

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50301021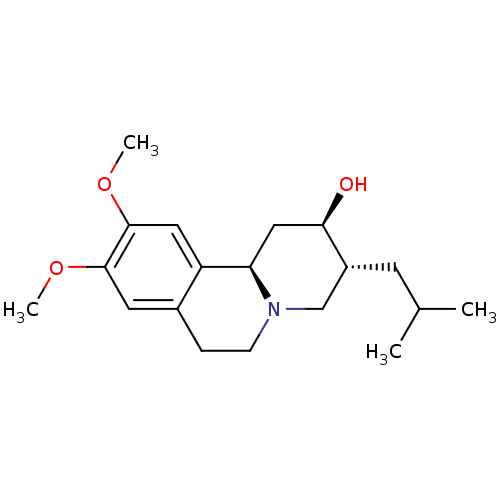 ((+)-dihydrotetrabenzaine | CHEMBL576222 | US110532...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of VMAT2 in rat brain | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00202 BindingDB Entry DOI: 10.7270/Q2NZ8CCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50301021 ((+)-dihydrotetrabenzaine | CHEMBL576222 | US110532...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of VMAT2 in rat brain | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00202 BindingDB Entry DOI: 10.7270/Q2NZ8CCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31801 (2-aminobenzimidazole deriv., 12) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 7 | -46.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50125977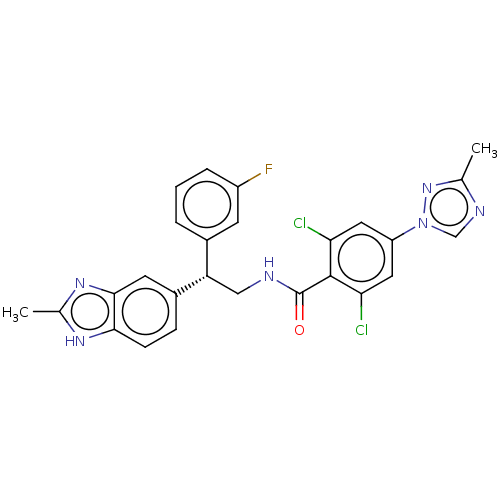 (CHEMBL3627897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... | J Med Chem 59: 1818-29 (2016) Article DOI: 10.1021/acs.jmedchem.5b01293 BindingDB Entry DOI: 10.7270/Q29Z96S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50342820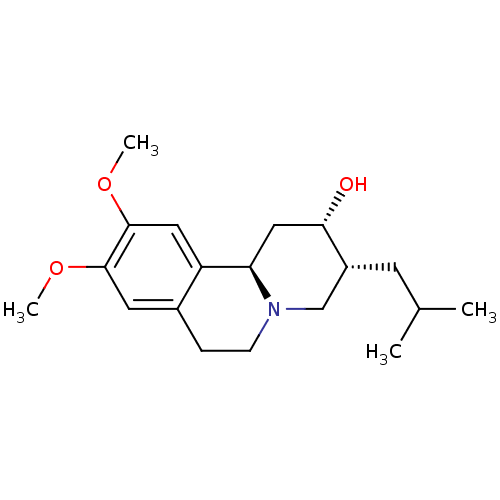 ((2S,3R,11bR)-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of VMAT2 in rat brain | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00202 