindingDB Entry DOI: 10.7270/Q2M908N4 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285670

((1-{(R)-1-{3-[(E)-2-(2,3-Dichloro-thieno[3,2-b]pyr...)Show SMILES OC(=O)CC1(CS[C@H](CCc2ccccc2C2(O)CCC2)c2cccc(\C=C\c3ccc4sc(Cl)c(Cl)c4n3)c2)CC1 Show InChI InChI=1S/C34H33Cl2NO3S2/c35-30-31-28(42-32(30)36)14-12-25(37-31)11-9-22-5-3-7-24(19-22)27(41-21-33(17-18-33)20-29(38)39)13-10-23-6-1-2-8-26(23)34(40)15-4-16-34/h1-3,5-9,11-12,14,19,27,40H,4,10,13,15-18,20-21H2,(H,38,39)/b11-9+/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285672
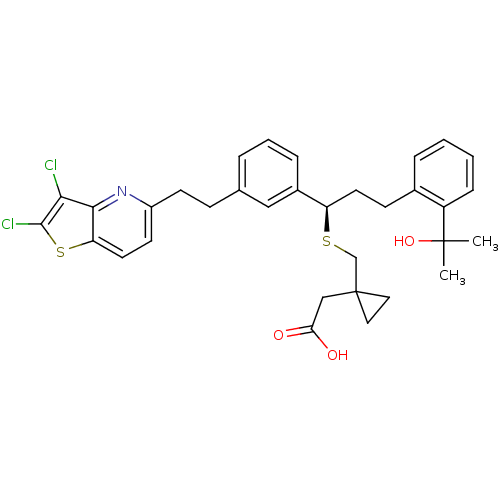
((1-{(R)-1-{3-[2-(2,3-Dichloro-thieno[3,2-b]pyridin...)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(CCc2ccc3sc(Cl)c(Cl)c3n2)c1 Show InChI InChI=1S/C33H35Cl2NO3S2/c1-32(2,39)25-9-4-3-7-22(25)11-14-26(40-20-33(16-17-33)19-28(37)38)23-8-5-6-21(18-23)10-12-24-13-15-27-30(36-24)29(34)31(35)41-27/h3-9,13,15,18,26,39H,10-12,14,16-17,19-20H2,1-2H3,(H,37,38)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185890
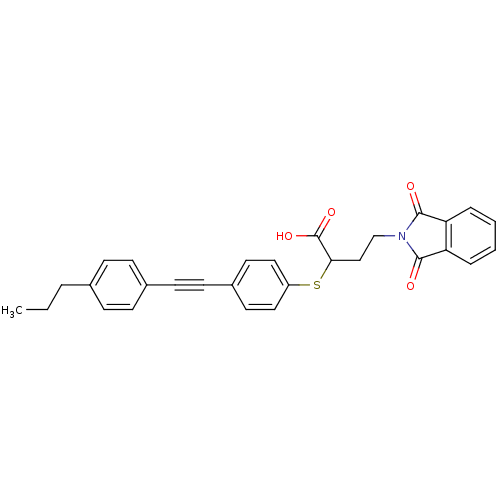
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-pr...)Show SMILES CCCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C29H25NO4S/c1-2-5-20-8-10-21(11-9-20)12-13-22-14-16-23(17-15-22)35-26(29(33)34)18-19-30-27(31)24-6-3-4-7-25(24)28(30)32/h3-4,6-11,14-17,26H,2,5,18-19H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285673
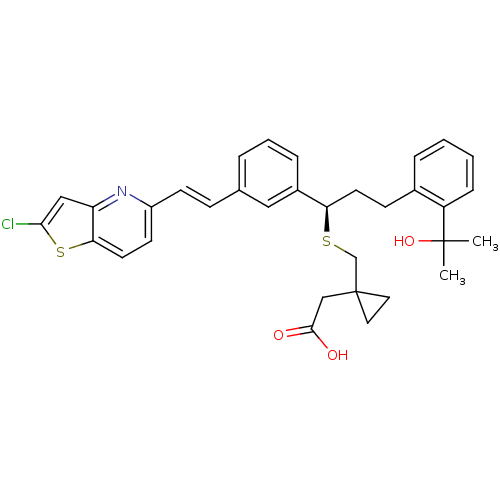
((1-{(R)-1-{3-[(E)-2-(2-Chloro-thieno[3,2-b]pyridin...)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3sc(Cl)cc3n2)c1 Show InChI InChI=1S/C33H34ClNO3S2/c1-32(2,38)26-9-4-3-7-23(26)11-14-28(39-21-33(16-17-33)20-31(36)37)24-8-5-6-22(18-24)10-12-25-13-15-29-27(35-25)19-30(34)40-29/h3-10,12-13,15,18-19,28,38H,11,14,16-17,20-21H2,1-2H3,(H,36,37)/b12-10+/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185877
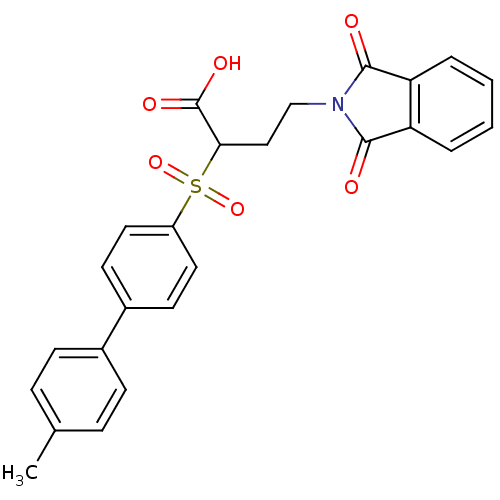
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-meth...)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C25H21NO6S/c1-16-6-8-17(9-7-16)18-10-12-19(13-11-18)33(31,32)22(25(29)30)14-15-26-23(27)20-4-2-3-5-21(20)24(26)28/h2-13,22H,14-15H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185899
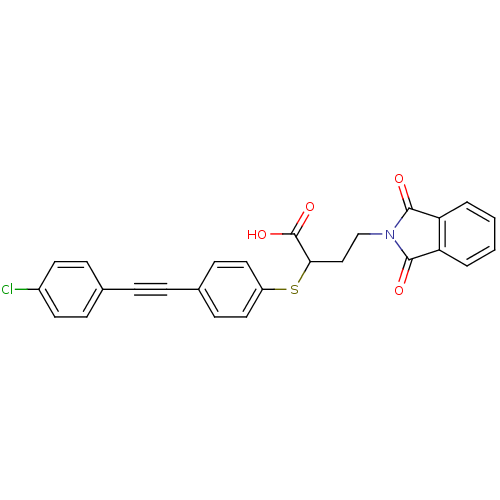
(2-[4-(4-chloro-phenylethynyl)-phenylsulfanyl]-4-(1...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(Cl)cc1 Show InChI InChI=1S/C26H18ClNO4S/c27-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)33-23(26(31)32)15-16-28-24(29)21-3-1-2-4-22(21)25(28)30/h1-4,7-14,23H,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185896

