

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629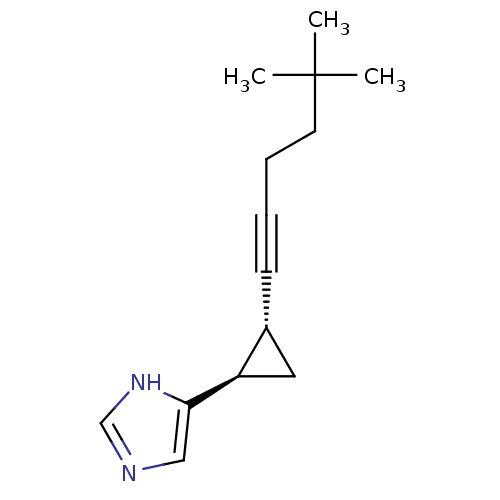 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916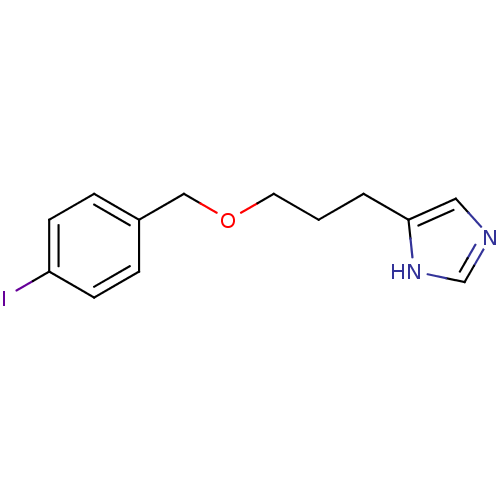 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074627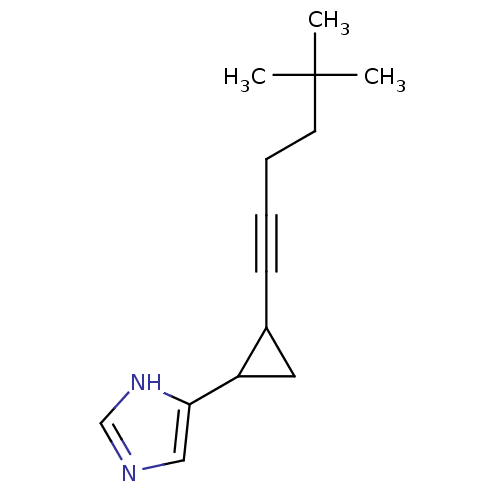 (4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50604668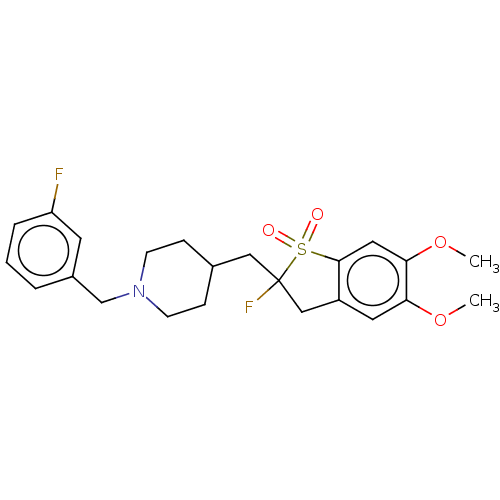 (CHEMBL5180947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114305 BindingDB Entry DOI: 10.7270/Q26M3BX2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070217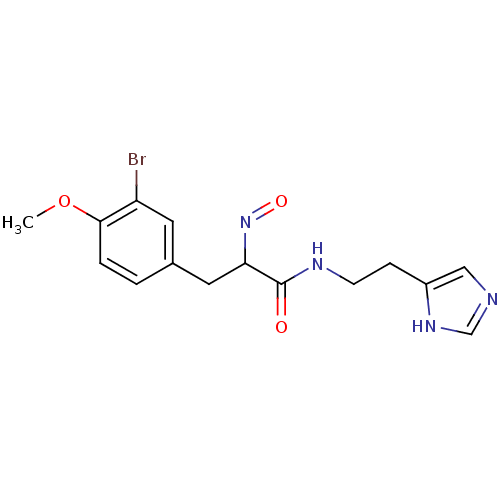 (3-(3-Bromo-4-methoxy-phenyl)-2-[(E)-hydroxyimino]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038757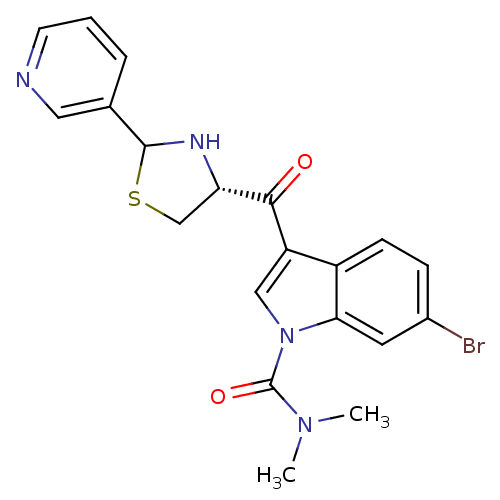 (6-Bromo-3-((R)-2-pyridin-3-yl-thiazolidine-4-carbo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211457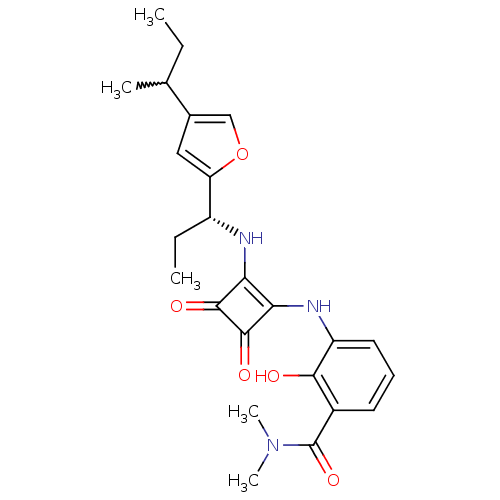 (3-(2-((R)-1-(4-sec-butylfuran-2-yl)propylamino)-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214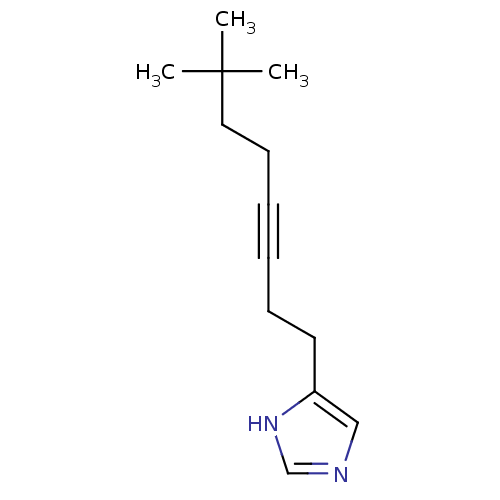 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211449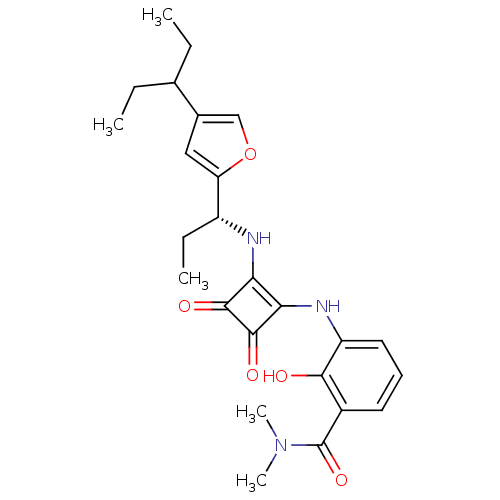 ((R)-3-(3,4-dioxo-2-(1-(4-(pentan-3-yl)furan-2-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038809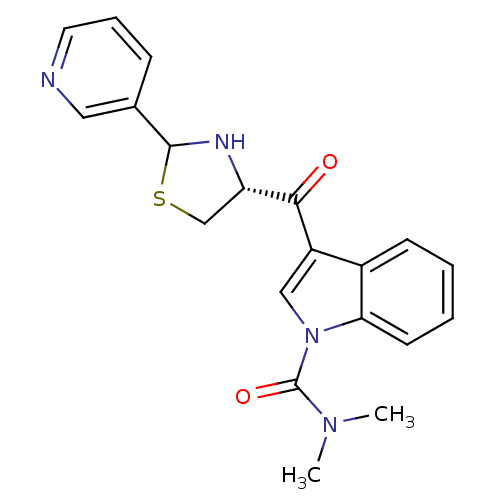 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220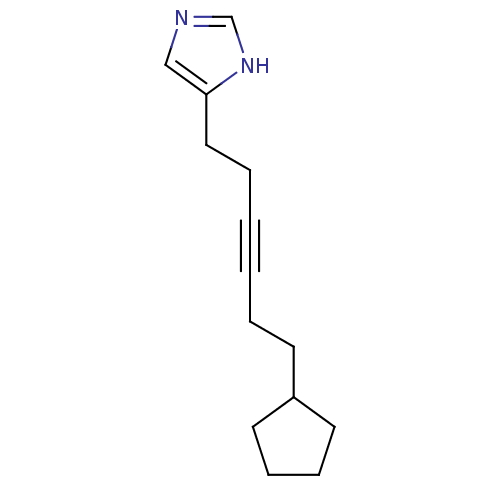 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM7967 (1-methyl-5-(beta-aminoethyl)-imidazole | 2-(1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038750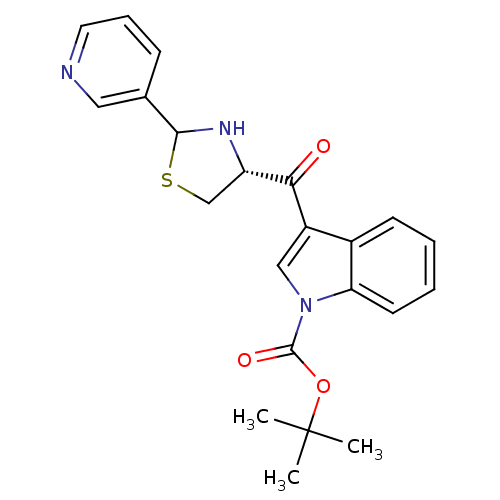 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038834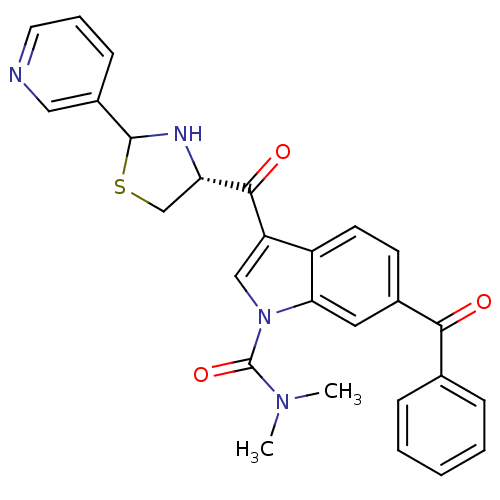 (6-Benzoyl-3-((R)-2-pyridin-3-yl-thiazolidine-4-car...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038792 (6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211454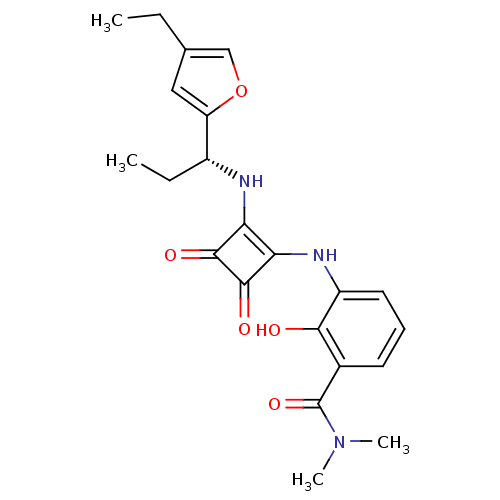 ((R)-3-(2-(1-(4-ethylfuran-2-yl)propylamino)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211458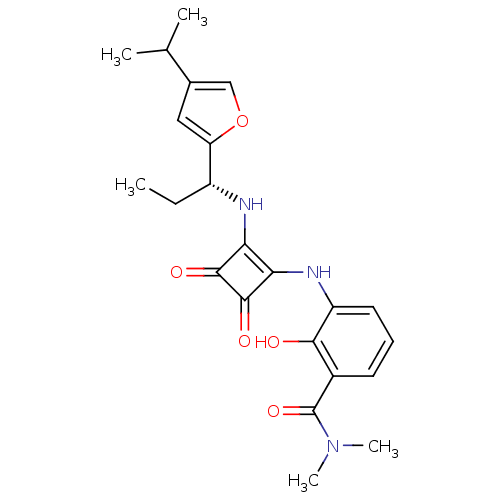 ((R)-2-hydroxy-3-(2-(1-(4-isopropylfuran-2-yl)propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM85407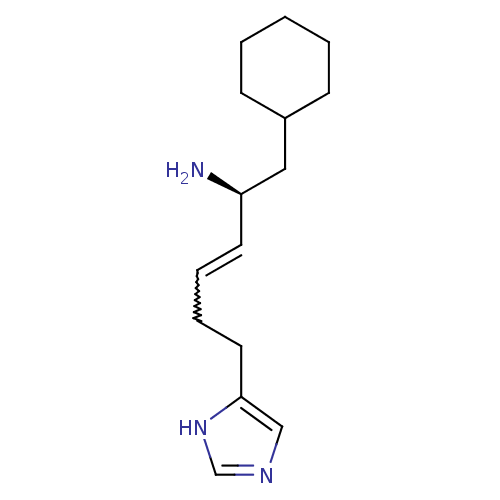 (GT 