

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203205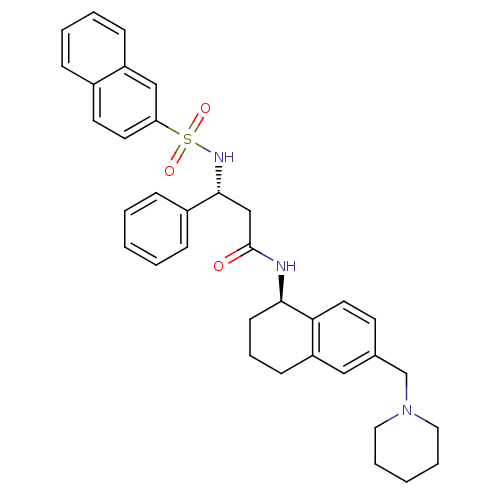 ((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200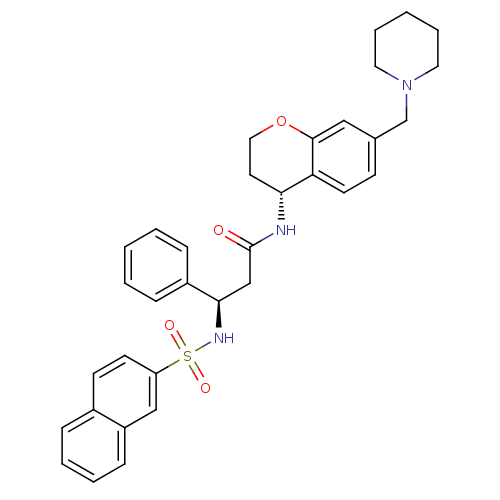 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203199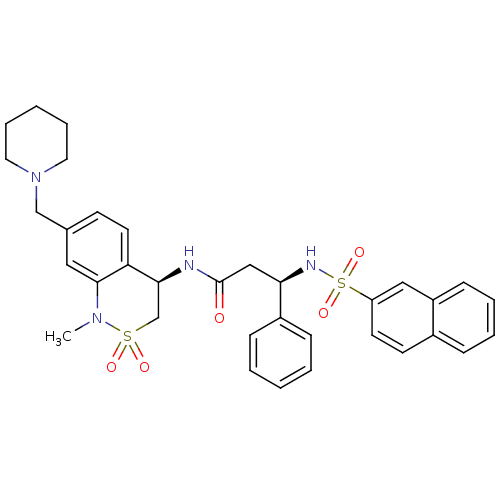 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203211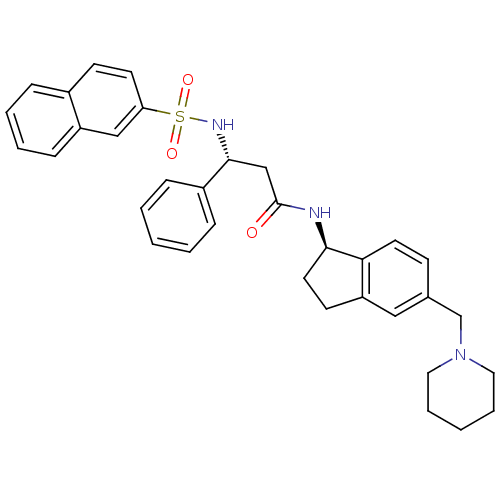 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203210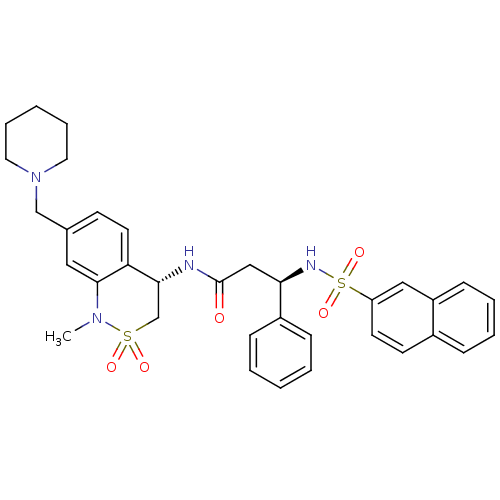 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203206 ((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203197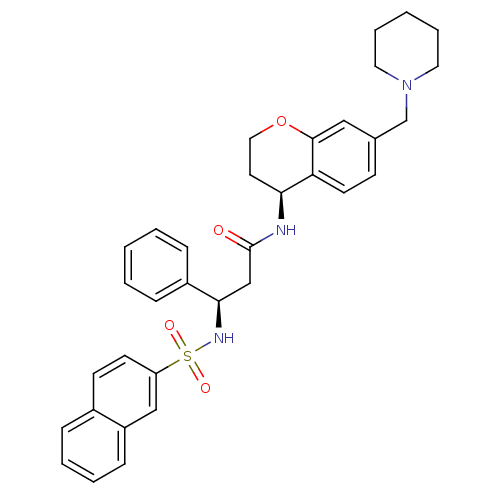 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203208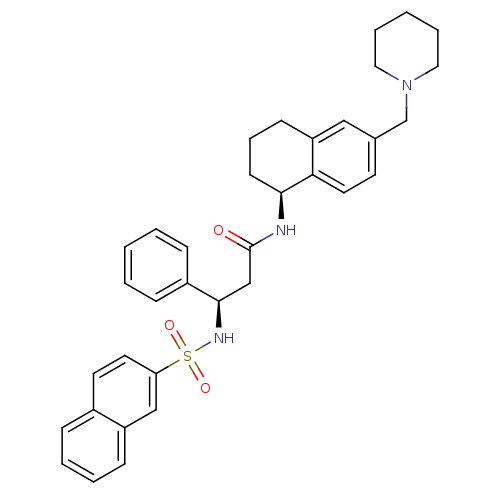 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203202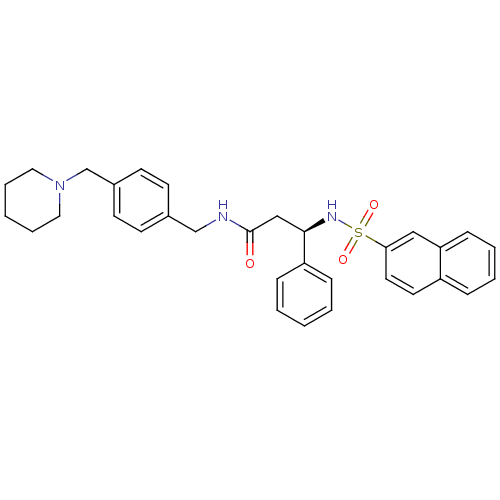 ((R)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(naphthal...