BindingDB Entry DOI: 10.7270/Q2NZ8CCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50151405 (CHEMBL3775211 | US10189819, Example 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... | J Med Chem 59: 1818-29 (2016) Article DOI: 10.1021/acs.jmedchem.5b01293 BindingDB Entry DOI: 10.7270/Q29Z96S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31800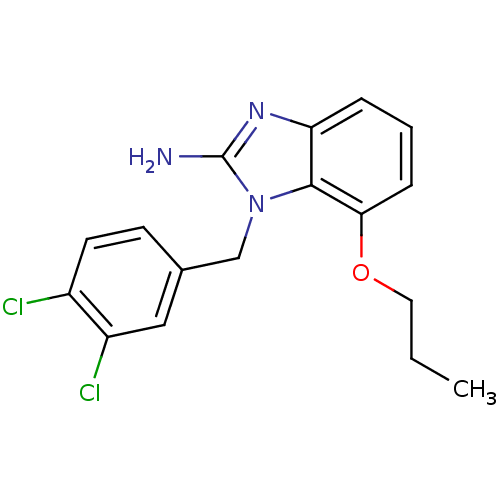 (2-aminobenzimidazole deriv., 11) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204495 (CHEMBL3921061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of human serum BChE using S-Butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition... | Bioorg Med Chem 25: 360-371 (2017) Article DOI: 10.1016/j.bmc.2016.11.002 BindingDB Entry DOI: 10.7270/Q2NK3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Rattus norvegicus (Rat)) | BDBM50561748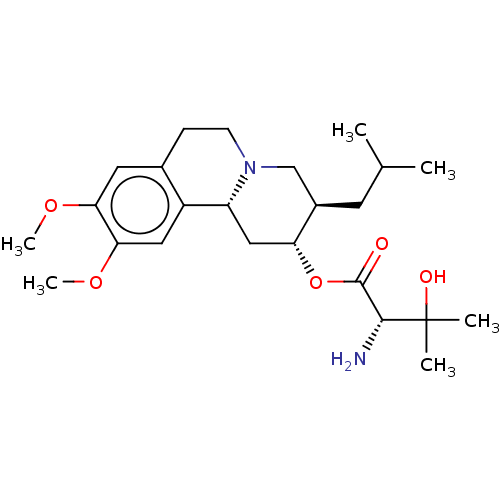 (CHEMBL4752068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of VMAT2 in rat brain | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00202 BindingDB Entry DOI: 10.7270/Q2NZ8CCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486243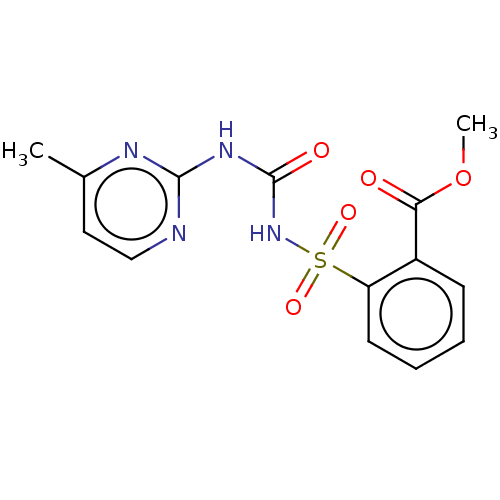 (MONOSULFURON ESTER) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268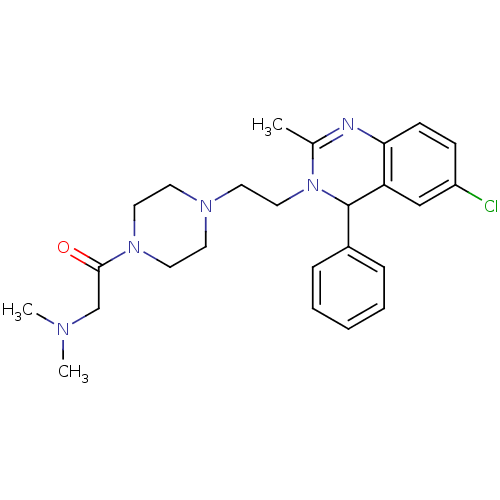 