

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316980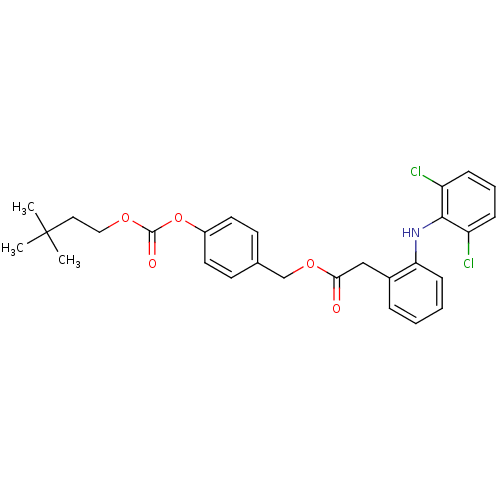 (4-((3,3-Dimethylbutoxy)carbonyloxy)benzyl 2-(2-(2,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316977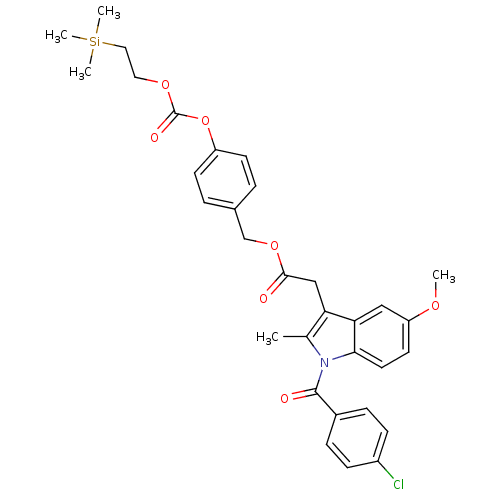 (4-((2-(Trimethylsilyl)ethoxy)carbonyloxy)benzyl 2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316975 ((S)-4-((2-(Trimethylsilyl)ethoxy)carbonyloxy)benzy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316973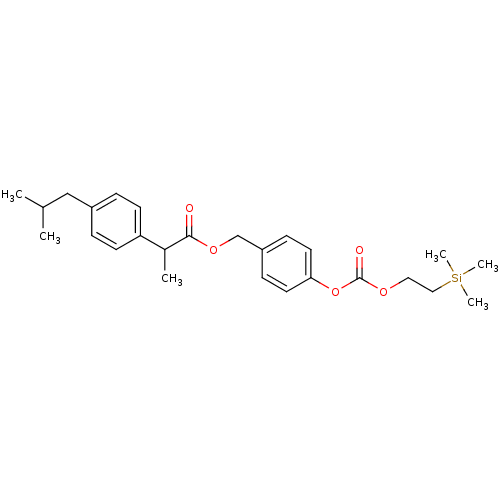 (4-((2-(Trimethylsilyl)ethoxy)carbonyloxy)benzyl 2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258426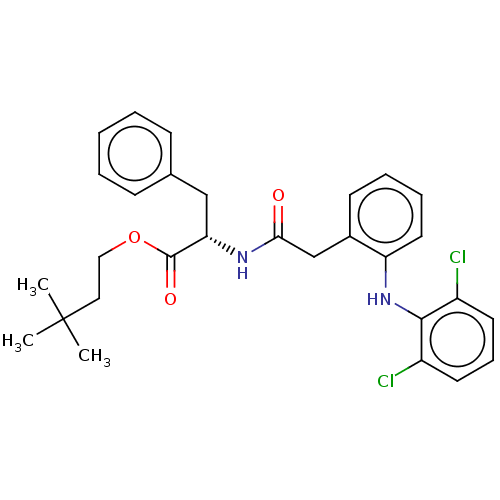 (US9512068, NDH4578) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316979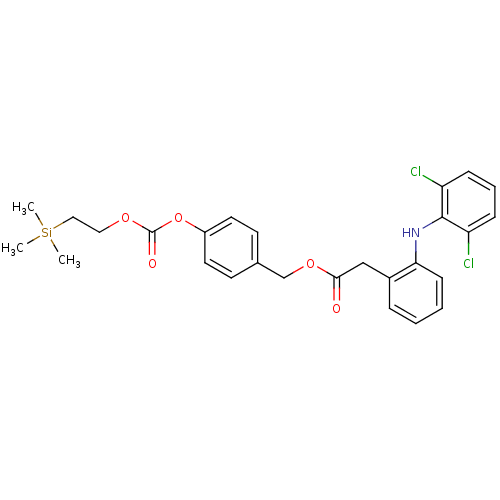 (4-((2-(Trimethylsilyl)ethoxy)carbonyloxy)benzyl 2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316976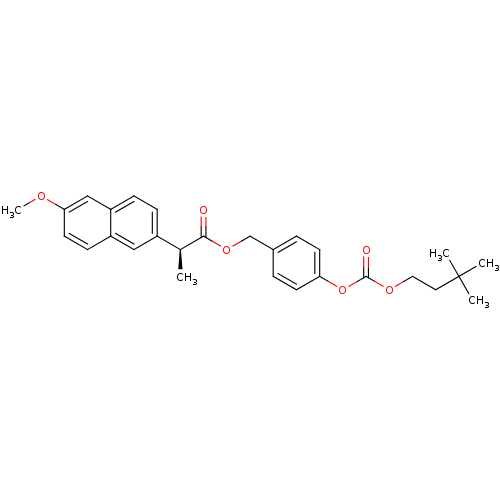 ((S)-4-((3,3-Dimethylbutoxy)carbonyloxy)benzyl 2-(6...