2231) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038806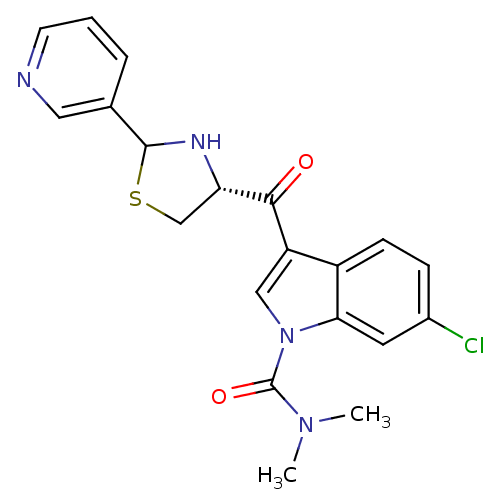 (6-Chloro-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038777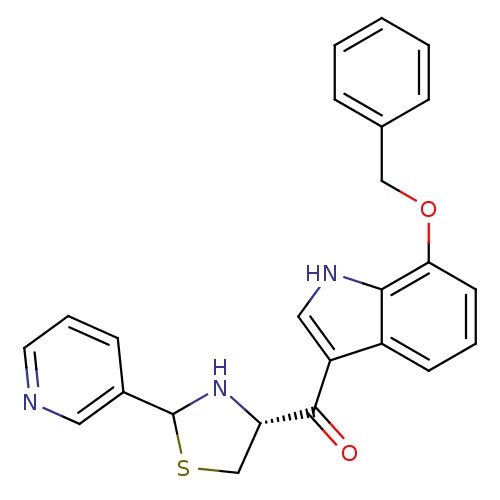 ((7-Benzyloxy-1H-indol-3-yl)-((R)-2-pyridin-3-yl-th...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038780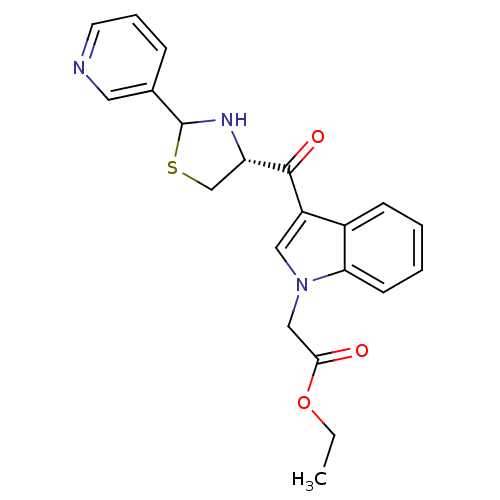 (CHEMBL64428 | [3-((R)-2-Pyridin-3-yl-thiazolidine-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038786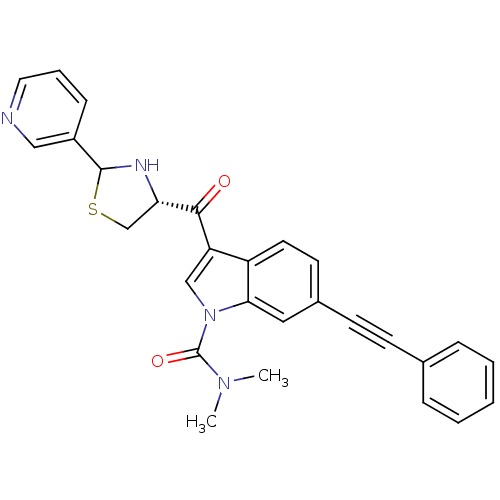 (6-Phenylethynyl-3-((R)-2-pyridin-3-yl-thiazolidine...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038825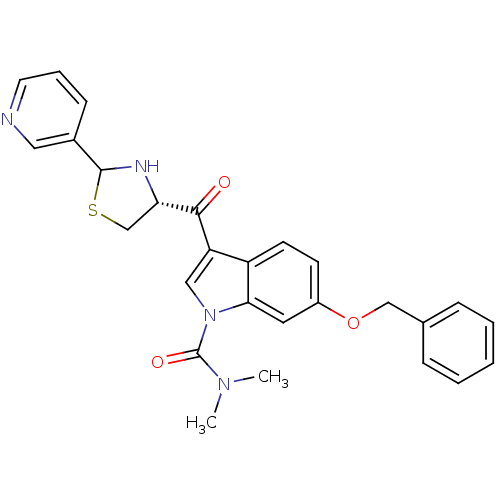 (6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038810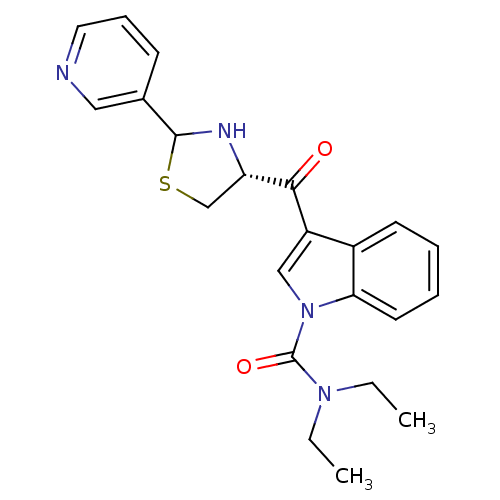 