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354299 (CHEMBL1836378) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31798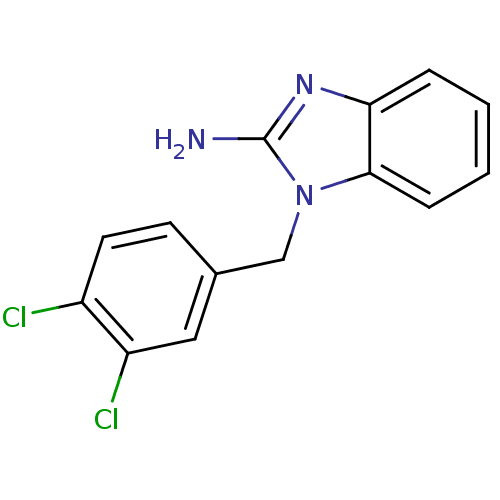 (2-aminobenzimidazole deriv., 9) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 400 | -36.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM50468050 (CHEMBL4287599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center Curated by ChEMBL | Assay Description Irreversible inhibition of KDM5A ARID/PhD1 domain deletion mutant (1 to 739 residues) (unknown origin) | J Med Chem 61: 10588-10601 (2018) Article DOI: 10.1021/acs.jmedchem.8b01219 BindingDB Entry DOI: 10.7270/Q2R49TGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50103521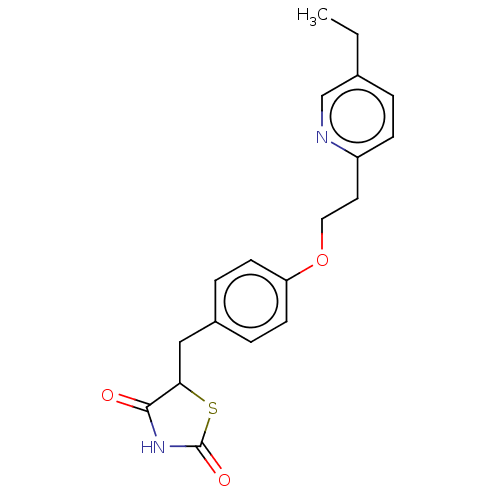 (Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50103521 (Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257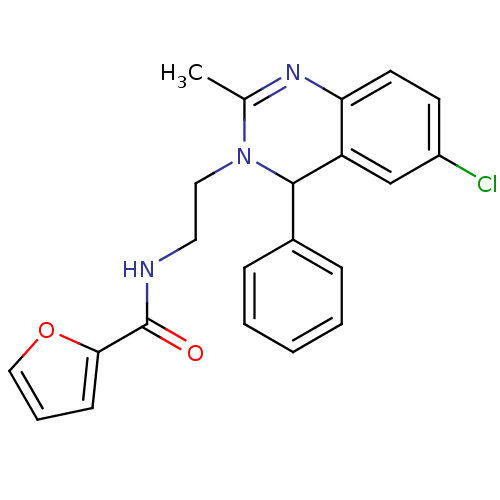 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31799 (2-aminobenzimidazole deriv., 10) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | -35.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50204495 (CHEMBL3921061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