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203203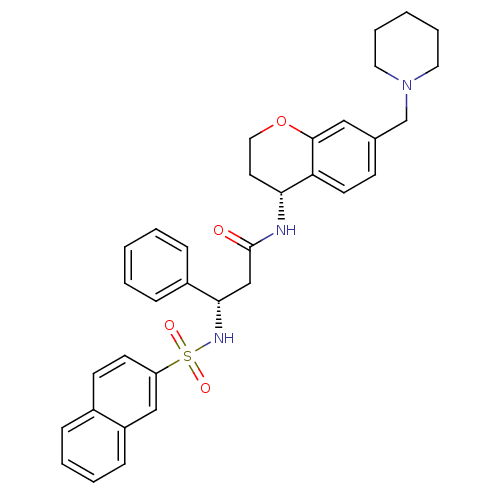 ((S)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203207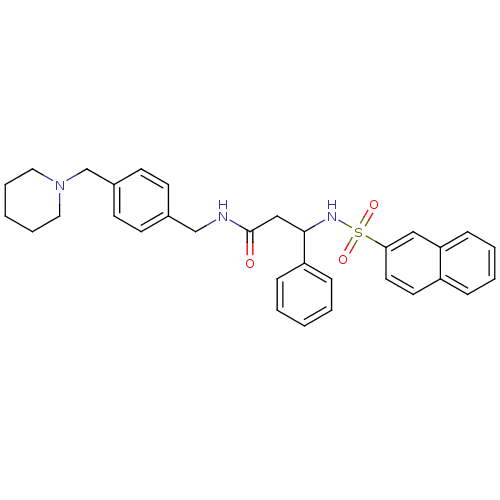 (3-(RS)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(napht...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203204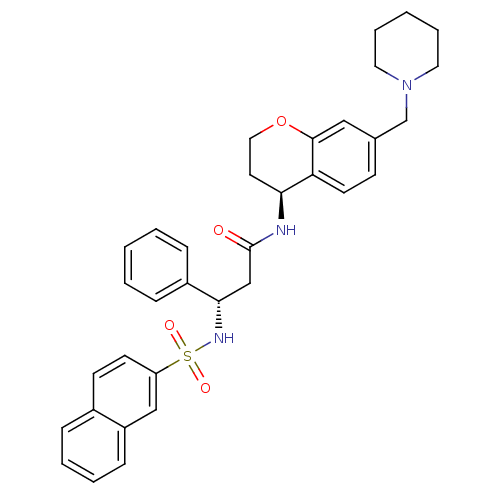 ((S)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203198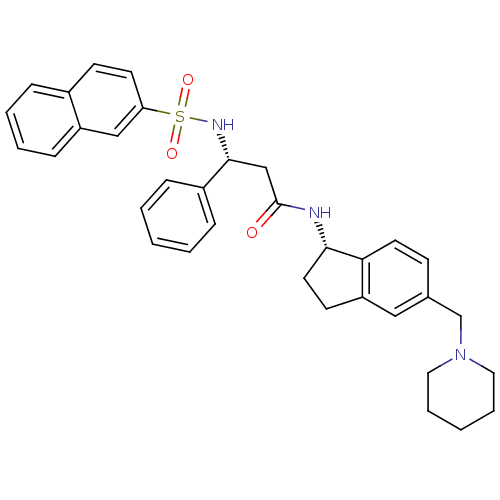 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203208 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203210 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203197 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203199 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203211 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203207 (3-(RS)-N-(4-(piperidin-1-ylmethyl)benzyl)-3-(napht...