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074630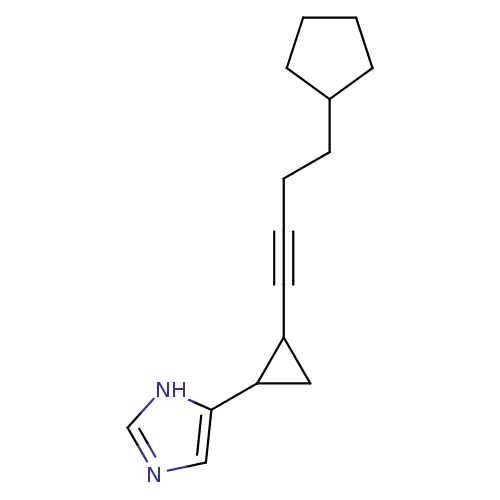 (4-[2-(4-Cyclopentyl-but-1-ynyl)-cyclopropyl]-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038819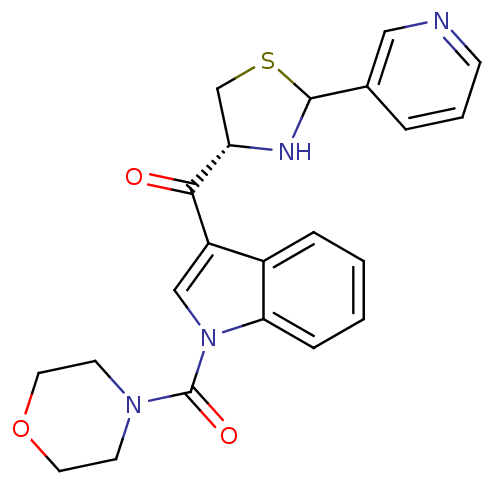 (CHEMBL64464 | Morpholin-4-yl-[3-((R)-2-pyridin-3-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Antagonistic activity of the compound was evaluated against histamine H3 receptor using [3H]- N-alpha-methylhistamine as radioligand in experiment 2 | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038801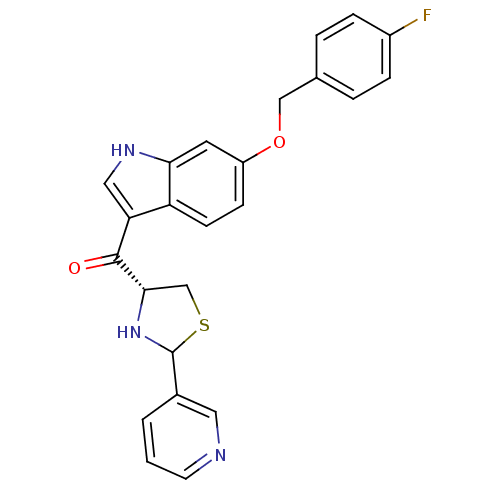 (CHEMBL62830 | [6-(4-Fluoro-benzyloxy)-1H-indol-3-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038749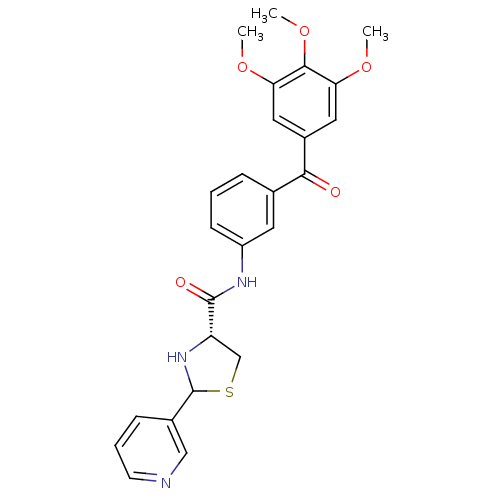 ((R)-2-Pyridin-3-yl-thiazolidine-4-carboxylic acid ...