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-tr...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Sc1ccc(cc1)C#Cc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H18F3NO5S/c28-27(29,30)36-19-11-7-17(8-12-19)5-6-18-9-13-20(14-10-18)37-23(26(34)35)15-16-31-24(32)21-3-1-2-4-22(21)25(31)33/h1-4,7-14,23H,15-16H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185888

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-et...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C28H23NO4S/c1-2-19-7-9-20(10-8-19)11-12-21-13-15-22(16-14-21)34-25(28(32)33)17-18-29-26(30)23-5-3-4-6-24(23)27(29)31/h3-10,13-16,25H,2,17-18H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50374494

(CHEMBL271736)Show SMILES CN1CCN(CCN(c2cccn(O)c2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C24H27ClN4O5S/c1-26-13-15-27(16-14-26)17-18-29(23-3-2-12-28(31)24(23)30)35(32,33)22-10-8-21(9-11-22)34-20-6-4-19(25)5-7-20/h2-12,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185883

(2-(4'-chloro-biphenyl-4-sulfonyl)-4-(1,3-dioxo-1,3...)Show SMILES ONC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN2O6S/c25-17-9-5-15(6-10-17)16-7-11-18(12-8-16)34(32,33)21(22(28)26-31)13-14-27-23(29)19-3-1-2-4-20(19)24(27)30/h1-12,21,31H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185887
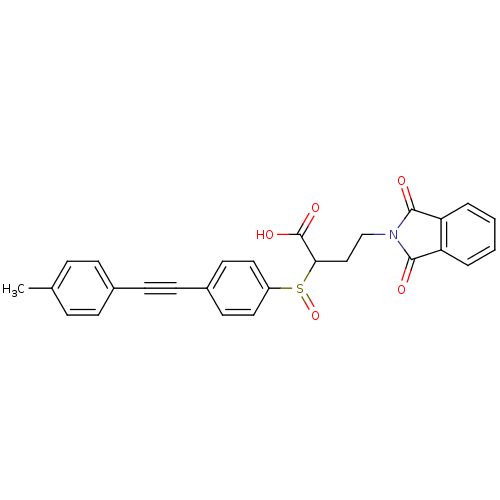
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO5S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)34(33)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50185884