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258427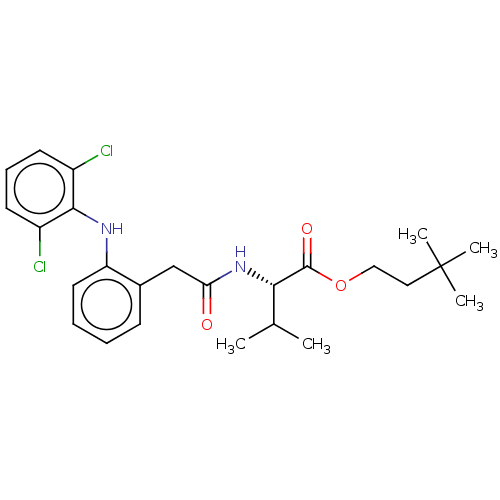 (US9512068, NDH4591) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316974 (4-((3,3-Dimethylbutoxy)carbonyloxy)benzyl 2-(4-Iso...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316978 (4-((3,3-Dimethylbutoxy)carbonyloxy)benzyl 2-(1-(4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258425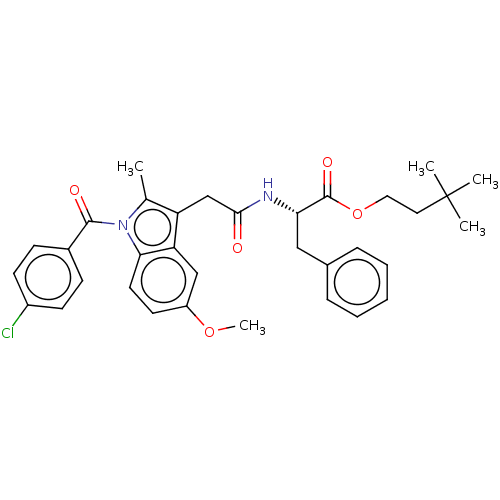 (US9512068, NDH4577) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316990 (2-(Trimethylsilyl)ethyl 2-(2-(2,6-Dichlorophenylam...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316991 (3,3-Dimethylbutyl 2-(2-(2,6-Dichlorophenylamino)ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258422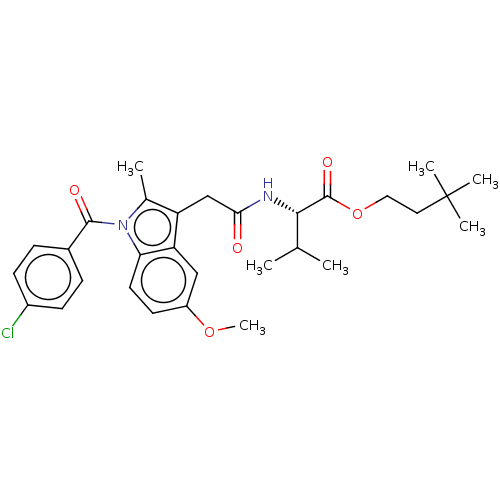 (US9512068, NDH4537) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316986 (2-(Trimethylsilyl)ethyl 