 Found 758 hits with Last Name = 'chenard' and Initial = 'bl'
Found 758 hits with Last Name = 'chenard' and Initial = 'bl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806
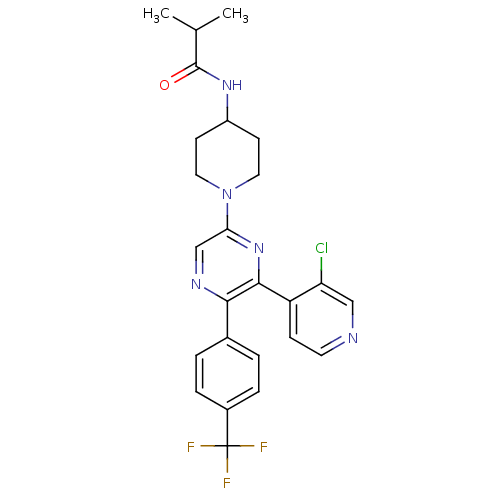
(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202412
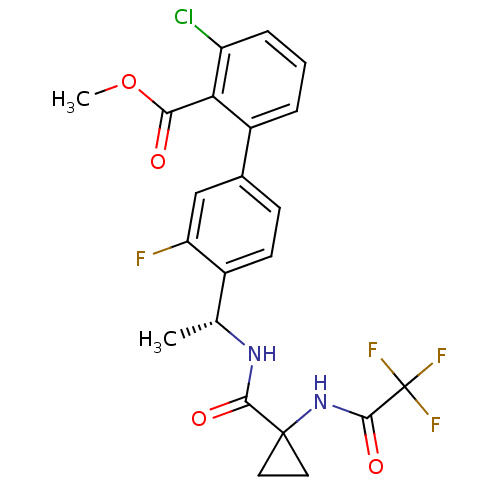
(3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H19ClF4N2O4/c1-11(28-19(31)21(8-9-21)29-20(32)22(25,26)27)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)18(30)33-2/h3-7,10-11H,8-9H2,1-2H3,(H,28,31)(H,29,32)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127438
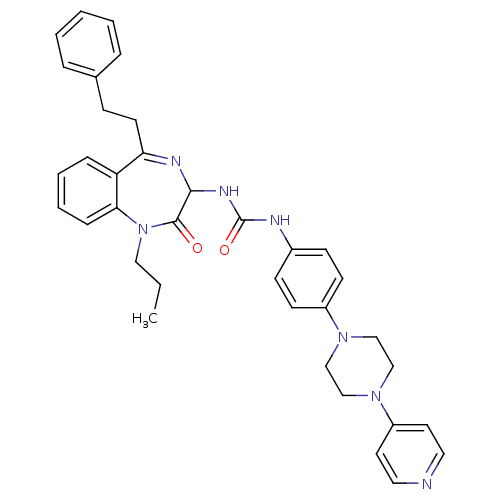
(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260767
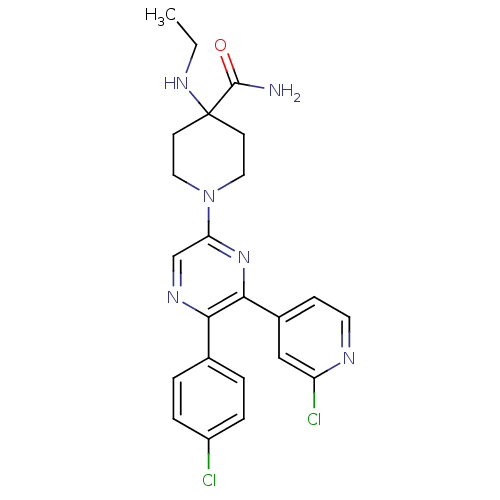
(1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C23H24Cl2N6O/c1-2-29-23(22(26)32)8-11-31(12-9-23)19-14-28-20(15-3-5-17(24)6-4-15)21(30-19)16-7-10-27-18(25)13-16/h3-7,10,13-14,29H,2,8-9,11-12H2,1H3,(H2,26,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260805
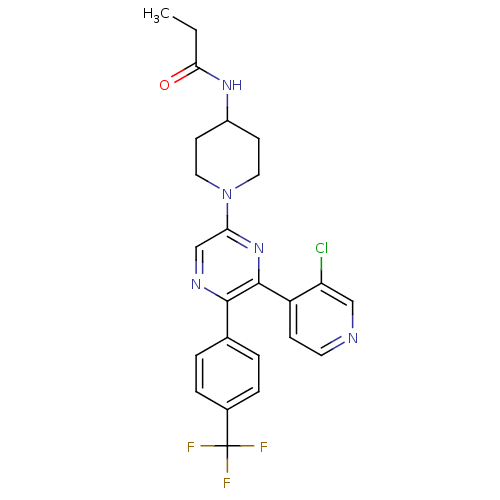
(CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CCC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C24H23ClF3N5O/c1-2-21(34)31-17-8-11-33(12-9-17)20-14-30-22(15-3-5-16(6-4-15)24(26,27)28)23(32-20)18-7-10-29-13-19(18)25/h3-7,10,13-14,17H,2,8-9,11-12H2,1H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260681
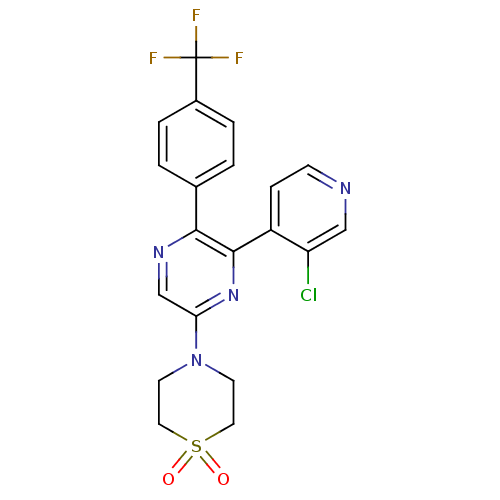
(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260681

(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260768
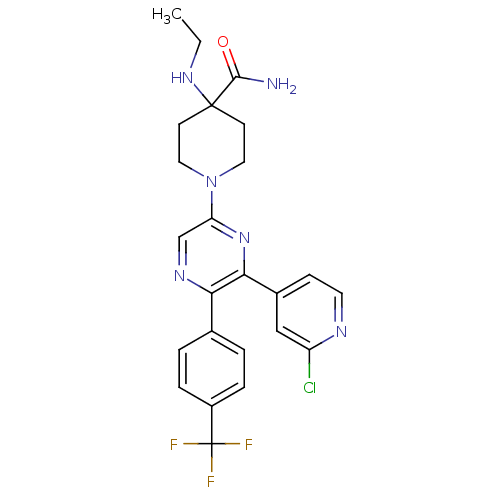
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C24H24ClF3N6O/c1-2-32-23(22(29)35)8-11-34(12-9-23)19-14-31-20(15-3-5-17(6-4-15)24(26,27)28)21(33-19)16-7-10-30-18(25)13-16/h3-7,10,13-14,32H,2,8-9,11-12H2,1H3,(H2,29,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260682