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038759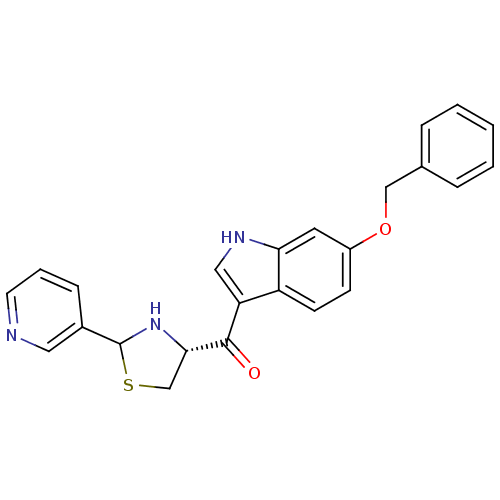 ((6-Benzyloxy-1H-indol-3-yl)-((R)-2-pyridin-3-yl-th...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038835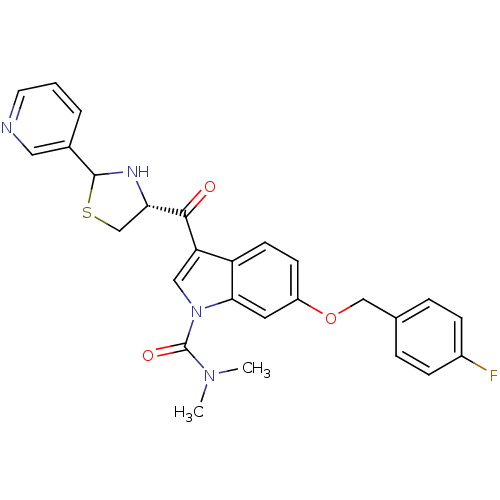 (6-(4-Fluoro-benzyloxy)-3-((R)-2-pyridin-3-yl-thiaz...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074624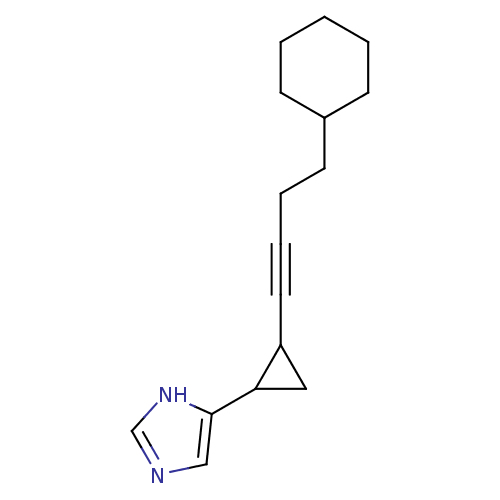 (4-[2-(4-Cyclohexyl-but-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 275: 598-604 (1995) BindingDB Entry DOI: 10.7270/Q21G0JSP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038752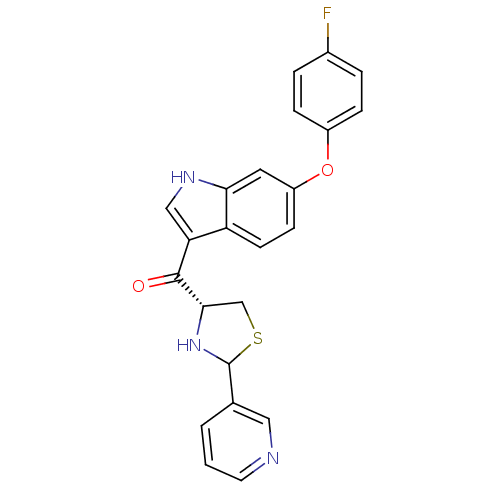 (CHEMBL64636 | [6-(4-Fluoro-phenoxy)-1H-indol-3-yl]...