 Found 204 hits with Last Name = 'hayler' and Initial = 'j'
Found 204 hits with Last Name = 'hayler' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
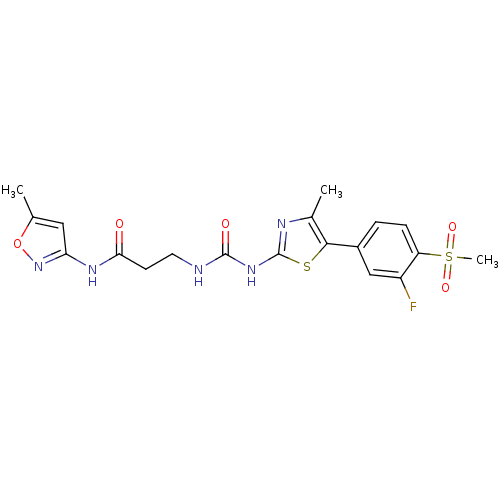
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
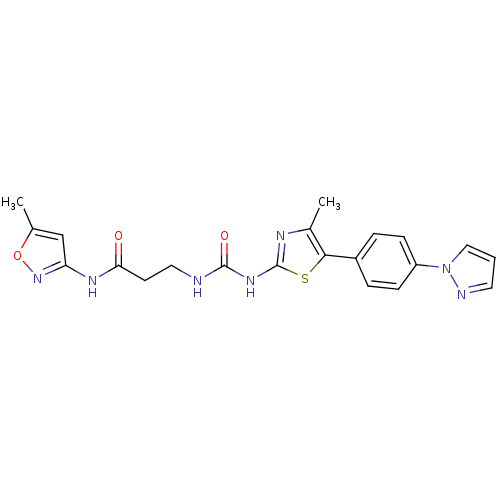
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
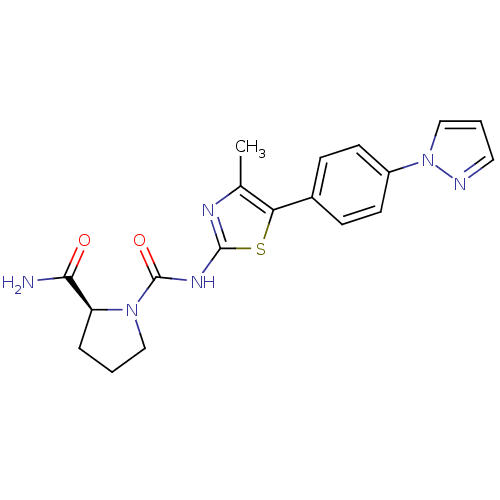
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082611
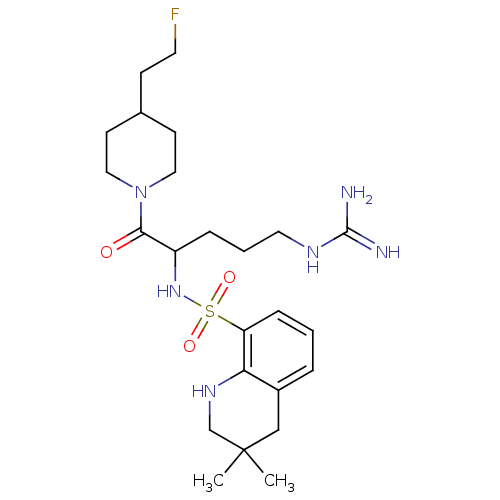
(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C24H39FN6O3S/c1-24(2)15-18-5-3-7-20(21(18)29-16-24)35(33,34)30-19(6-4-12-28-23(26)27)22(32)31-13-9-17(8-11-25)10-14-31/h3,5,7,17,19,29-30H,4,6,8-16H2,1-2H3,(H4,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090249
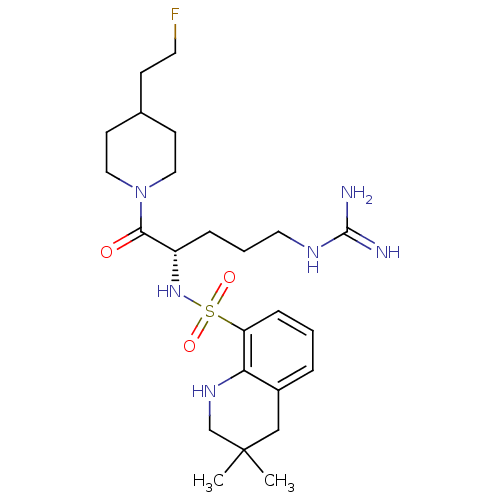
(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C24H39FN6O3S/c1-24(2)15-18-5-3-7-20(21(18)29-16-24)35(33,34)30-19(6-4-12-28-23(26)27)22(32)31-13-9-17(8-11-25)10-14-31/h3,5,7,17,19,29-30H,4,6,8-16H2,1-2H3,(H4,26,27,28)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082575

(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CC(F)F)CC1 Show InChI InChI=1S/C24H38F2N6O3S/c1-24(2)14-17-5-3-7-19(21(17)30-15-24)36(34,35)31-18(6-4-10-29-23(27)28)22(33)32-11-8-16(9-12-32)13-20(25)26/h3,5,7,16,18,20,30-31H,4,6,8-15H2,1-2H3,(H4,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082578
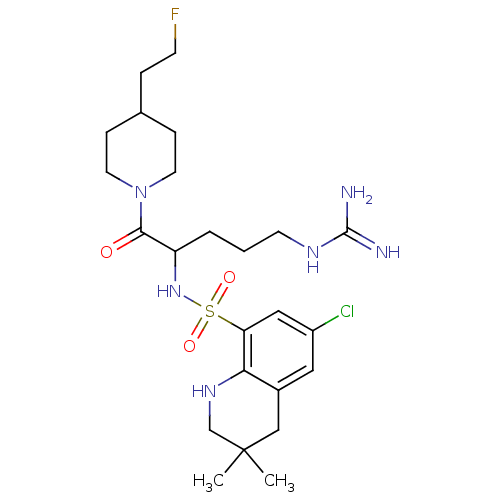
(6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...)Show SMILES CC1(C)CNc2c(C1)cc(Cl)cc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C24H38ClFN6O3S/c1-24(2)14-17-12-18(25)13-20(21(17)30-15-24)36(34,35)31-19(4-3-9-29-23(27)28)22(33)32-10-6-16(5-8-26)7-11-32/h12-13,16,19,30-31H,3-11,14-15H2,1-2H3,(H4,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390426
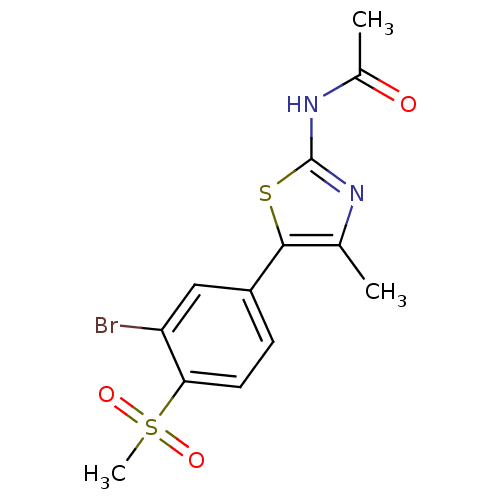
(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50082589
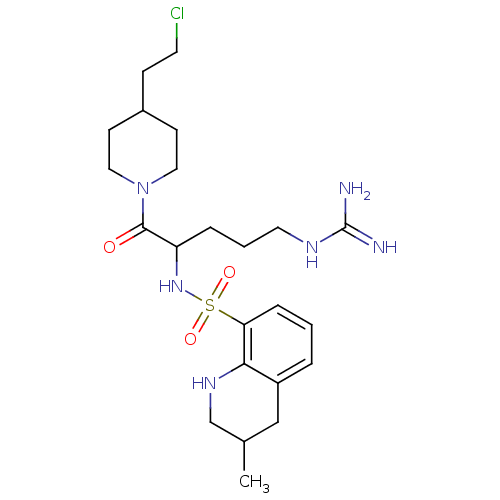
(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCCl)CC1 Show InChI InChI=1S/C23H37ClN6O3S/c1-16-14-18-4-2-6-20(21(18)28-15-16)34(32,33)29-19(5-3-11-27-23(25)26)22(31)30-12-8-17(7-10-24)9-13-30/h2,4,6,16-17,19,28-29H,3,5,7-15H2,1H3,(H4,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibitory activity against human thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082579