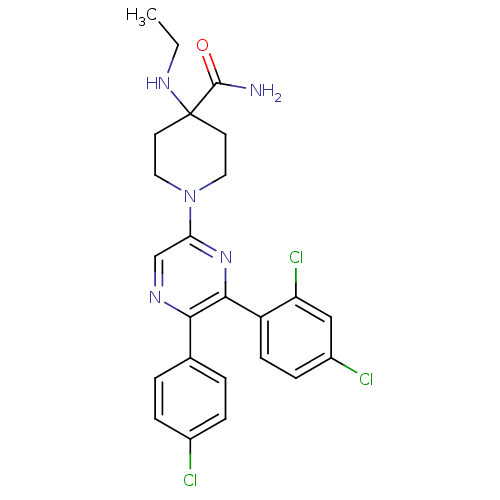
(1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl3N5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-5-16(25)6-4-15)22(31-20)18-8-7-17(26)13-19(18)27/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50127438

(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260803
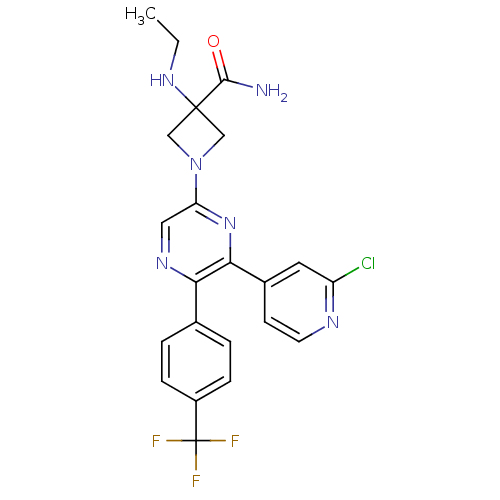
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260718

(1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccccc1Cl)C(N)=O Show InChI InChI=1S/C24H25Cl2N5O/c1-2-29-24(23(27)32)11-13-31(14-12-24)20-15-28-21(16-7-9-17(25)10-8-16)22(30-20)18-5-3-4-6-19(18)26/h3-10,15,29H,2,11-14H2,1H3,(H2,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260717
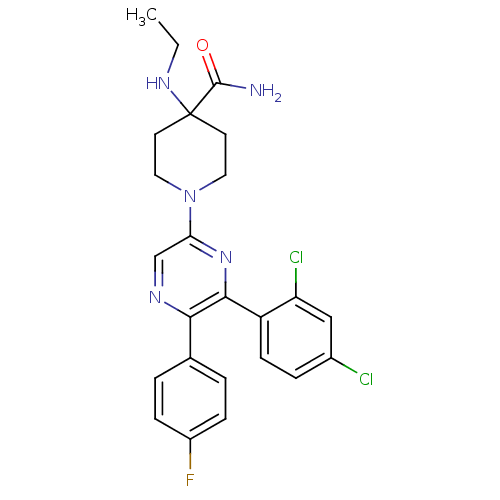
(1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(F)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl2FN5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-6-17(27)7-4-15)22(31-20)18-8-5-16(25)13-19(18)26/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260804
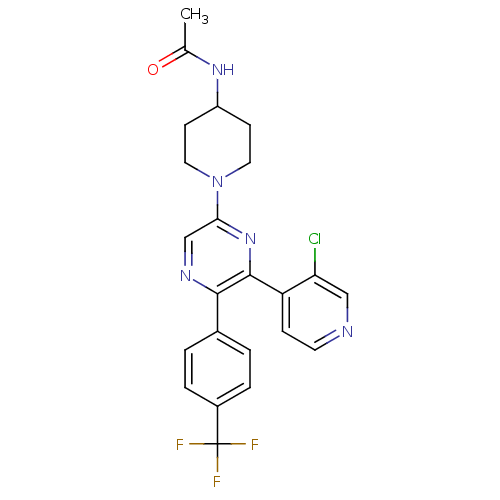
(CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C23H21ClF3N5O/c1-14(33)30-17-7-10-32(11-8-17)20-13-29-21(15-2-4-16(5-3-15)23(25,26)27)22(31-20)18-6-9-28-12-19(18)24/h2-6,9,12-13,17H,7-8,10-11H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260803

(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260720
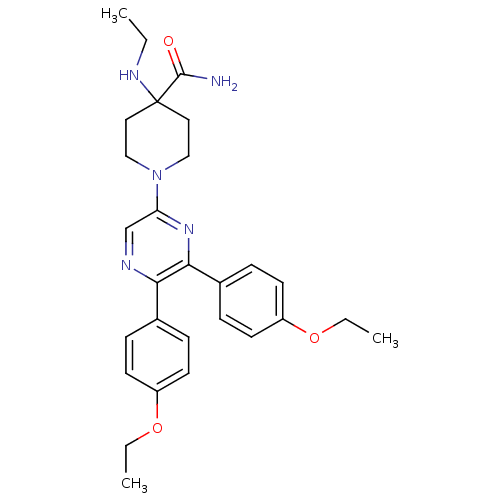
(1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(OCC)cc2)c(n1)-c1ccc(OCC)cc1)C(N)=O Show InChI InChI=1S/C28H35N5O3/c1-4-31-28(27(29)34)15-17-33(18-16-28)24-19-30-25(20-7-11-22(12-8-20)35-5-2)26(32-24)21-9-13-23(14-10-21)36-6-3/h7-14,19,31H,4-6,15-18H2,1-3H3,(H2,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50096326