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50203205 ((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203198 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonistic activity at African green monkey bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378442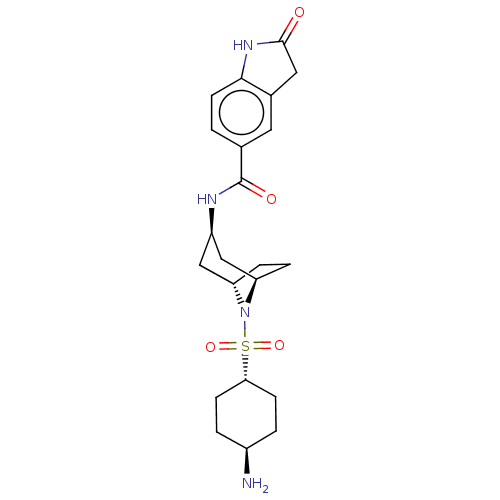 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378443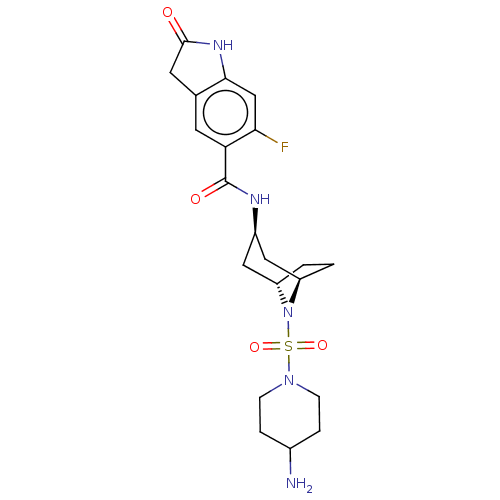 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444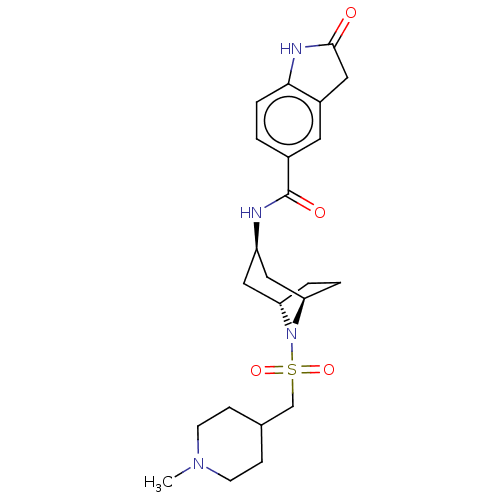 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446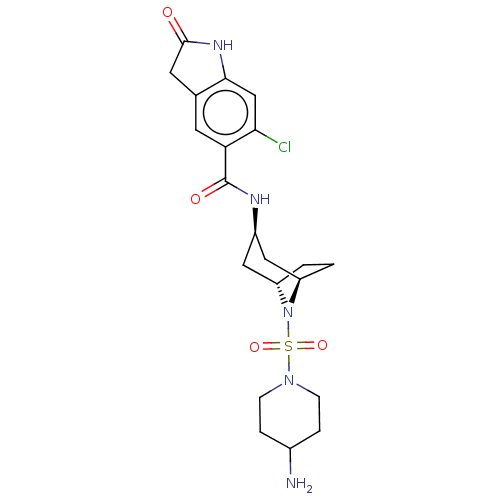 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378447 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378448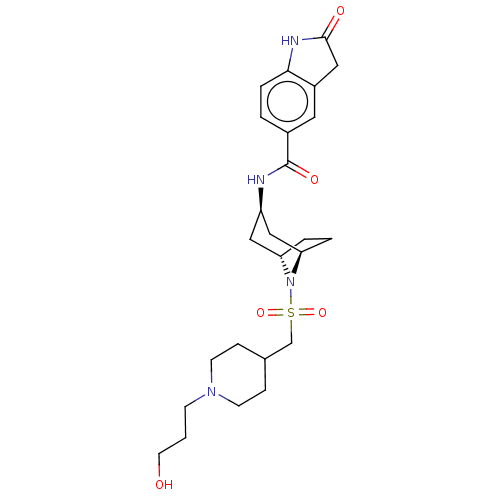 (N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)piperidin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378449 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378450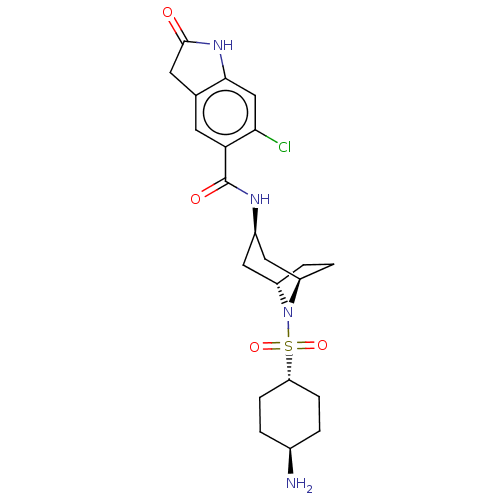 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378746 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378451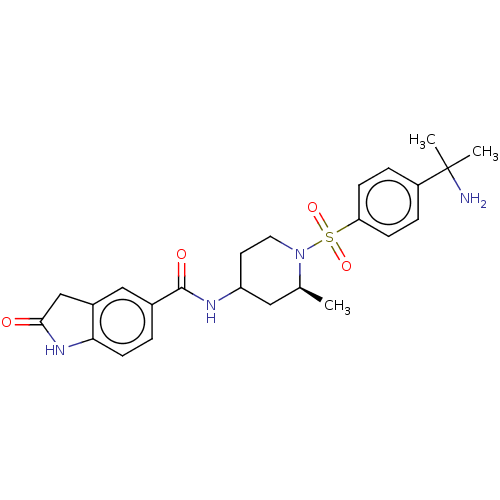 (N-((2S)-1-((4-(2-aminopropan-2-yl)phenyl)sulfonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378452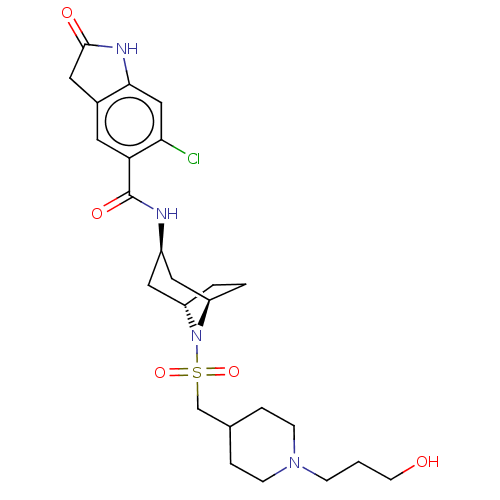 (6-chloro-N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378453 (6-chloro-N-((1R,3r,5S)-8-((4-(methylamino)piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378455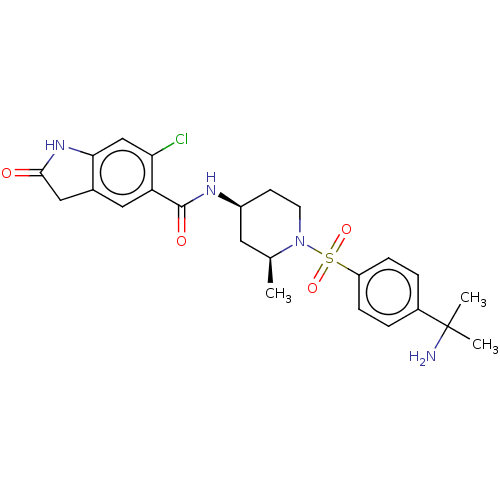 (N-((2S,4S)-1-((4-(2-aminopropan-2-yl)phenyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378454 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378456 (N-((1R,3r,5S)-8-((4-(methylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378457 (2-oxo-N-((1R,3r,5S)-8-((piperidin-3-ylmethyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378458 (N-((1R,3R,5S)-8-(((1s,4S)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378460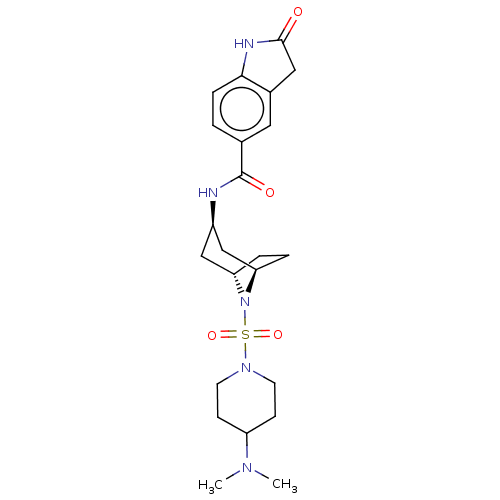 (N-((1R,3r,5S)-8-((4-(dimethylamino)piperidin-1-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.89 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD2 (Homo sapiens (Human)) | BDBM50582891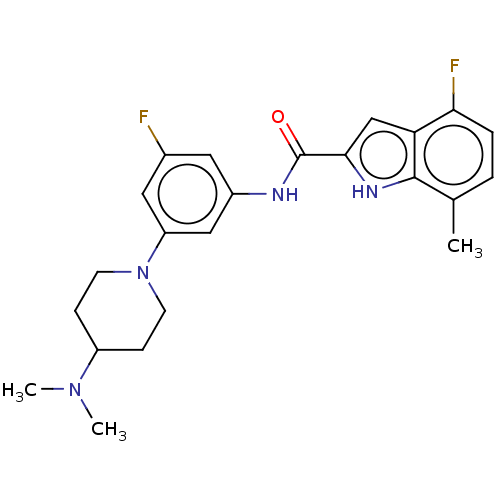 (CHEMBL5078908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SETD2 (unknown origin) preincubated for 30 mins followed by SAM substrate addition measured after 2 hrs by plate reader method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00272 BindingDB Entry DOI: 10.7270/Q2Z03D29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378747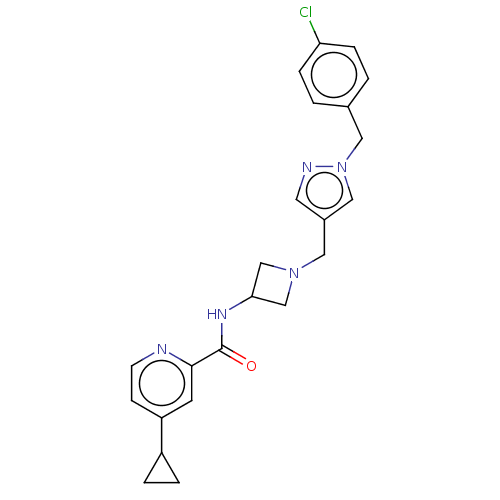 