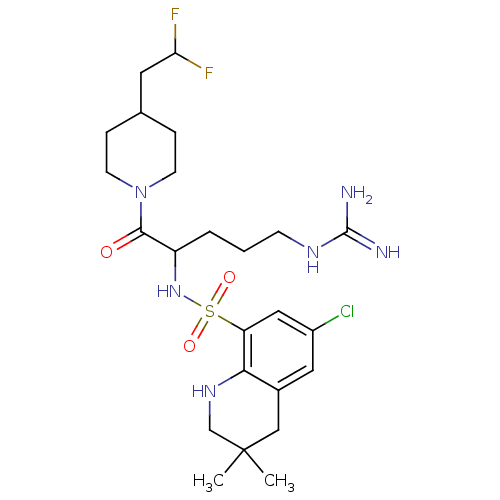
(6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...)Show SMILES CC1(C)CNc2c(C1)cc(Cl)cc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CC(F)F)CC1 Show InChI InChI=1S/C24H37ClF2N6O3S/c1-24(2)13-16-11-17(25)12-19(21(16)31-14-24)37(35,36)32-18(4-3-7-30-23(28)29)22(34)33-8-5-15(6-9-33)10-20(26)27/h11-12,15,18,20,31-32H,3-10,13-14H2,1-2H3,(H4,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082583
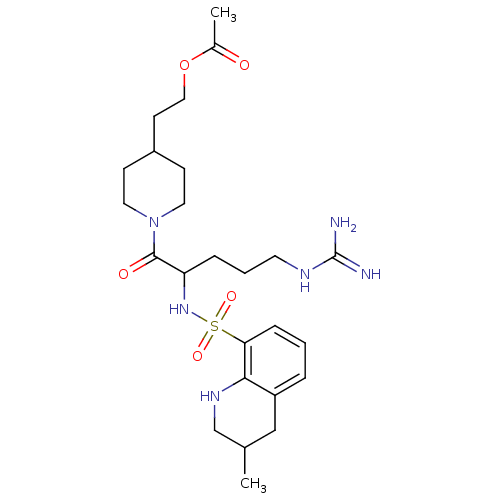
(Acetic acid 2-{1-[5-guanidino-2-(3-methyl-1,2,3,4-...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCOC(C)=O)CC1 Show InChI InChI=1S/C25H40N6O5S/c1-17-15-20-5-3-7-22(23(20)29-16-17)37(34,35)30-21(6-4-11-28-25(26)27)24(33)31-12-8-19(9-13-31)10-14-36-18(2)32/h3,5,7,17,19,21,29-30H,4,6,8-16H2,1-2H3,(H4,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073297
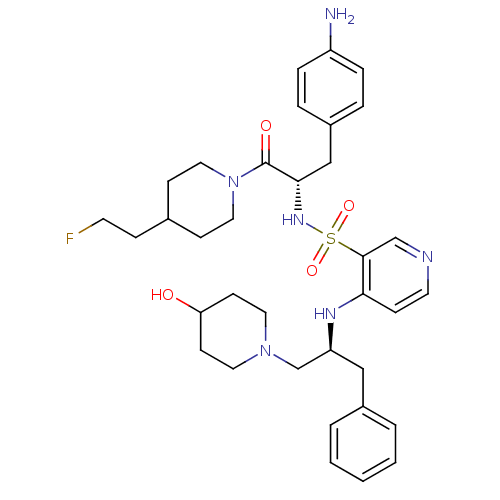
(4-[(S)-1-(4-Hydroxy-piperidin-1-ylmethyl)-2-phenyl...)Show SMILES Nc1ccc(C[C@H](NS(=O)(=O)c2cnccc2N[C@H](CN2CCC(O)CC2)Cc2ccccc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C35H47FN6O4S/c36-16-10-26-12-20-42(21-13-26)35(44)33(23-28-6-8-29(37)9-7-28)40-47(45,46)34-24-38-17-11-32(34)39-30(22-27-4-2-1-3-5-27)25-41-18-14-31(43)15-19-41/h1-9,11,17,24,26,30-31,33,40,43H,10,12-16,18-23,25,37H2,(H,38,39)/t30-,33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073283
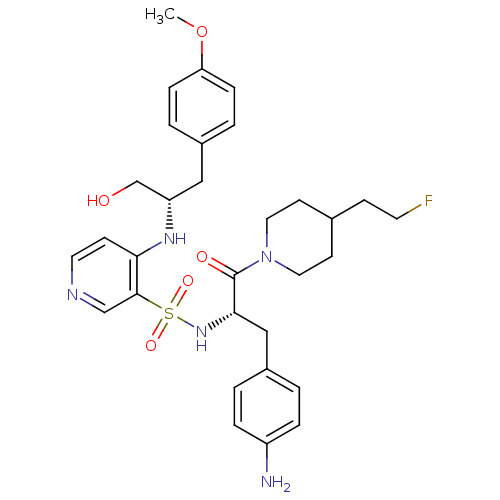
(4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...)Show SMILES COc1ccc(C[C@@H](CO)Nc2ccncc2S(=O)(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C31H40FN5O5S/c1-42-27-8-4-23(5-9-27)18-26(21-38)35-28-11-15-34-20-30(28)43(40,41)36-29(19-24-2-6-25(33)7-3-24)31(39)37-16-12-22(10-14-32)13-17-37/h2-9,11,15,20,22,26,29,36,38H,10,12-14,16-19,21,33H2,1H3,(H,34,35)/t26-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073283

(4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...)Show SMILES COc1ccc(C[C@@H](CO)Nc2ccncc2S(=O)(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C31H40FN5O5S/c1-42-27-8-4-23(5-9-27)18-26(21-38)35-28-11-15-34-20-30(28)43(40,41)36-29(19-24-2-6-25(33)7-3-24)31(39)37-16-12-22(10-14-32)13-17-37/h2-9,11,15,20,22,26,29,36,38H,10,12-14,16-19,21,33H2,1H3,(H,34,35)/t26-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390419