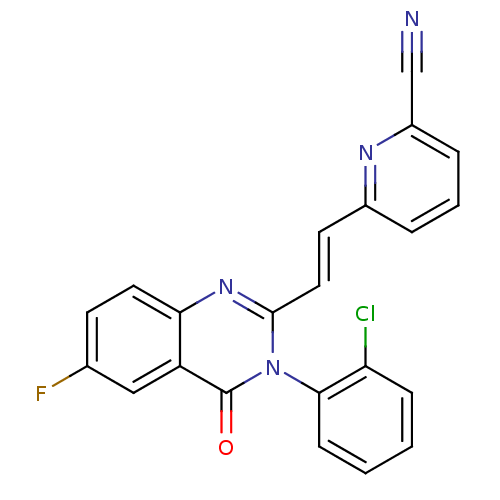
(6-{(E)-2-[3-(2-Chloro-phenyl)-6-fluoro-4-oxo-3,4-d...)Show SMILES Fc1ccc2nc(\C=C\c3cccc(n3)C#N)n(-c3ccccc3Cl)c(=O)c2c1 |(1.2,-4.46,;2.55,-5.21,;2.55,-6.75,;3.88,-7.52,;5.21,-6.75,;6.56,-7.52,;7.91,-6.73,;9.25,-7.5,;9.25,-9.04,;10.6,-9.81,;10.6,-11.35,;11.93,-12.12,;13.26,-11.35,;13.26,-9.78,;11.92,-9.04,;14.58,-8.99,;15.91,-8.22,;7.91,-5.18,;9.25,-4.41,;9.22,-2.88,;10.53,-2.1,;11.88,-2.85,;11.91,-4.39,;10.57,-5.18,;10.57,-6.72,;6.56,-4.41,;6.54,-2.87,;5.21,-5.19,;3.88,-4.42,)| Show InChI InChI=1S/C22H12ClFN4O/c23-18-6-1-2-7-20(18)28-21(11-9-15-4-3-5-16(13-25)26-15)27-19-10-8-14(24)12-17(19)22(28)29/h1-12H/b11-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260766
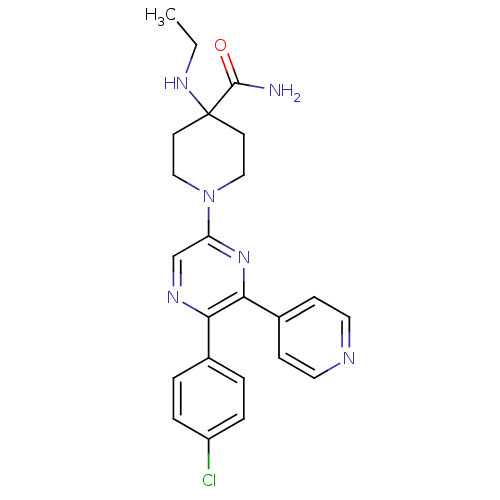
(1-(5-(4-chlorophenyl)-6-(pyridin-4-yl)pyrazin-2-yl...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccncc1)C(N)=O Show InChI InChI=1S/C23H25ClN6O/c1-2-28-23(22(25)31)9-13-30(14-10-23)19-15-27-20(16-3-5-18(24)6-4-16)21(29-19)17-7-11-26-12-8-17/h3-8,11-12,15,28H,2,9-10,13-14H2,1H3,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260719
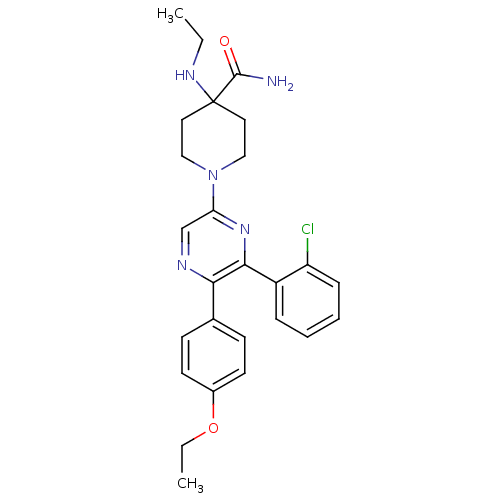
(1-(6-(2-chlorophenyl)-5-(4-ethoxyphenyl)pyrazin-2-...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(OCC)cc2)c(n1)-c1ccccc1Cl)C(N)=O Show InChI InChI=1S/C26H30ClN5O2/c1-3-30-26(25(28)33)13-15-32(16-14-26)22-17-29-23(18-9-11-19(12-10-18)34-4-2)24(31-22)20-7-5-6-8-21(20)27/h5-12,17,30H,3-4,13-16H2,1-2H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50096327
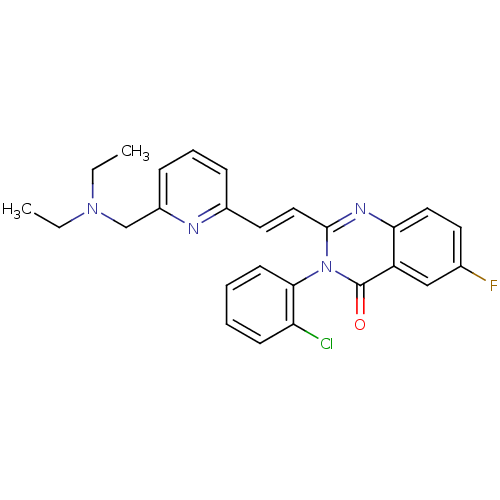