2-(1-(4-Chlorobenzoyl)-5-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258424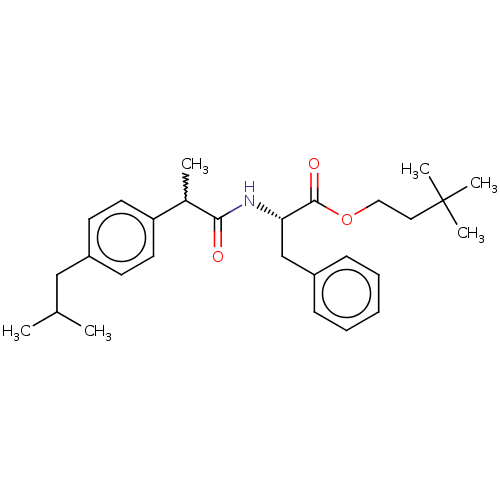 (US9512068, NDH4576) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258423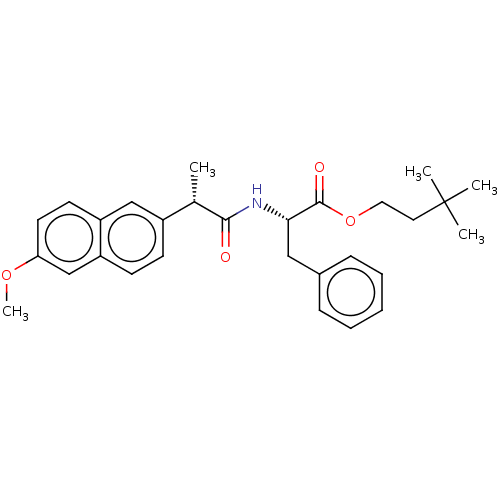 (US9512068, NDH4572) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.77E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258431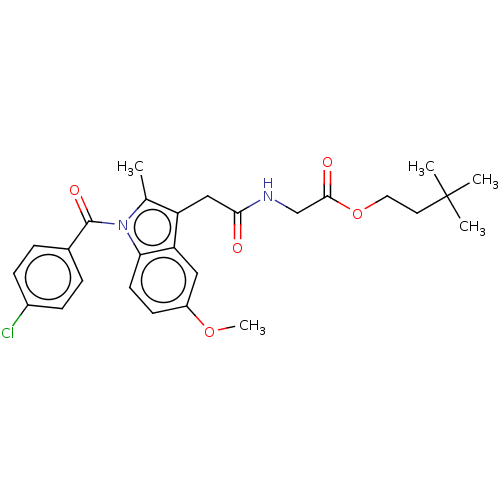 (US9512068, NDH4615) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.63E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258428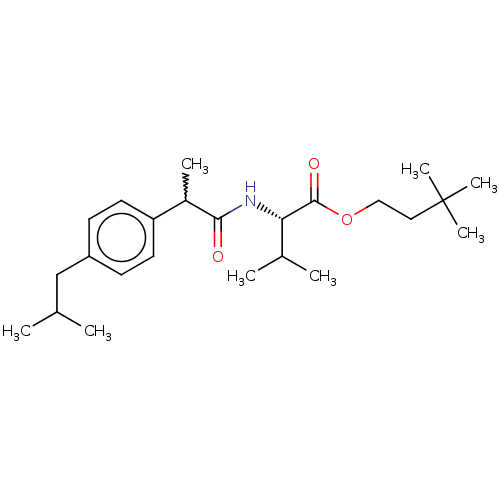 (US9512068, NDH4595) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316987 (3,3-Dimethylbutyl 2-(1-(4-Chlorobenzoyl)-5-methoxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316984 ((S)-2-(Trimethylsilyl)ethyl 2-(6-Methoxynaphthalen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258435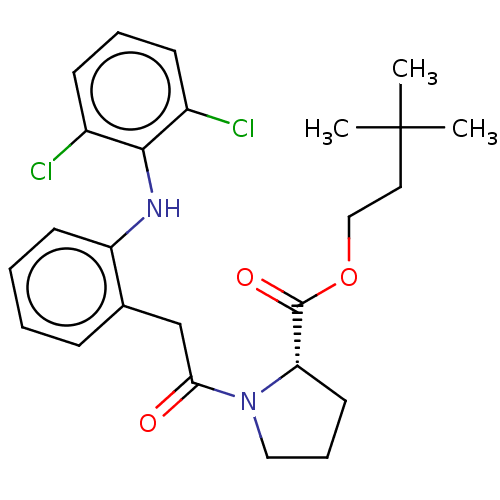 (US9512068, NDH4628) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258429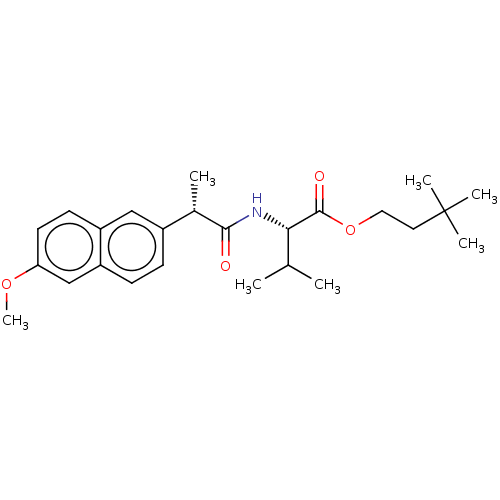 (US9512068, NDH4596) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316985 ((S)-3,3-Dimethylbutyl 2-(6-Methoxynaphthalen-2-yl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316983 (3,3-Dimethylbutyl 2-(4-Isobutylphenyl)propanoate |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258432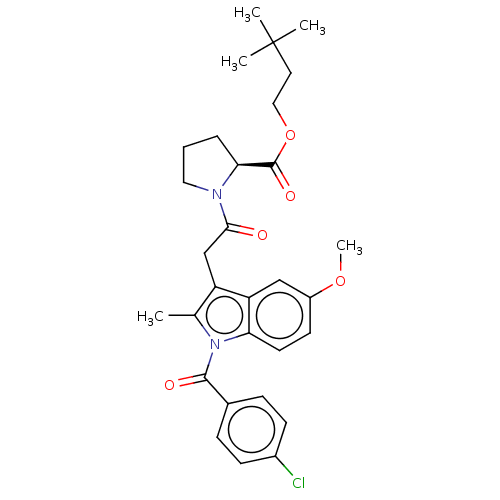 (US9512068, NDH4617) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316982 (2-(Trimethylsilyl)ethyl 2-(4-Isobutylphenyl)propan...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258430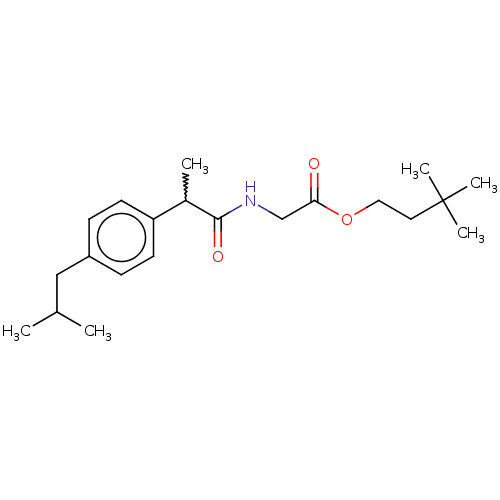 (US9512068, NDH4614) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133608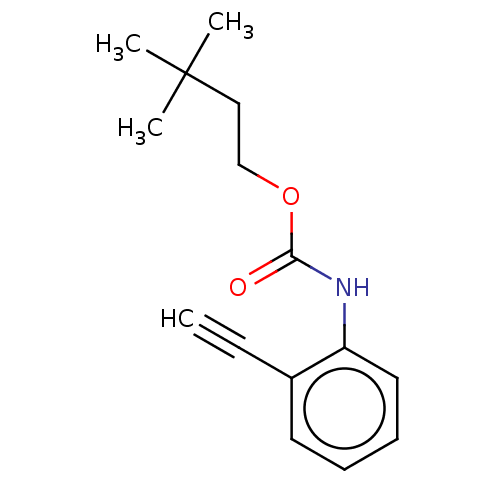 (CHEMBL3633470) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133607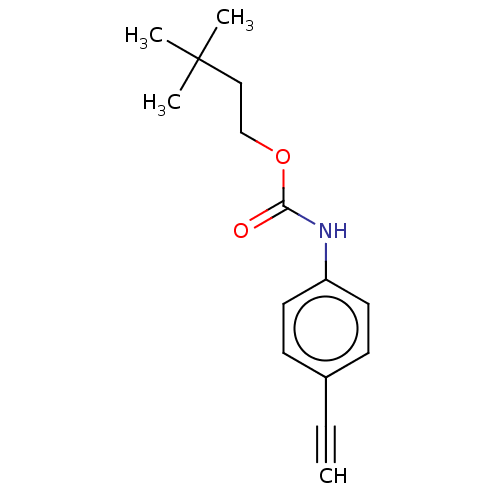 (CHEMBL3633471) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133605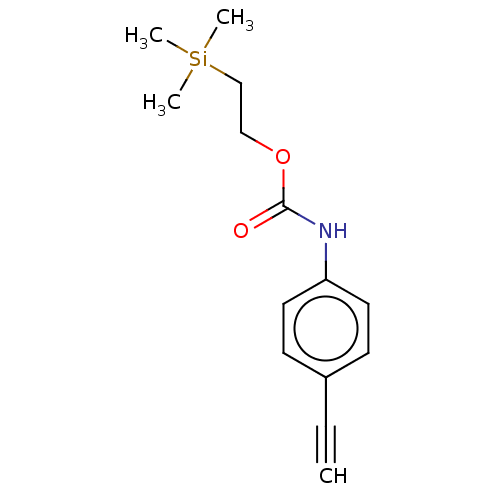 (CHEMBL3633473) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133606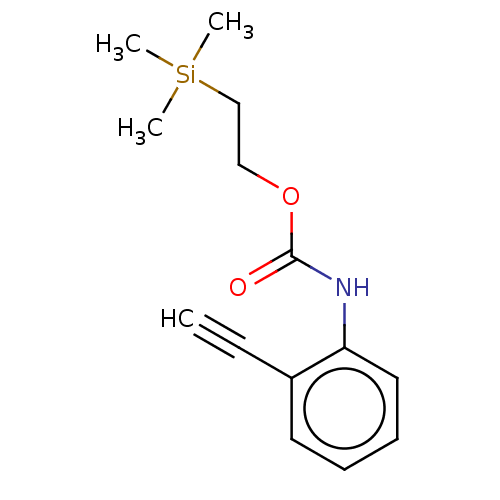 (CHEMBL3633472) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258434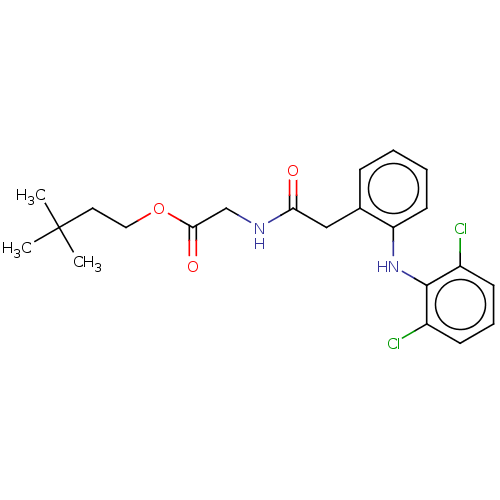 (US9512068, NDH4627) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133611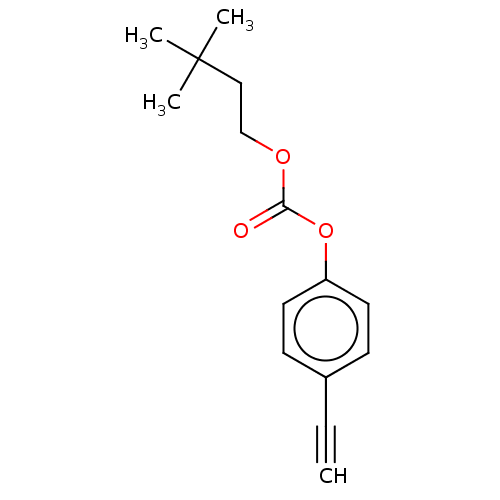 (CHEMBL3633467) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133609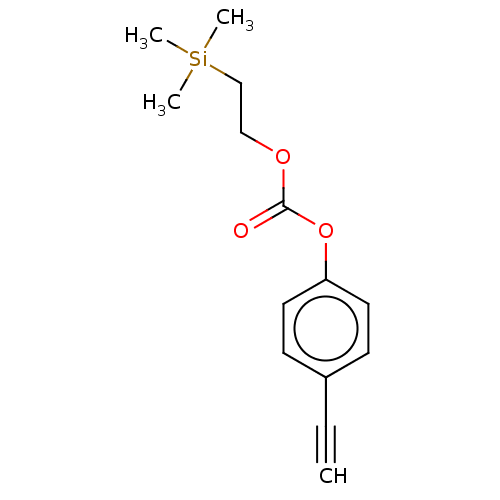 (CHEMBL3633469) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133610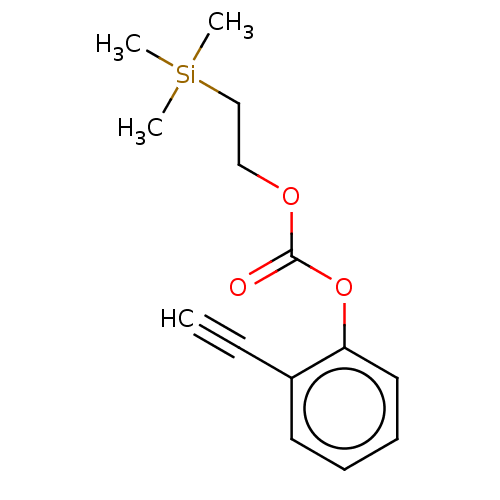 (CHEMBL3633468) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50133612 (CHEMBL3633466) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholineiodide as substrate measured at 30 secs intervals over 9 mins by E... | Bioorg Med Chem Lett 25: 5609-12 (2015) Article DOI: 10.1016/j.bmcl.2015.10.039 BindingDB Entry DOI: 