South China University of Technology Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addit... | Bioorg Med Chem 25: 360-371 (2017) Article DOI: 10.1016/j.bmc.2016.11.002 BindingDB Entry DOI: 10.7270/Q2NK3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50530214 (CHEBI:82937 | Leriglitazone | Min-102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50530214 (CHEBI:82937 | Leriglitazone | Min-102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486242 (CHEMBL2230130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50125977 (CHEMBL3627897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human factor 10a using n-Acetyl-KPR-AFC as substrate preinubated for 30 mins followed by substrate addition measured after 1 hr by fluo... | J Med Chem 59: 1818-29 (2016) Article DOI: 10.1021/acs.jmedchem.5b01293 BindingDB Entry DOI: 10.7270/Q29Z96S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486241 (CHEBI:5869 | IMAZAQUIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486245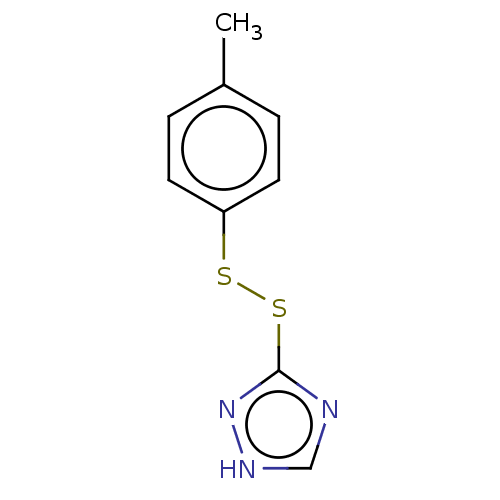 (CHEMBL2230131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486244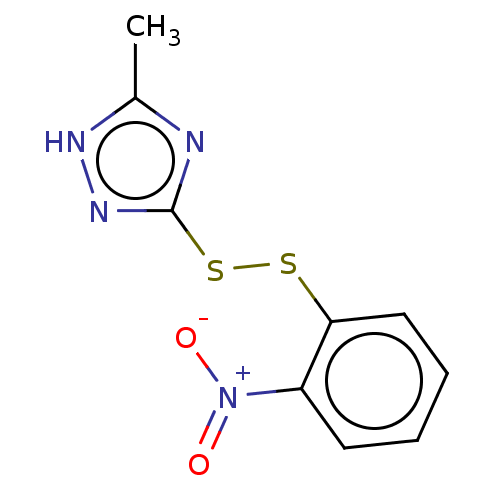 (CHEMBL2229985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486243 (MONOSULFURON ESTER) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31795 (2-aminobenzimidazole deriv., 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | -28.