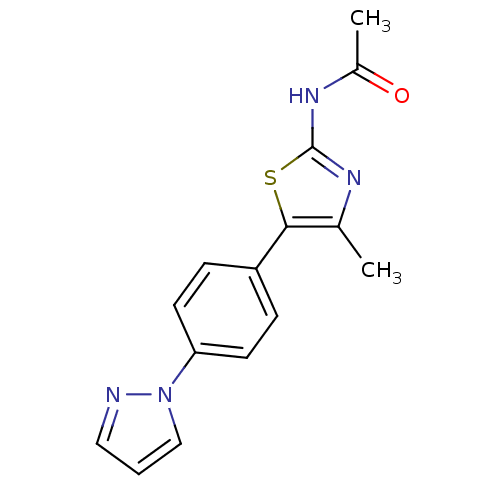
(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082589

(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCCl)CC1 Show InChI InChI=1S/C23H37ClN6O3S/c1-16-14-18-4-2-6-20(21(18)28-15-16)34(32,33)29-19(5-3-11-27-23(25)26)22(31)30-12-8-17(7-10-24)9-13-30/h2,4,6,16-17,19,28-29H,3,5,7-15H2,1H3,(H4,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390423
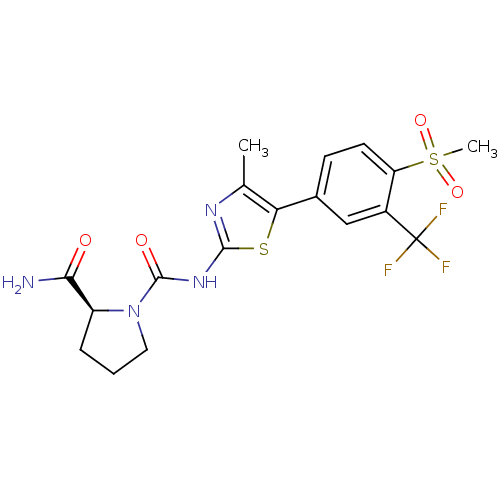
(CHEMBL2071337)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C18H19F3N4O4S2/c1-9-14(10-5-6-13(31(2,28)29)11(8-10)18(19,20)21)30-16(23-9)24-17(27)25-7-3-4-12(25)15(22)26/h5-6,8,12H,3-4,7H2,1-2H3,(H2,22,26)(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390425
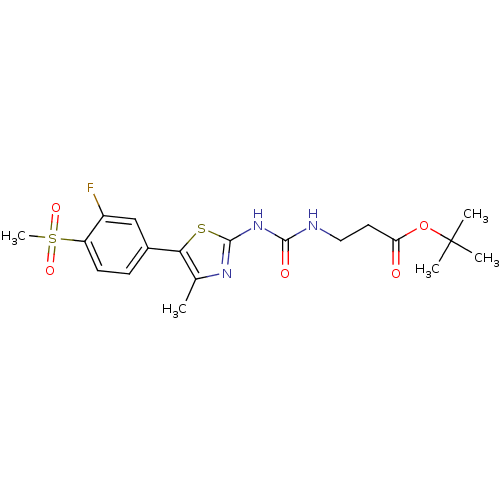
(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073298
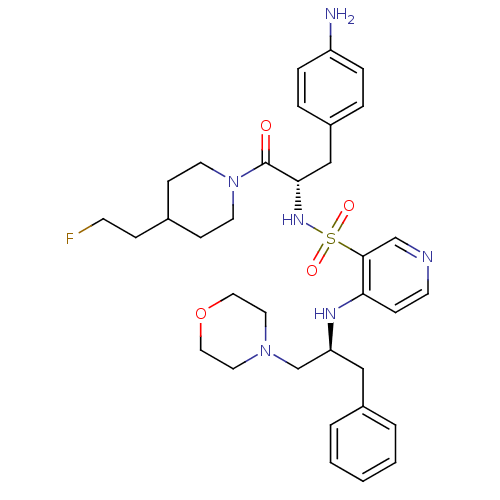
(4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...)Show SMILES Nc1ccc(C[C@H](NS(=O)(=O)c2cnccc2N[C@H](CN2CCOCC2)Cc2ccccc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C34H45FN6O4S/c35-14-10-26-12-16-41(17-13-26)34(42)32(23-28-6-8-29(36)9-7-28)39-46(43,44)33-24-37-15-11-31(33)38-30(22-27-4-2-1-3-5-27)25-40-18-20-45-21-19-40/h1-9,11,15,24,26,30,32,39H,10,12-14,16-23,25,36H2,(H,37,38)/t30-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073298

(4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...)Show SMILES Nc1ccc(C[C@H](NS(=O)(=O)c2cnccc2N[C@H](CN2CCOCC2)Cc2ccccc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C34H45FN6O4S/c35-14-10-26-12-16-41(17-13-26)34(42)32(23-28-6-8-29(36)9-7-28)39-46(43,44)33-24-37-15-11-31(33)38-30(22-27-4-2-1-3-5-27)25-40-18-20-45-21-19-40/h1-9,11,15,24,26,30,32,39H,10,12-14,16-23,25,36H2,(H,37,38)/t30-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082598
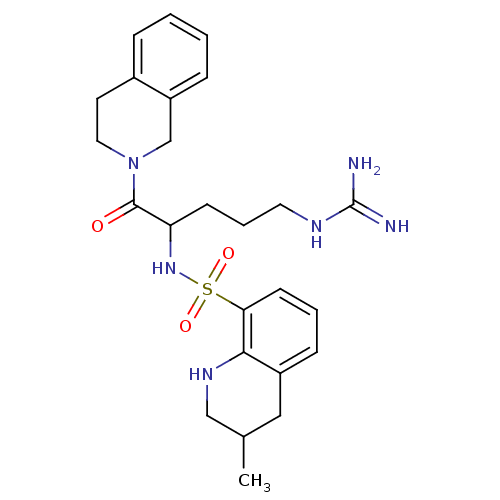
(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCc2ccccc2C1 Show InChI InChI=1S/C25H34N6O3S/c1-17-14-19-8-4-10-22(23(19)29-15-17)35(33,34)30-21(9-5-12-28-25(26)27)24(32)31-13-11-18-6-2-3-7-20(18)16-31/h2-4,6-8,10,17,21,29-30H,5,9,11-16H2,1H3,(H4,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082577
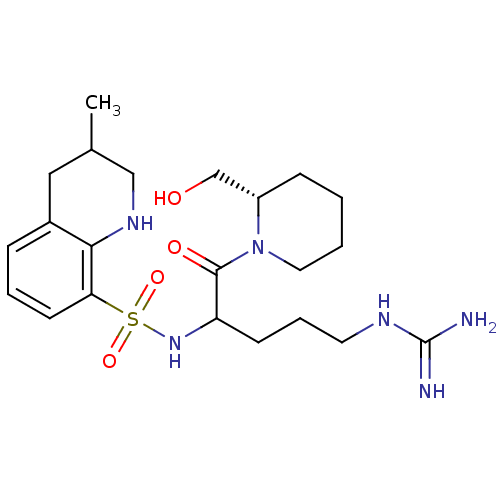