((R)-3-(2-Chloro-phenyl)-2-[(E)-2-(6-diethylaminome...)Show SMILES CCN(CC)Cc1cccc(\C=C\c2nc3ccc(F)cc3c(=O)n2-c2ccccc2Cl)n1 |(13.07,-4.53,;11.73,-3.76,;10.41,-4.56,;10.42,-6.09,;9.1,-6.86,;9.06,-3.81,;7.73,-4.59,;7.75,-6.13,;6.41,-6.9,;5.09,-6.14,;5.09,-4.6,;3.74,-3.86,;3.74,-2.32,;2.4,-1.55,;1.06,-2.32,;-.3,-1.55,;-1.63,-2.32,;-2.95,-1.55,;-2.95,-.01,;-4.31,.76,;-1.63,.76,;-.3,-.01,;1.05,.81,;1.03,2.35,;2.4,.04,;3.72,.81,;3.72,2.32,;5.04,3.12,;6.37,2.35,;6.37,.81,;5.07,.04,;5.06,-1.52,;6.4,-3.82,)| Show InChI InChI=1S/C26H24ClFN4O/c1-3-31(4-2)17-20-9-7-8-19(29-20)13-15-25-30-23-14-12-18(28)16-21(23)26(33)32(25)24-11-6-5-10-22(24)27/h5-16H,3-4,17H2,1-2H3/b15-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260765
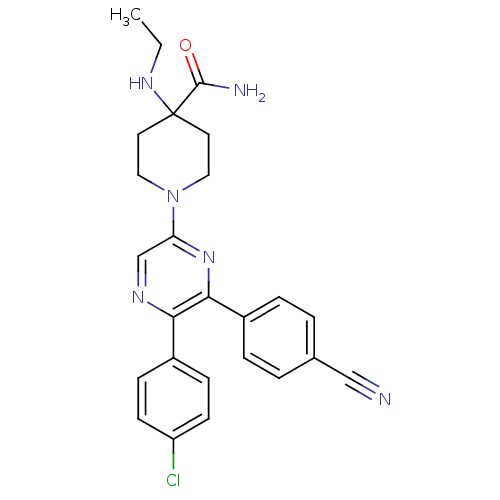
(1-(5-(4-chlorophenyl)-6-(4-cyanophenyl)pyrazin-2-y...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(cc1)C#N)C(N)=O Show InChI InChI=1S/C25H25ClN6O/c1-2-30-25(24(28)33)11-13-32(14-12-25)21-16-29-22(18-7-9-20(26)10-8-18)23(31-21)19-5-3-17(15-27)4-6-19/h3-10,16,30H,2,11-14H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85710
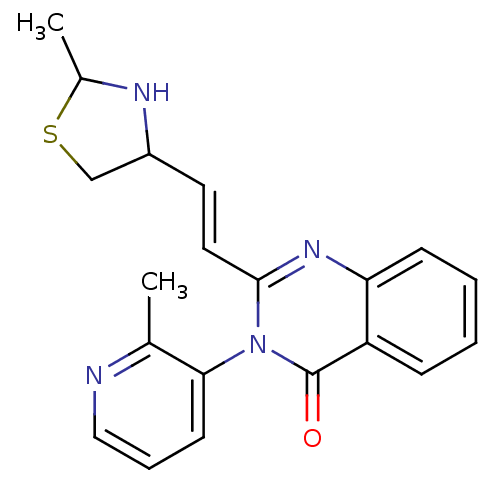
(CP-471236)Show SMILES CC1NC(CS1)\C=C\c1nc2ccccc2c(=O)n1-c1cccnc1C |(16.26,-2.66,;14.78,-3.06,;13.53,-2.16,;12.28,-3.06,;12.76,-4.52,;14.3,-4.52,;11.08,-2.44,;11.08,-.9,;9.75,-.13,;8.41,-.9,;7.08,-.13,;5.75,-.9,;4.41,-.13,;4.41,1.41,;5.75,2.18,;7.08,1.41,;8.41,2.18,;8.41,3.72,;9.75,1.41,;11.08,2.18,;12.42,1.41,;13.75,2.18,;13.75,3.72,;12.42,4.49,;11.08,3.72,;9.75,4.49,)| Show InChI InChI=1S/C20H20N4OS/c1-13-18(8-5-11-21-13)24-19(10-9-15-12-26-14(2)22-15)23-17-7-4-3-6-16(17)20(24)25/h3-11,14-15,22H,12H2,1-2H3/b10-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50202412