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31794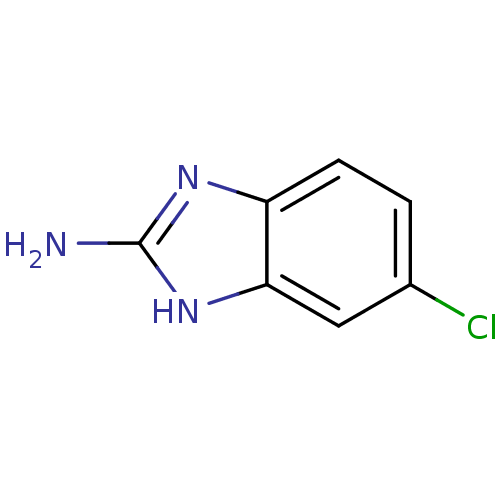 (2-aminobenzimidazole deriv., 4 | 5-Chloro-1H-benzo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.06E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31791 (2-aminobenzothiazole deriv., 2) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.11E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31797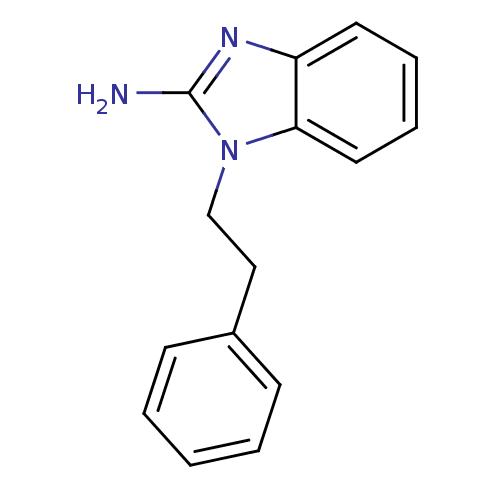 (2-aminobenzimidazole deriv., 8) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.39E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486242 (CHEMBL2230130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486245 (CHEMBL2230131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486244 (CHEMBL2229985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31793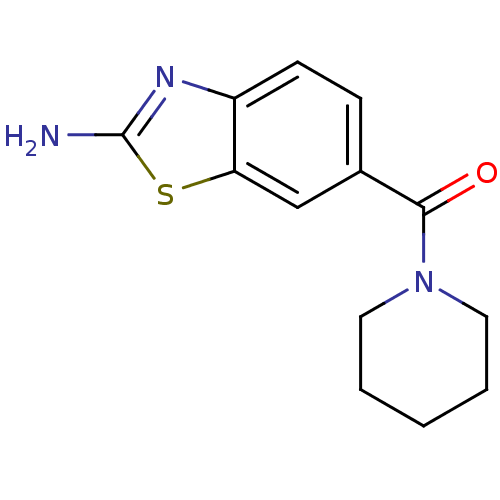 (2-aminobenzothiazole deriv., 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.41E+5 | -21.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31796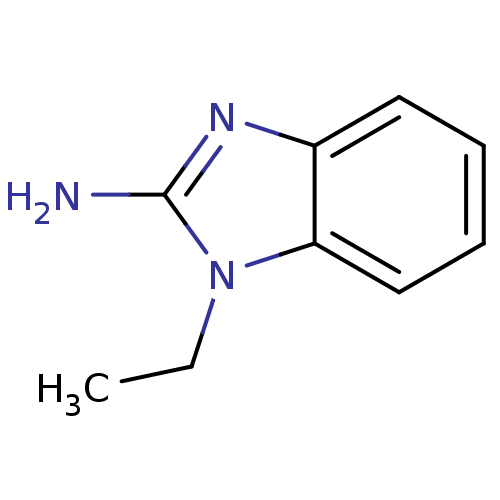 (2-aminobenzimidazole deriv., 7) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | >-21.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM7960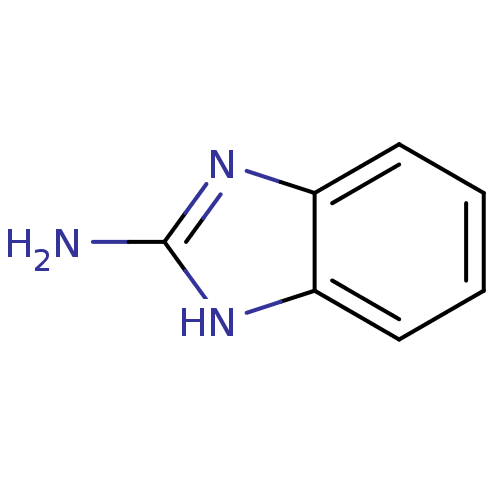 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 2.88E+5 | -20.