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038760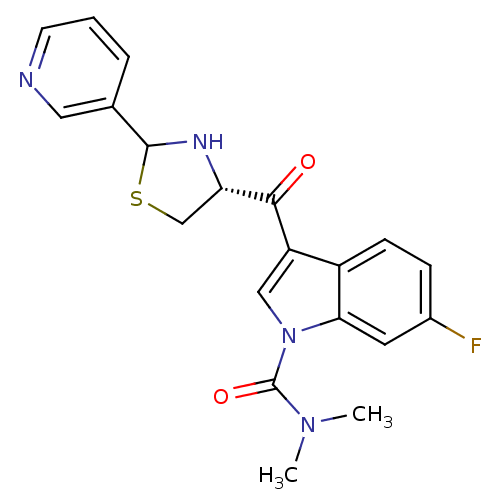 (6-Fluoro-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211453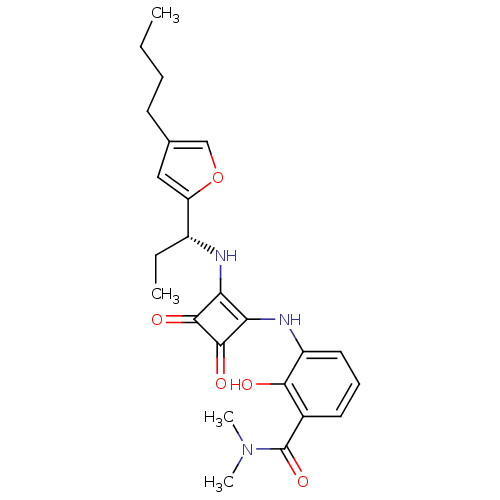 ((R)-3-(2-(1-(4-butylfuran-2-yl)propylamino)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038788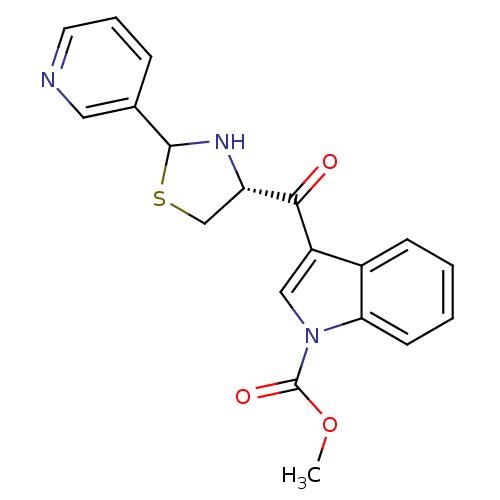 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50211456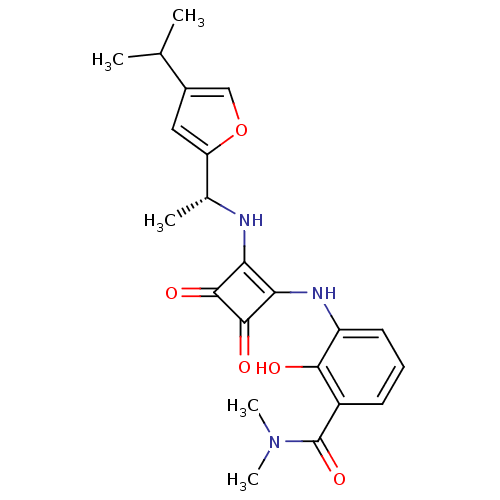 ((R)-2-hydroxy-3-(2-(1-(4-isopropylfuran-2-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA | Bioorg Med Chem Lett 17: 3778-83 (2007) Article DOI: 10.1016/j.bmcl.2007.04.016 BindingDB Entry DOI: 10.7270/Q25D8RH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038754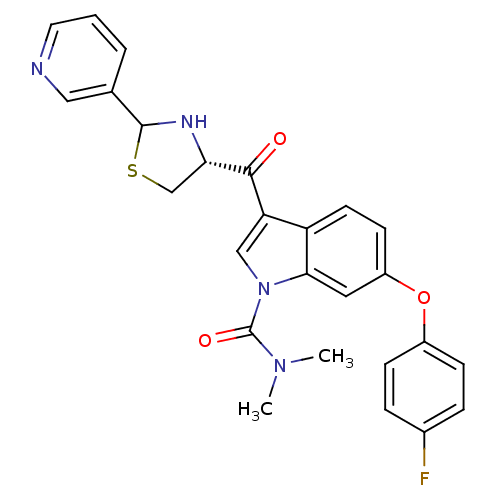 (6-(4-Fluoro-phenoxy)-3-((R)-2-pyridin-3-yl-thiazol...