(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCCC[C@H]1CO Show InChI InChI=1S/C22H36N6O4S/c1-15-12-16-6-4-9-19(20(16)26-13-15)33(31,32)27-18(8-5-10-25-22(23)24)21(30)28-11-3-2-7-17(28)14-29/h4,6,9,15,17-18,26-27,29H,2-3,5,7-8,10-14H2,1H3,(H4,23,24,25)/t15?,17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50077057

(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CNC(=O)NCCC1CCN(CC1)C(=O)[C@H](Cc1nc2ccccc2s1)NS(=O)(=O)c1cccc2CC(C)(C)CNc12 Show InChI InChI=1S/C30H40N6O4S2/c1-30(2)18-21-7-6-10-25(27(21)33-19-30)42(39,40)35-23(17-26-34-22-8-4-5-9-24(22)41-26)28(37)36-15-12-20(13-16-36)11-14-32-29(38)31-3/h4-10,20,23,33,35H,11-19H2,1-3H3,(H2,31,32,38)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin (in vitro) |
Bioorg Med Chem Lett 9: 1317-22 (1999)
BindingDB Entry DOI: 10.7270/Q2ZW1K31 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073294
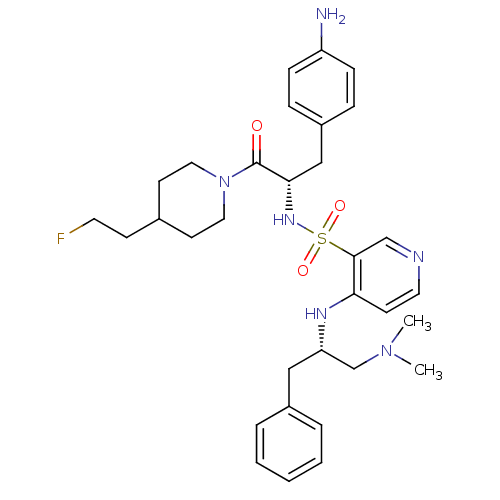
(4-((S)-1-Benzyl-2-dimethylamino-ethylamino)-pyridi...)Show SMILES CN(C)C[C@H](Cc1ccccc1)Nc1ccncc1S(=O)(=O)N[C@@H](Cc1ccc(N)cc1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C32H43FN6O3S/c1-38(2)23-28(20-25-6-4-3-5-7-25)36-29-13-17-35-22-31(29)43(41,42)37-30(21-26-8-10-27(34)11-9-26)32(40)39-18-14-24(12-16-33)15-19-39/h3-11,13,17,22,24,28,30,37H,12,14-16,18-21,23,34H2,1-2H3,(H,35,36)/t28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50077058
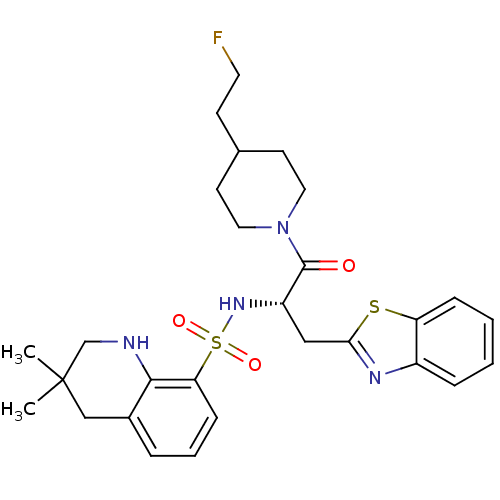
(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C28H35FN4O3S2/c1-28(2)17-20-6-5-9-24(26(20)30-18-28)38(35,36)32-22(16-25-31-21-7-3-4-8-23(21)37-25)27(34)33-14-11-19(10-13-29)12-15-33/h3-9,19,22,30,32H,10-18H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50077058

(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C28H35FN4O3S2/c1-28(2)17-20-6-5-9-24(26(20)30-18-28)38(35,36)32-22(16-25-31-21-7-3-4-8-23(21)37-25)27(34)33-14-11-19(10-13-29)12-15-33/h3-9,19,22,30,32H,10-18H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin (in vitro) |
Bioorg Med Chem Lett 9: 1317-22 (1999)
BindingDB Entry DOI: 10.7270/Q2ZW1K31 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082612
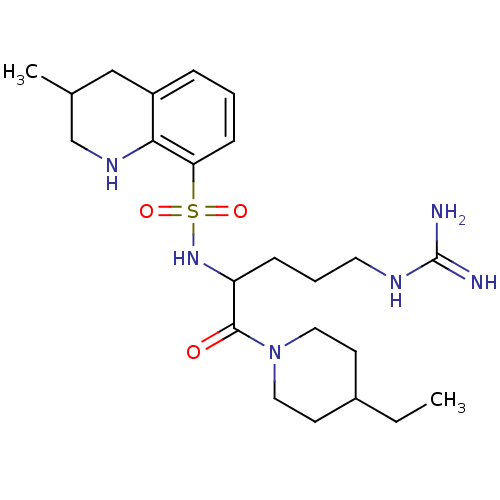