(3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H19ClF4N2O4/c1-11(28-19(31)21(8-9-21)29-20(32)22(25,26)27)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)18(30)33-2/h3-7,10-11H,8-9H2,1-2H3,(H,28,31)(H,29,32)/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260681

(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50048389
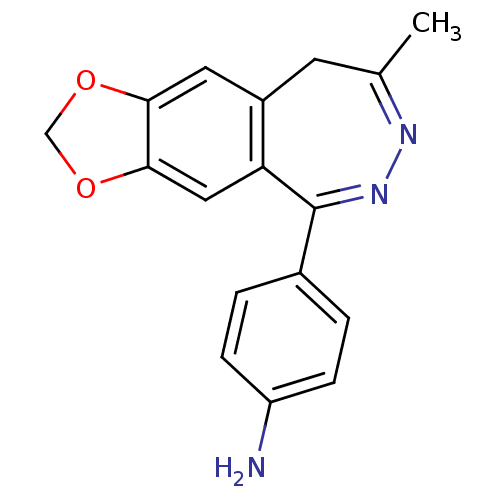
(4-(8-Methyl-9H-1,3-dioxa-6,7-diaza-cyclohepta[f]in...)Show SMILES CC1=NN=C(c2ccc(N)cc2)c2cc3OCOc3cc2C1 |t:1,3| Show InChI InChI=1S/C17H15N3O2/c1-10-6-12-7-15-16(22-9-21-15)8-14(12)17(20-19-10)11-2-4-13(18)5-3-11/h2-5,7-8H,6,9,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85711
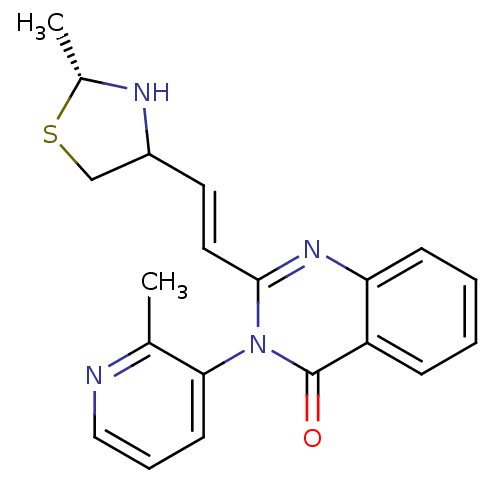
(CP-471237)Show SMILES C[C@@H]1NC(CS1)\C=C\c1nc2ccccc2c(=O)n1-c1cccnc1C |r,wU:1.0,(16.26,-2.66,;14.78,-3.06,;13.53,-2.16,;12.28,-3.06,;12.76,-4.52,;14.3,-4.52,;11.08,-2.44,;11.08,-.9,;9.75,-.13,;8.41,-.9,;7.08,-.13,;5.75,-.9,;4.41,-.13,;4.41,1.41,;5.75,2.18,;7.08,1.41,;8.41,2.18,;8.41,3.72,;9.75,1.41,;11.08,2.18,;12.42,1.41,;13.75,2.18,;13.75,3.72,;12.42,4.49,;11.08,3.72,;9.75,4.49,)| Show InChI InChI=1S/C20H20N4OS/c1-13-18(8-5-11-21-13)24-19(10-9-15-12-26-14(2)22-15)23-17-7-4-3-6-16(17)20(24)25/h3-11,14-15,22H,12H2,1-2H3/b10-9+/t14-,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260803

(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50323836
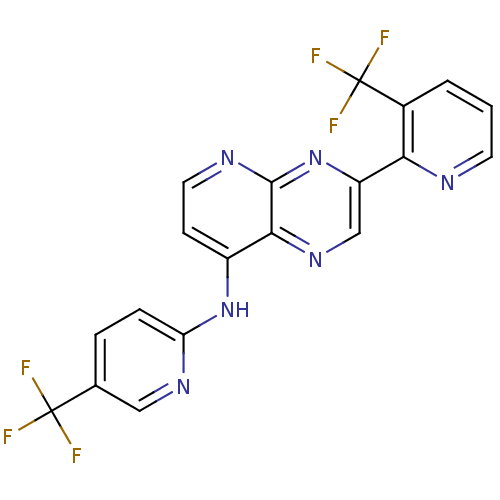
(3-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(cnc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C19H10F6N6/c20-18(21,22)10-3-4-14(28-8-10)30-12-5-7-27-17-16(12)29-9-13(31-17)15-11(19(23,24)25)2-1-6-26-15/h1-9H,(H,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315610
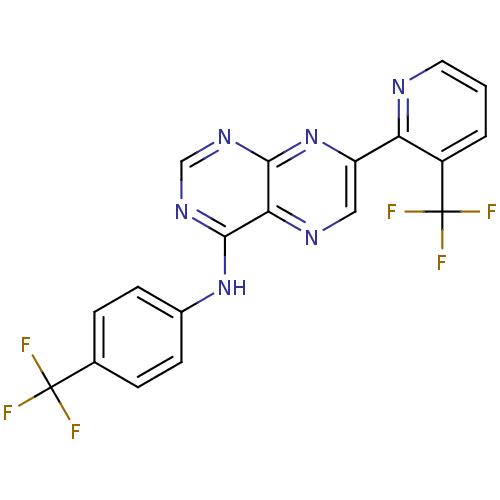