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038763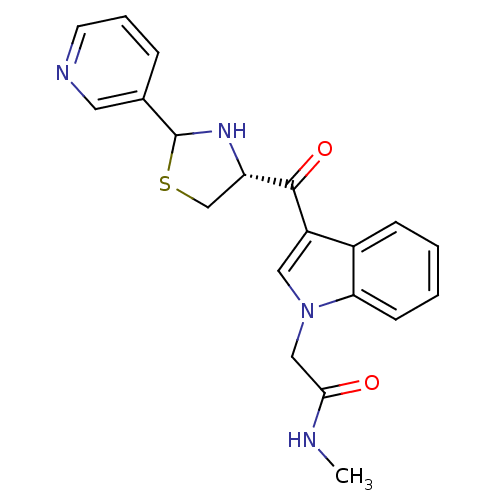 (CHEMBL293259 | N-Methyl-2-[3-((R)-2-pyridin-3-yl-t...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038830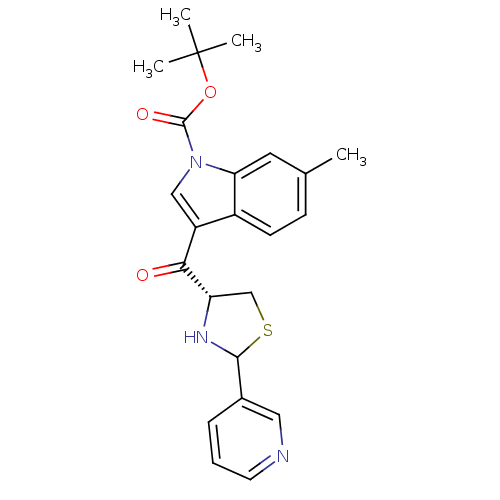 (6-Methyl-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074628 (4-Undec-3-ynyl-1H-imidazole | CHEMBL170723) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070211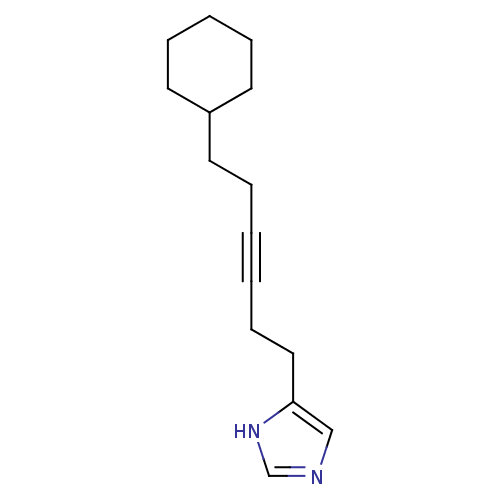 (4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070211 (4-(6-Cyclohexyl-hex-3-ynyl)-1H-imidazole | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1478 total ) | Next | Last >> |