(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CCC1CCN(CC1)C(=O)C(CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H38N6O3S/c1-3-17-9-12-29(13-10-17)22(30)19(7-5-11-26-23(24)25)28-33(31,32)20-8-4-6-18-14-16(2)15-27-21(18)20/h4,6,8,16-17,19,27-28H,3,5,7,9-15H2,1-2H3,(H4,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090244
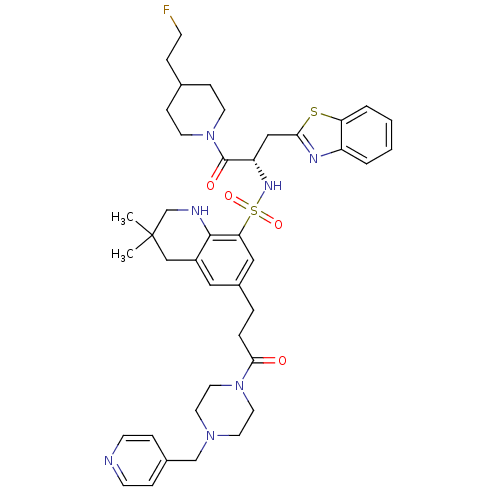
(3,3-Dimethyl-6-[3-oxo-3-(4-pyridin-4-ylmethyl-pipe...)Show SMILES CC1(C)CNc2c(C1)cc(CCC(=O)N1CCN(Cc3ccncc3)CC1)cc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C41H52FN7O4S2/c1-41(2)26-32-23-31(7-8-38(50)48-21-19-47(20-22-48)27-30-10-15-43-16-11-30)24-36(39(32)44-28-41)55(52,53)46-34(25-37-45-33-5-3-4-6-35(33)54-37)40(51)49-17-12-29(9-14-42)13-18-49/h3-6,10-11,15-16,23-24,29,34,44,46H,7-9,12-14,17-22,25-28H2,1-2H3/t34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390418
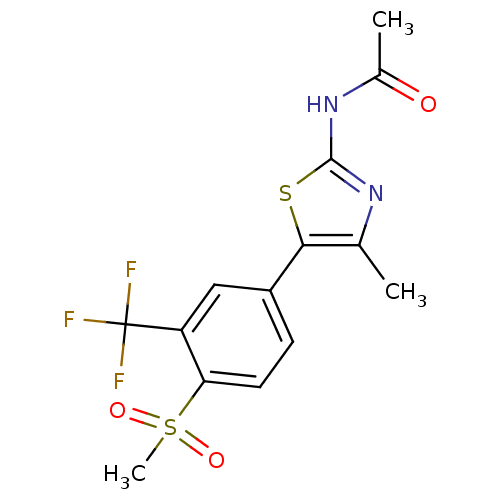
(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075870
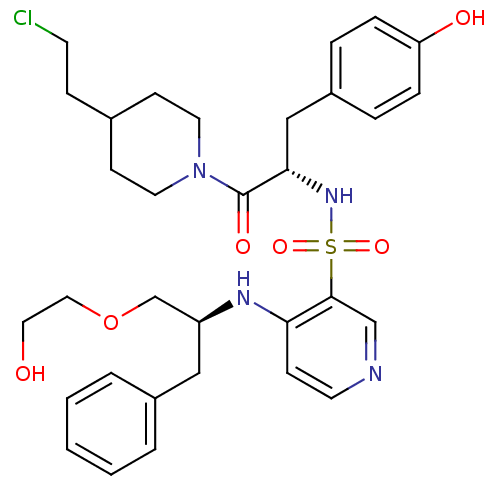
(4-[(S)-1-(2-Hydroxy-ethoxymethyl)-2-phenyl-ethylam...)Show SMILES OCCOC[C@H](Cc1ccccc1)Nc1ccncc1S(=O)(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC(CCCl)CC1 Show InChI InChI=1S/C32H41ClN4O6S/c33-14-10-24-12-16-37(17-13-24)32(40)30(21-26-6-8-28(39)9-7-26)36-44(41,42)31-22-34-15-11-29(31)35-27(23-43-19-18-38)20-25-4-2-1-3-5-25/h1-9,11,15,22,24,27,30,36,38-39H,10,12-14,16-21,23H2,(H,34,35)/t27-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082580
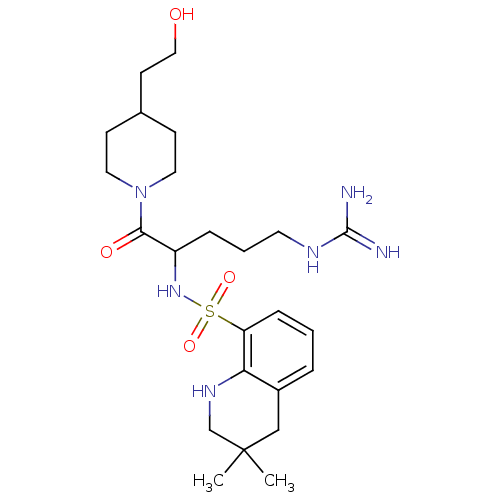
(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCO)CC1 Show InChI InChI=1S/C24H40N6O4S/c1-24(2)15-18-5-3-7-20(21(18)28-16-24)35(33,34)29-19(6-4-11-27-23(25)26)22(32)30-12-8-17(9-13-30)10-14-31/h3,5,7,17,19,28-29,31H,4,6,8-16H2,1-2H3,(H4,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibitory activity against human thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090250
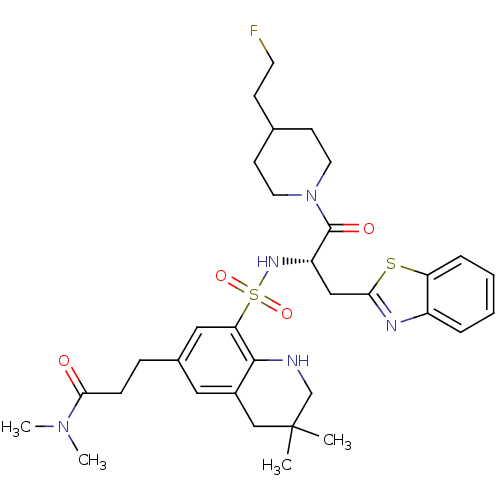
(3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...)Show SMILES CN(C)C(=O)CCc1cc2CC(C)(C)CNc2c(c1)S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C33H44FN5O4S2/c1-33(2)20-24-17-23(9-10-30(40)38(3)4)18-28(31(24)35-21-33)45(42,43)37-26(19-29-36-25-7-5-6-8-27(25)44-29)32(41)39-15-12-22(11-14-34)13-16-39/h5-8,17-18,22,26,35,37H,9-16,19-21H2,1-4H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082608