10.7270/Q2NZ89FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM258433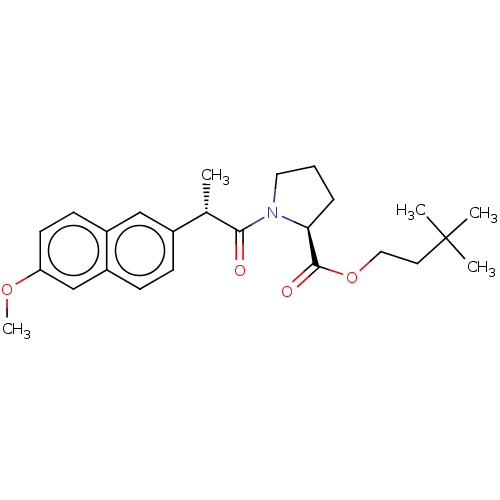 (US9512068, NDH4618) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; LEHIGH UNIVERSITY US Patent | Assay Description The modified Ellman assay for inhibition of acetylcholinesterase (AChE) and the mouse ear vesication assay (MEVA) have been described in detail by us... | US Patent US9512068 (2016) BindingDB Entry DOI: 10.7270/Q2HH6J0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316989 (2-[[2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-i...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316988 ((S)-2-(2-(6-methoxynaphthalen-2-yl)propanoyloxy)-N...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50316981 (CHEMBL1098454 | N,N,N-Trimethyl-2-[2-[4-(2-methylp...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 20: 2987-90 (2010) Article DOI: 10.1016/j.bmcl.2010.02.102 BindingDB Entry DOI: 10.7270/Q2M045MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||