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3nc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C19H10F6N6/c20-18(21,22)10-3-5-11(6-4-10)30-16-15-17(29-9-28-16)31-13(8-27-15)14-12(19(23,24)25)2-1-7-26-14/h1-9H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315616

(3-[3-(Trifluoromethyl)pyridin-2-yl]-N-[5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-5-12(6-4-11)30-14-7-9-28-18-17(14)29-10-15(31-18)16-13(20(24,25)26)2-1-8-27-16/h1-10H,(H,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272889

(2-(isopropoxymethyl)-N-(4-(trifluoromethyl)phenyl)...)Show SMILES CC(C)OCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C25H20F6N4O/c1-14(2)36-13-21-34-20-12-15(22-19(25(29,30)31)4-3-11-32-22)5-10-18(20)23(35-21)33-17-8-6-16(7-9-17)24(26,27)28/h3-12,14H,13H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272976

(2-((2,6-dimethylmorpholino)methyl)-N-(4-(trifluoro...)Show SMILES CC1CN(Cc2nc(Nc3ccc(cc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)CC(C)O1 Show InChI InChI=1S/C28H25F6N5O/c1-16-13-39(14-17(2)40-16)15-24-37-23-12-18(25-22(28(32,33)34)4-3-11-35-25)5-10-21(23)26(38-24)36-20-8-6-19(7-9-20)27(29,30)31/h3-12,16-17H,13-15H2,1-2H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272848
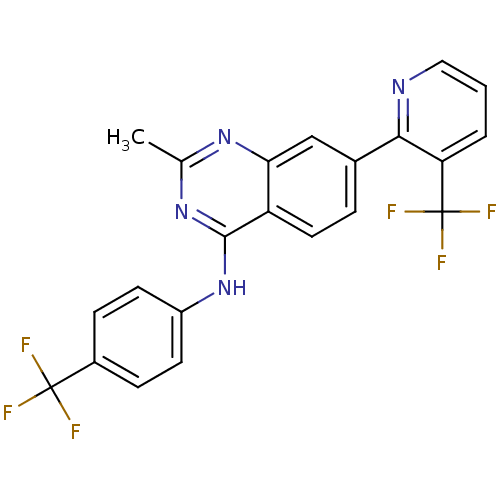
(2-methyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(trifl...)Show SMILES Cc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H14F6N4/c1-12-30-18-11-13(19-17(22(26,27)28)3-2-10-29-19)4-9-16(18)20(31-12)32-15-7-5-14(6-8-15)21(23,24)25/h2-11H,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272890
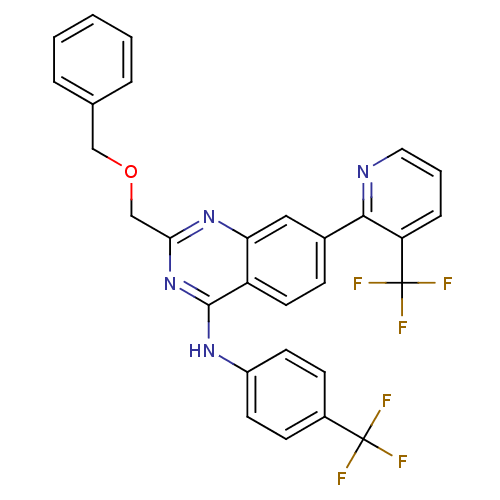