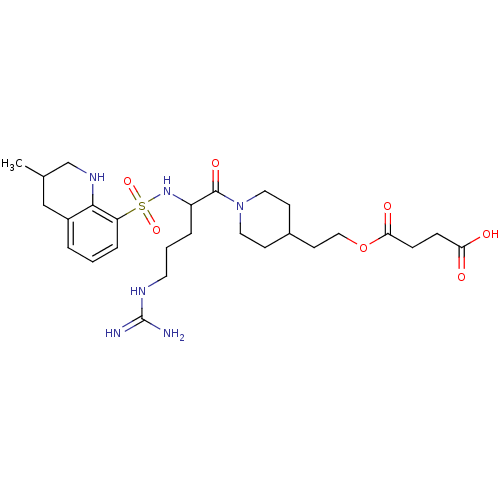
(CHEMBL139718 | Succinic acid mono-(2-{1-[5-guanidi...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCOC(=O)CCC(O)=O)CC1 Show InChI InChI=1S/C27H42N6O7S/c1-18-16-20-4-2-6-22(25(20)31-17-18)41(38,39)32-21(5-3-12-30-27(28)29)26(37)33-13-9-19(10-14-33)11-15-40-24(36)8-7-23(34)35/h2,4,6,18-19,21,31-32H,3,5,7-17H2,1H3,(H,34,35)(H4,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090247

(6-[3-(4-Acetyl-piperazin-1-yl)-3-oxo-propyl]-3,3-d...)Show SMILES CC(=O)N1CCN(CC1)C(=O)CCc1cc2CC(C)(C)CNc2c(c1)S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C37H49FN6O5S2/c1-25(45)42-16-18-43(19-17-42)34(46)9-8-27-20-28-23-37(2,3)24-39-35(28)32(21-27)51(48,49)41-30(22-33-40-29-6-4-5-7-31(29)50-33)36(47)44-14-11-26(10-13-38)12-15-44/h4-7,20-21,26,30,39,41H,8-19,22-24H2,1-3H3/t30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390415
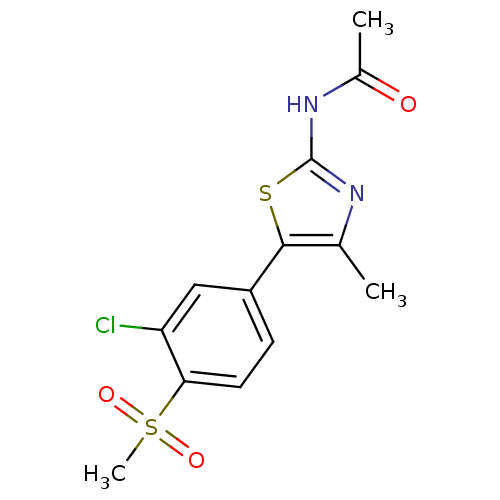
(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390411
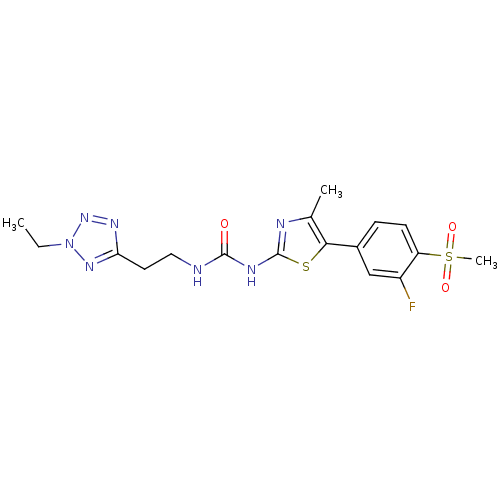
(CHEMBL2071342)Show SMILES CCn1nnc(CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)n1 Show InChI InChI=1S/C17H20FN7O3S2/c1-4-25-23-14(22-24-25)7-8-19-16(26)21-17-20-10(2)15(29-17)11-5-6-13(12(18)9-11)30(3,27)28/h5-6,9H,4,7-8H2,1-3H3,(H2,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50077049
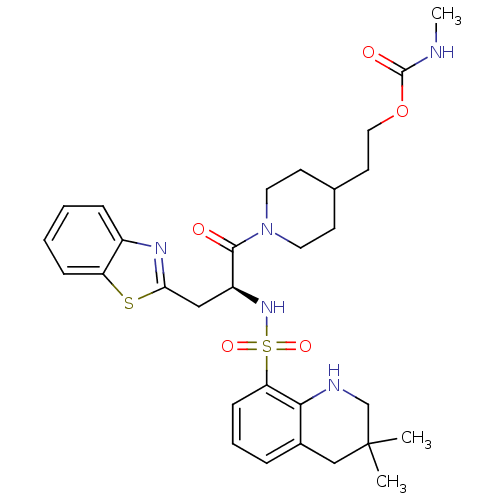
(CHEMBL24186 | Methyl-carbamic acid 2-{1-[(S)-3-ben...)Show SMILES CNC(=O)OCCC1CCN(CC1)C(=O)[C@H](Cc1nc2ccccc2s1)NS(=O)(=O)c1cccc2CC(C)(C)CNc12 Show InChI InChI=1S/C30H39N5O5S2/c1-30(2)18-21-7-6-10-25(27(21)32-19-30)42(38,39)34-23(17-26-33-22-8-4-5-9-24(22)41-26)28(36)35-14-11-20(12-15-35)13-16-40-29(37)31-3/h4-10,20,23,32,34H,11-19H2,1-3H3,(H,31,37)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin (in vitro) |
Bioorg Med Chem Lett 9: 1317-22 (1999)
BindingDB Entry DOI: 10.7270/Q2ZW1K31 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073295
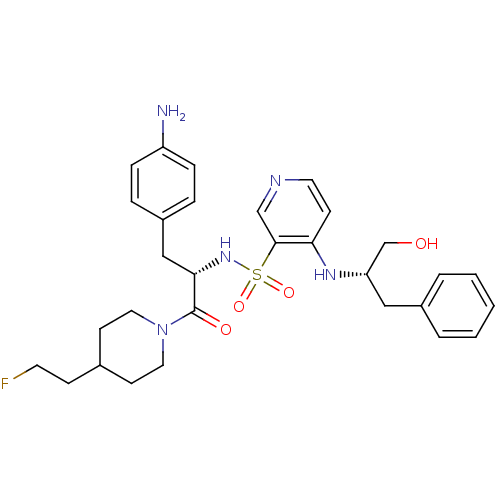
(4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...)Show SMILES Nc1ccc(C[C@H](NS(=O)(=O)c2cnccc2N[C@H](CO)Cc2ccccc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C30H38FN5O4S/c31-14-10-22-12-16-36(17-13-22)30(38)28(19-24-6-8-25(32)9-7-24)35-41(39,40)29-20-33-15-11-27(29)34-26(21-37)18-23-4-2-1-3-5-23/h1-9,11,15,20,22,26,28,35,37H,10,12-14,16-19,21,32H2,(H,33,34)/t26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090243