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetolactate synthase, chloroplastic (Arabidopsis thaliana) | BDBM50486241 (CHEBI:5869 | IMAZAQUIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay | J Agric Food Chem 60: 8286-93 (2012) Article DOI: 10.1021/jf302206x BindingDB Entry DOI: 10.7270/Q2FX7DB2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144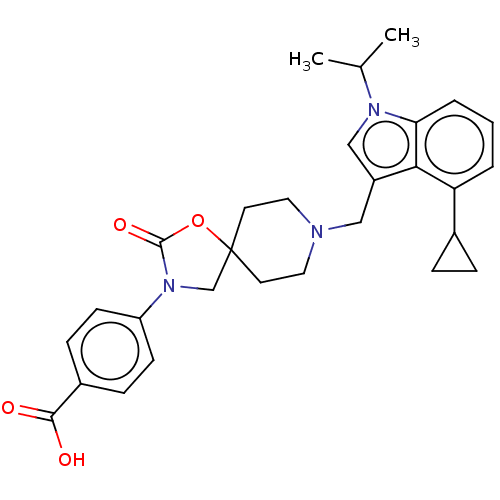 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468136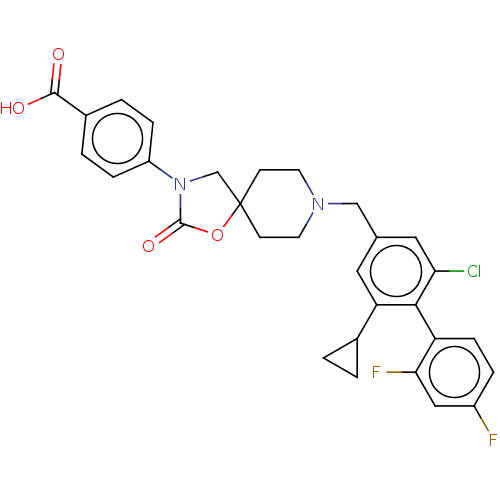 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160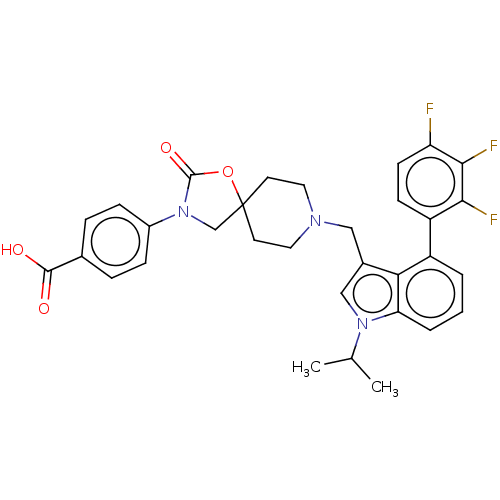 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50374938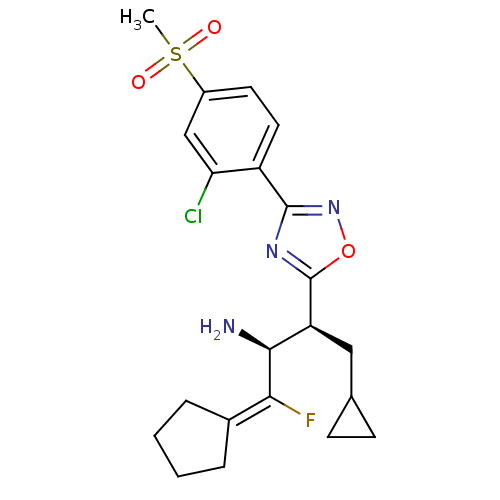 (CHEMBL402163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 expressed in insect cell | Bioorg Med Chem Lett 18: 2409-13 (2008) Article DOI: 10.1016/j.bmcl.2008.02.050 BindingDB Entry DOI: 10.7270/Q2HD7WJ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM123216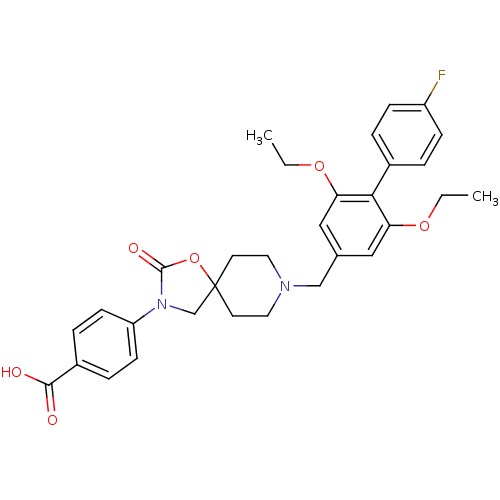 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468146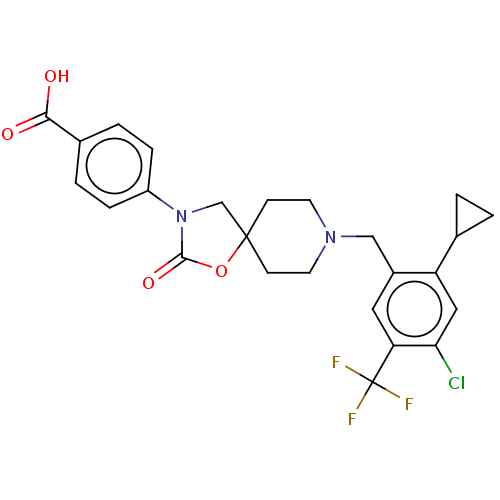 (CHEMBL4285917) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468157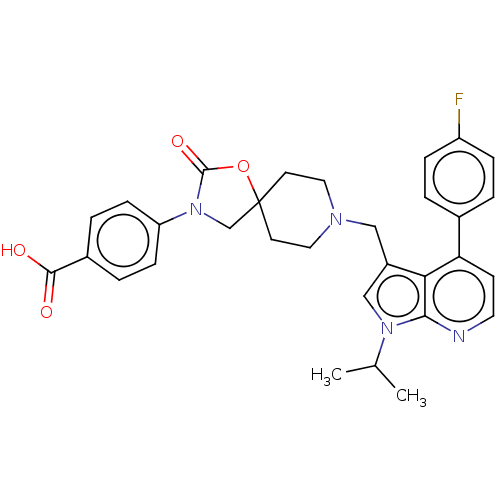 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3411 total ) | Next | Last >> |