(2-(benzyloxymethyl)-N-(4-(trifluoromethyl)phenyl)-...)Show SMILES FC(F)(F)c1ccc(Nc2nc(COCc3ccccc3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C29H20F6N4O/c30-28(31,32)20-9-11-21(12-10-20)37-27-22-13-8-19(26-23(29(33,34)35)7-4-14-36-26)15-24(22)38-25(39-27)17-40-16-18-5-2-1-3-6-18/h1-15H,16-17H2,(H,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor assessed as inhibition of low pH-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315608
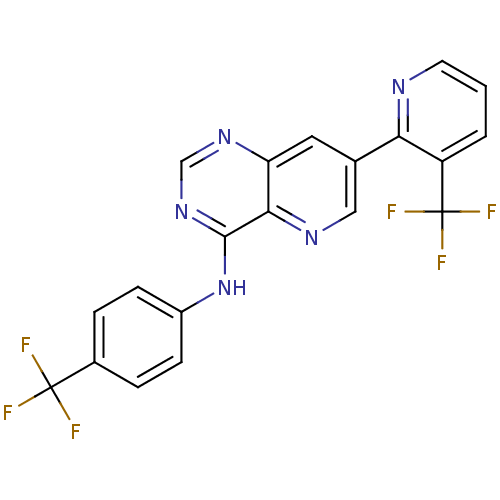
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-3-5-13(6-4-12)31-18-17-15(29-10-30-18)8-11(9-28-17)16-14(20(24,25)26)2-1-7-27-16/h1-10H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50264141
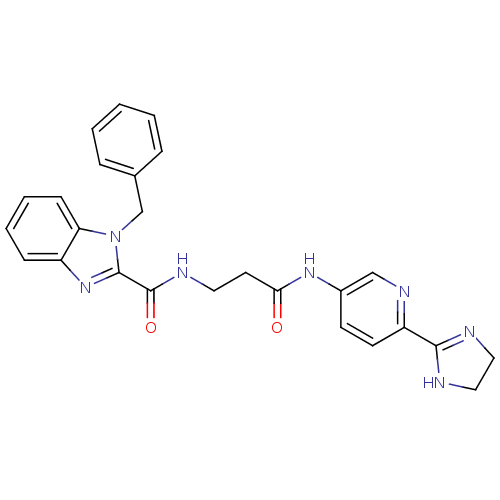
(1-Benzyl-1H-benzoimidazole-2-carboxylic acid {2-[6...)Show SMILES O=C(CCNC(=O)c1nc2ccccc2n1Cc1ccccc1)Nc1ccc(nc1)C1=NCCN1 |t:34| Show InChI InChI=1S/C26H25N7O2/c34-23(31-19-10-11-21(30-16-19)24-27-14-15-28-24)12-13-29-26(35)25-32-20-8-4-5-9-22(20)33(25)17-18-6-2-1-3-7-18/h1-11,16H,12-15,17H2,(H,27,28)(H,29,35)(H,31,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50323839
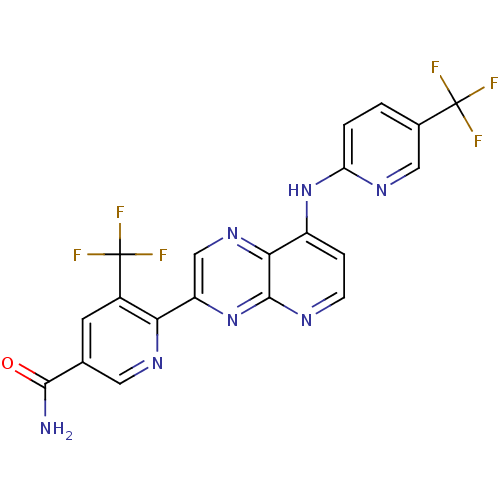
(5-(trifluoromethyl)-6-(8-(5-(trifluoromethyl)pyrid...)Show SMILES NC(=O)c1cnc(-c2cnc3c(Nc4ccc(cn4)C(F)(F)F)ccnc3n2)c(c1)C(F)(F)F Show InChI InChI=1S/C20H11F6N7O/c21-19(22,23)10-1-2-14(29-7-10)32-12-3-4-28-18-16(12)31-8-13(33-18)15-11(20(24,25)26)5-9(6-30-15)17(27)34/h1-8H,(H2,27,34)(H,28,29,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726
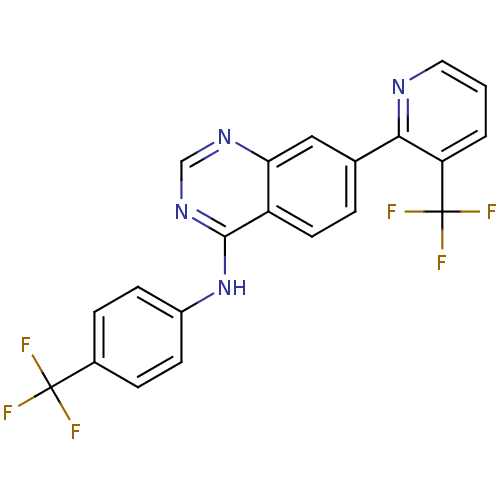
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315609

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3nc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-5-12(6-4-11)30-17-13-7-8-15(31-18(13)29-10-28-17)16-14(20(24,25)26)2-1-9-27-16/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273167
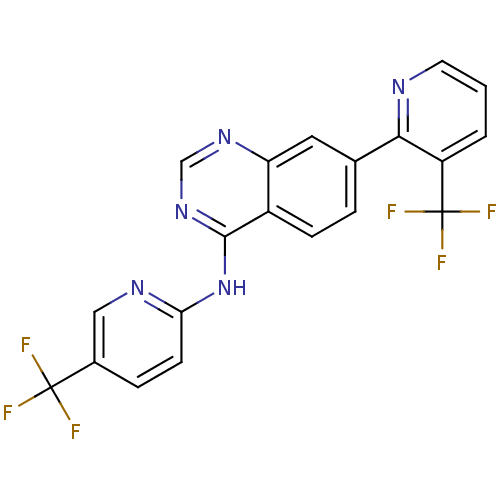