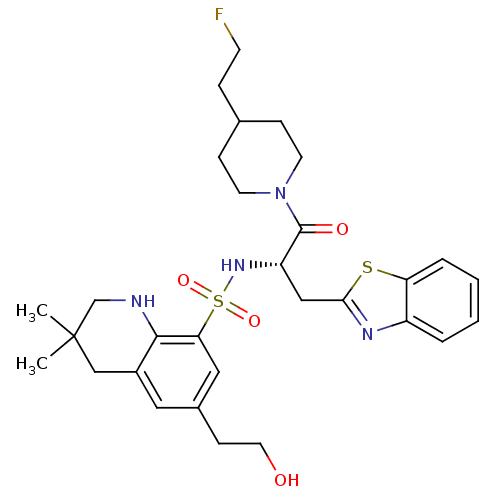
(6-(2-Hydroxy-ethyl)-3,3-dimethyl-1,2,3,4-tetrahydr...)Show SMILES CC1(C)CNc2c(C1)cc(CCO)cc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C30H39FN4O4S2/c1-30(2)18-22-15-21(10-14-36)16-26(28(22)32-19-30)41(38,39)34-24(17-27-33-23-5-3-4-6-25(23)40-27)29(37)35-12-8-20(7-11-31)9-13-35/h3-6,15-16,20,24,32,34,36H,7-14,17-19H2,1-2H3/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073295

(4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...)Show SMILES Nc1ccc(C[C@H](NS(=O)(=O)c2cnccc2N[C@H](CO)Cc2ccccc2)C(=O)N2CCC(CCF)CC2)cc1 Show InChI InChI=1S/C30H38FN5O4S/c31-14-10-22-12-16-36(17-13-22)30(38)28(19-24-6-8-25(32)9-7-24)35-41(39,40)29-20-33-15-11-27(29)34-26(21-37)18-23-4-2-1-3-5-23/h1-9,11,15,20,22,26,28,35,37H,10,12-14,16-19,21,32H2,(H,33,34)/t26-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075873
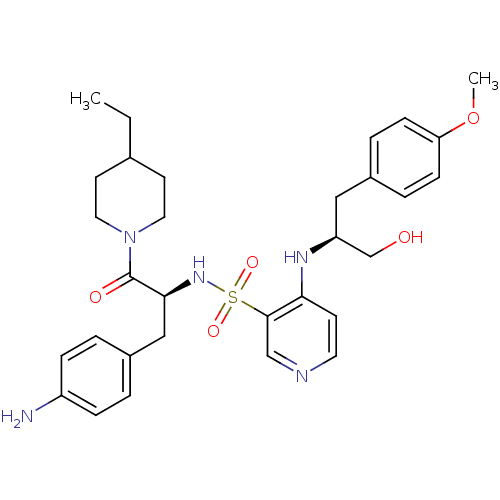
(4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...)Show SMILES CCC1CCN(CC1)C(=O)[C@H](Cc1ccc(N)cc1)NS(=O)(=O)c1cnccc1N[C@H](CO)Cc1ccc(OC)cc1 Show InChI InChI=1S/C31H41N5O5S/c1-3-22-13-16-36(17-14-22)31(38)29(19-24-4-8-25(32)9-5-24)35-42(39,40)30-20-33-15-12-28(30)34-26(21-37)18-23-6-10-27(41-2)11-7-23/h4-12,15,20,22,26,29,35,37H,3,13-14,16-19,21,32H2,1-2H3,(H,33,34)/t26-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibtion of human thrombin. |
Bioorg Med Chem Lett 9: 737-42 (1999)
BindingDB Entry DOI: 10.7270/Q2BG2N6V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082581
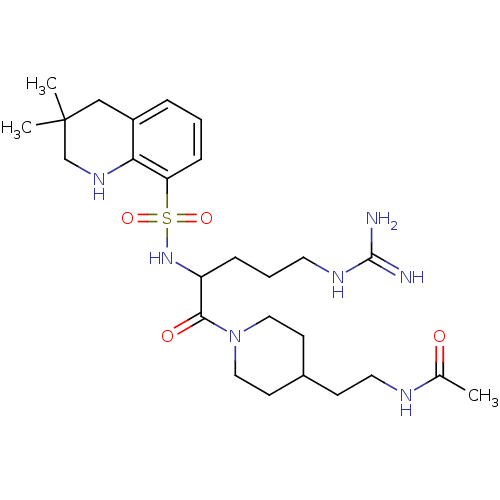
(CHEMBL143203 | N-(2-{1-[2-(3,3-Dimethyl-1,2,3,4-te...)Show SMILES CC(=O)NCCC1CCN(CC1)C(=O)C(CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)(C)CNc12 Show InChI InChI=1S/C26H43N7O4S/c1-18(34)29-13-9-19-10-14-33(15-11-19)24(35)21(7-5-12-30-25(27)28)32-38(36,37)22-8-4-6-20-16-26(2,3)17-31-23(20)22/h4,6,8,19,21,31-32H,5,7,9-17H2,1-3H3,(H,29,34)(H4,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082573