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonistic activity at human bradykinin B1 receptor expressed in CHO cells assessed as effect on DAK-mediated calcium mobilization | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378461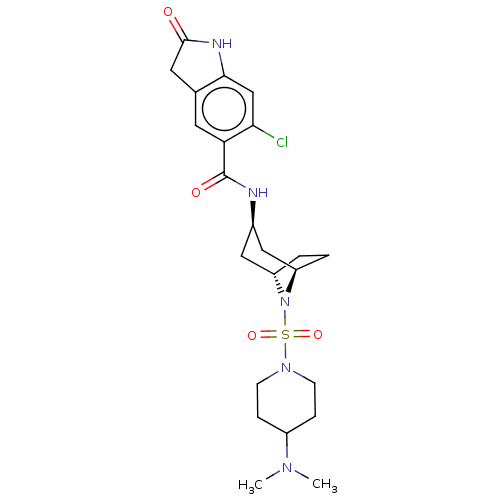 (6-chloro-N-((1R,3r,5S)-8-((4-(dimethylamino)piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.46 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462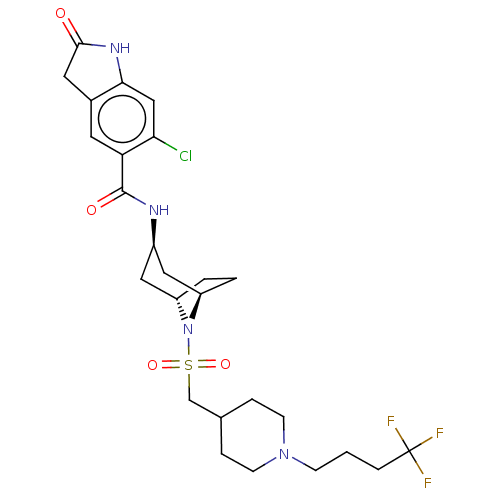 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.54 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378463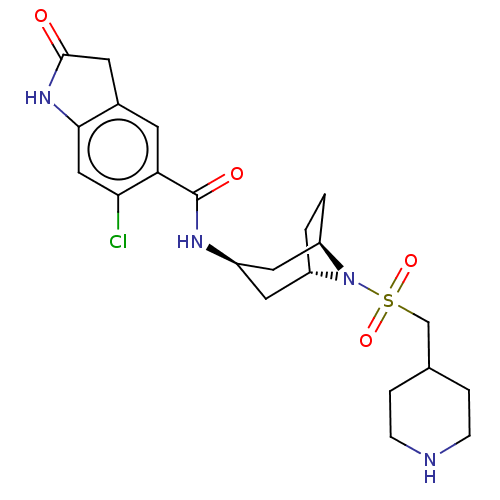 (6-chloro-2-oxo-N-((1R,3r,5S)-8-((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378802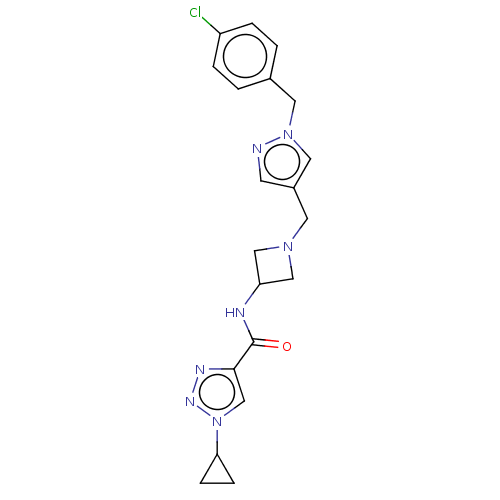 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2920 total ) | Next | Last >> |