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO6S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)35(33,34)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50052019
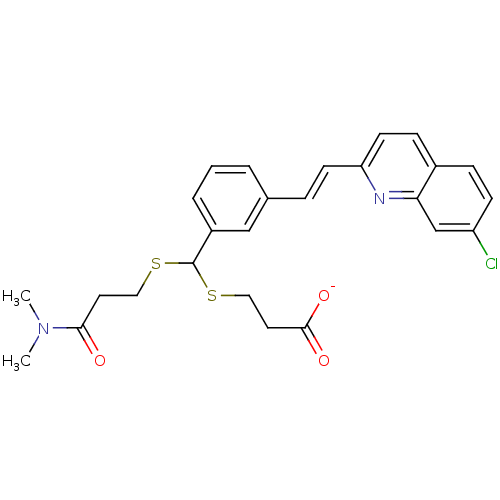
(CHEMBL89768 | L-660771 | MK-571 | Sodium; 3-[{3-[(...)Show SMILES CN(C)C(=O)CCSC(SCCC([O-])=O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C26H27ClN2O3S2/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32)/p-1/b10-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of binding of [3H]-LTD4 to guinea pig lung membranes |
Bioorg Med Chem Lett 5: 283-288 (1995)
Article DOI: 10.1016/0960-894X(95)00023-M
BindingDB Entry DOI: 10.7270/Q2KD1XW7 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50185887

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4-p-tol...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(cc1)S(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H21NO5S/c1-18-6-8-19(9-7-18)10-11-20-12-14-21(15-13-20)34(33)24(27(31)32)16-17-28-25(29)22-4-2-3-5-23(22)26(28)30/h2-9,12-15,24H,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185880

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-(4'-isop...)Show SMILES CC(C)c1ccc(cc1)-c1ccc(cc1)S(=O)(=O)C(CCN1C(=O)c2ccccc2C1=O)C(O)=O Show InChI InChI=1S/C27H25NO6S/c1-17(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)35(33,34)24(27(31)32)15-16-28-25(29)22-5-3-4-6-23(22)26(28)30/h3-14,17,24H,15-16H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50285676

(CHEMBL86302 | [1-((R)-1-{3-[(E)-2-(2,3-Dichloro-th...)Show SMILES OC(=O)CC1(CS[C@H](CCc2ccccc2)c2cccc(\C=C\c3ccc4sc(Cl)c(Cl)c4n3)c2)CC1 Show InChI InChI=1S/C30H27Cl2NO2S2/c31-27-28-25(37-29(27)32)14-12-23(33-28)11-9-21-7-4-8-22(17-21)24(13-10-20-5-2-1-3-6-20)36-19-30(15-16-30)18-26(34)35/h1-9,11-12,14,17,24H,10,13,15-16,18-19H2,(H,34,35)/b11-9+/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [ H]-LTD4 to DMSO differentiated U937 cell membranes |
Bioorg Med Chem Lett 5: 2551-2556 (1995)
Article DOI: 10.1016/0960-894X(95)00448-3
BindingDB Entry DOI: 10.7270/Q2W37W8Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50374494

(CHEMBL271736)Show SMILES CN1CCN(CCN(c2cccn(O)c2=O)S(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C24H27ClN4O5S/c1-26-13-15-27(16-14-26)17-18-29(23-3-2-12-28(31)24(23)30)35(32,33)22-10-8-21(9-11-22)34-20-6-4-19(25)5-7-20/h2-12,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 409-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.045
BindingDB Entry DOI: 10.7270/Q2833SWF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
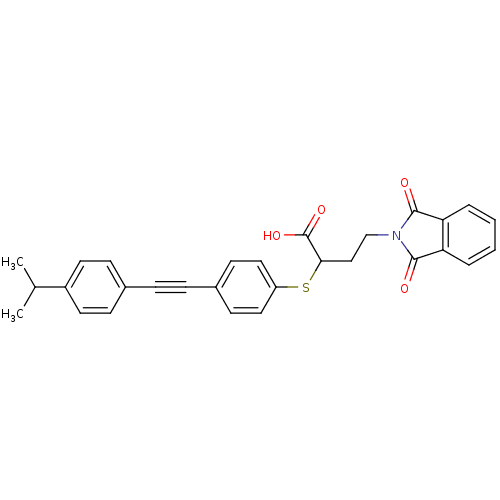
(Homo sapiens (Human)) | BDBM50185892
(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(4-is...)Show SMILES CC(C)c1ccc(cc1)C#Cc1ccc(SC(CCN2C(=O)c3ccccc3C2=O)C(O)=O)cc1 Show InChI InChI=1S/C29H25NO4S/c1-19(2)22-13-9-20(10-14-22)7-8-21-11-15-23(16-12-21)35-26(29(33)34)17-18-30-27(31)24-5-3-4-6-25(24)28(30)32/h3-6,9-16,19,26H,17-18H2,1-2H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50185871

(4-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-2-[4-(5-fl...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccc(cc1)-c1nc2ccc(F)cc2[nH]1 Show InChI InChI=1S/C25H18FN3O6S/c26-15-7-10-19-20(13-15)28-22(27-19)14-5-8-16(9-6-14)36(34,35)21(25(32)33)11-12-29-23(30)17-3-1-2-4-18(17)24(29)31/h1-10,13,21H,11-12H2,(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 3096-100 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.065
BindingDB Entry DOI: 10.7270/Q2M90886 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data