(3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCO)CC1 Show InChI InChI=1S/C23H38N6O4S/c1-16-14-18-4-2-6-20(21(18)27-15-16)34(32,33)28-19(5-3-10-26-23(24)25)22(31)29-11-7-17(8-12-29)9-13-30/h2,4,6,16-17,19,27-28,30H,3,5,7-15H2,1H3,(H4,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibitory activity against human thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082591
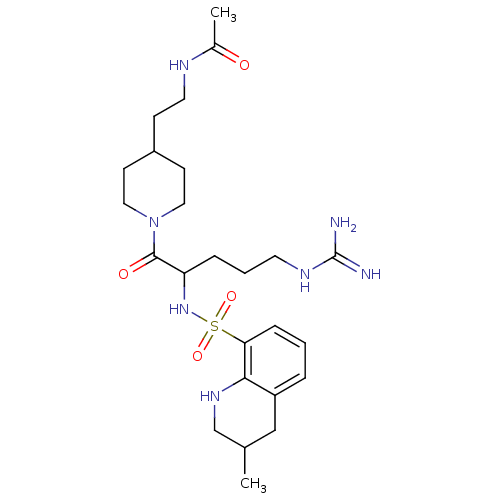
(CHEMBL139606 | N-(2-{1-[5-Guanidino-2-(3-methyl-1,...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCNC(C)=O)CC1 Show InChI InChI=1S/C25H41N7O4S/c1-17-15-20-5-3-7-22(23(20)30-16-17)37(35,36)31-21(6-4-11-29-25(26)27)24(34)32-13-9-19(10-14-32)8-12-28-18(2)33/h3,5,7,17,19,21,30-31H,4,6,8-16H2,1-2H3,(H,28,33)(H4,26,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390417
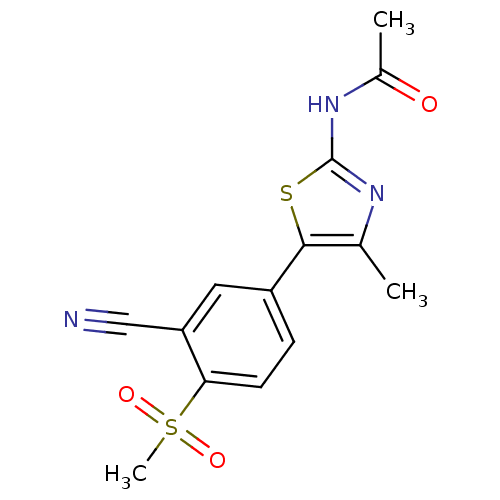
(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082603

((R)-3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES C[C@H]1CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCO)CC1 Show InChI InChI=1S/C23H38N6O4S/c1-16-14-18-4-2-6-20(21(18)27-15-16)34(32,33)28-19(5-3-10-26-23(24)25)22(31)29-11-7-17(8-12-29)9-13-30/h2,4,6,16-17,19,27-28,30H,3,5,7-15H2,1H3,(H4,24,25,26)/t16-,19?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50090246
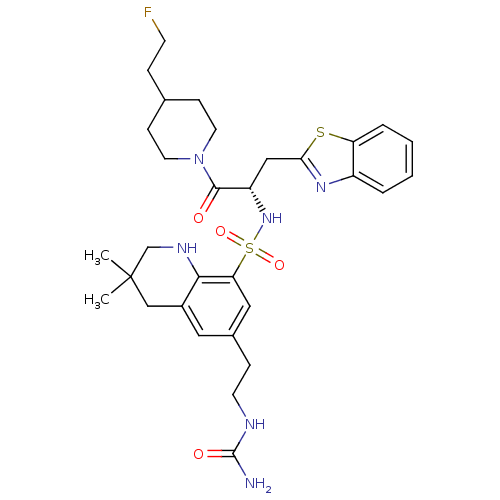
(3,3-Dimethyl-6-(2-ureido-ethyl)-1,2,3,4-tetrahydro...)Show SMILES CC1(C)CNc2c(C1)cc(CCNC(N)=O)cc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 Show InChI InChI=1S/C31H41FN6O4S2/c1-31(2)18-22-15-21(8-12-34-30(33)40)16-26(28(22)35-19-31)44(41,42)37-24(17-27-36-23-5-3-4-6-25(23)43-27)29(39)38-13-9-20(7-11-32)10-14-38/h3-6,15-16,20,24,35,37H,7-14,17-19H2,1-2H3,(H3,33,34,40)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity against thrombin |
Bioorg Med Chem Lett 10: 1567-70 (2000)
BindingDB Entry DOI: 10.7270/Q2RX9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50082580

(3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...)Show SMILES CC1(C)CNc2c(C1)cccc2S(=O)(=O)NC(CCCNC(N)=N)C(=O)N1CCC(CCO)CC1 Show InChI InChI=1S/C24H40N6O4S/c1-24(2)15-18-5-3-7-20(21(18)28-16-24)35(33,34)29-19(6-4-11-27-23(25)26)22(32)30-12-8-17(9-13-30)10-14-31/h3,5,7,17,19,28-29,31H,4,6,8-16H2,1-2H3,(H4,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine thrombin |
J Med Chem 42: 4584-603 (1999)
BindingDB Entry DOI: 10.7270/Q28G8MDS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data