((5-Trifluoromethylpyridin-2-yl)-[7-(3-trifluoromet...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-4-6-16(28-9-12)31-18-13-5-3-11(8-15(13)29-10-30-18)17-14(20(24,25)26)2-1-7-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272851
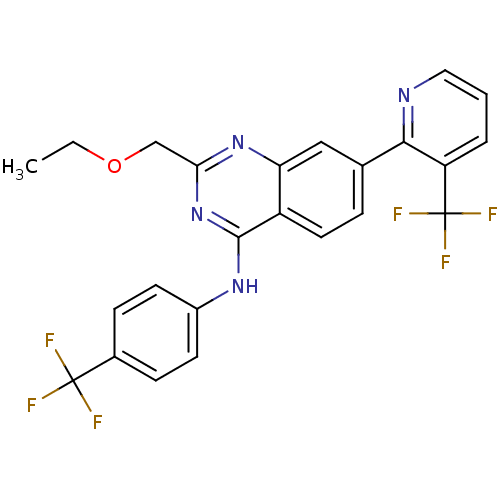
(2-(ethoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-(...)Show SMILES CCOCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H18F6N4O/c1-2-35-13-20-33-19-12-14(21-18(24(28,29)30)4-3-11-31-21)5-10-17(19)22(34-20)32-16-8-6-15(7-9-16)23(25,26)27/h3-12H,2,13H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20557
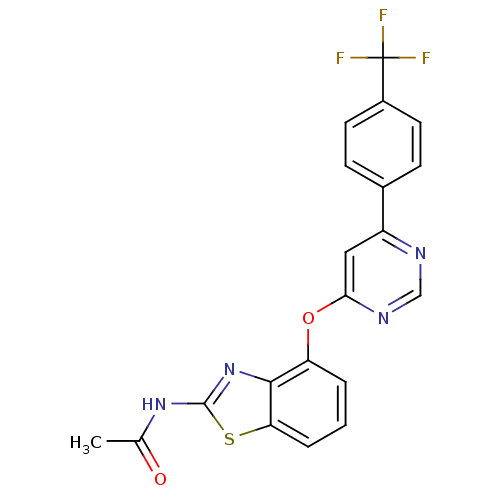
(AMG 517 | CHEMBL229430 | JMC503515 Compound 23 | N...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)-c3ccc(cc3)C(F)(F)F)cccc2s1 Show InChI InChI=1S/C20H13F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-10H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV1 |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM361763
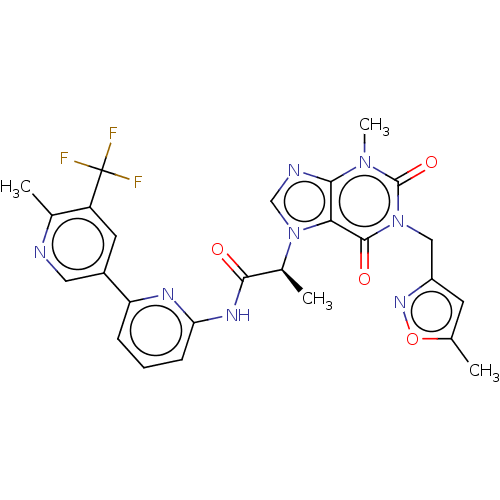
(US10221177, Compound 126)Show SMILES C[C@@H](C(=O)Nc1cccc(n1)-c1cnc(C)c(c1)C(F)(F)F)n1cnc2n(C)c(=O)n(Cc3cc(C)on3)c(=O)c12 |r| Show InChI InChI=1S/C26H23F3N8O4/c1-13-8-17(34-41-13)11-36-24(39)21-22(35(4)25(36)40)31-12-37(21)15(3)23(38)33-20-7-5-6-19(32-20)16-9-18(26(27,28)29)14(2)30-10-16/h5-10,12,15H,11H2,1-4H3,(H,32,33,38)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hydra Biosciences, Inc.
US Patent
| Assay Description
Compounds of Formula (I) inhibit the TRPA1 channel, as shown by measuring the in vitro inhibition of human TRPA1, provided in data tables shown in Ta... |
US Patent US10221177 (2019)
BindingDB Entry DOI: 10.7270/Q2445PR8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data